2 (4 Fluorophenyl) 1 (4 methylphenyl) 1H phenanthro[9,10 d]imidazole
Full text
Figure
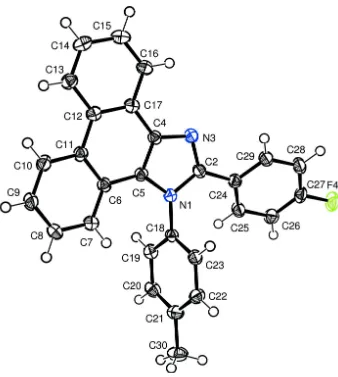
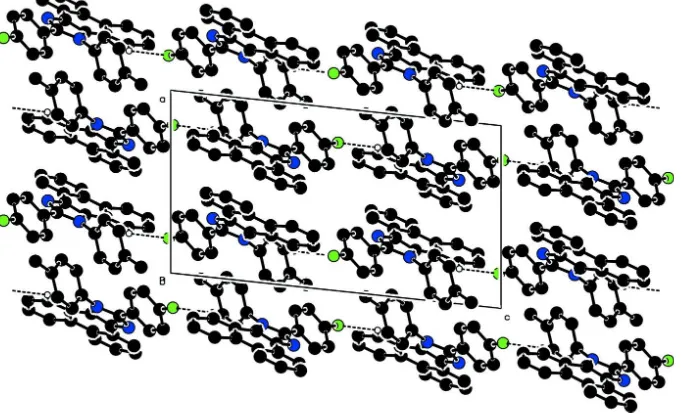
Related documents
H-atoms attached to carbon were placed in calculated positions (C—H = 0.95 - 0.98 Å) while that attached to nitrogen was placed in a location derived from a difference map and
All hydrogen atoms were observed in difference Fourier maps. The H atoms were refined using a riding model with a C —H distance of 0.99 Å for the methylene carbon atoms, 0.98 Å for
geometrically and allowed to ride on their parent atoms, with d(C—H) = 0.93 Å for aromatic and 0.96 for CH 3 atoms. The U iso values were constrained to be 1.5 U eq of the
All H atoms were located in a difference Fourier map, and were refined with distance restraints of C–H 1.00 Å and N–H. 0.88 Å; their temperature factors were
H atoms were placed at idealized positions and allowed to ride on their parent atoms with C—H distances in the range. 0.92–0.97 Å and O—H = 0.82 Å; U iso (H) values were set equal
C—H interactions (represented by dotted lines) along the [001] direction. H atoms not involved in these interactions have been omitted... The remaining H atoms were located in
The H atoms were positioned geometrically (C—H = 0.96 A ˚ for the methyl H atoms, 0.97 A ˚ for the methylene H atoms and 0.93 A˚ for the aromatic H atoms) and were included in
The carbon-bound H atoms were placed at calculated positions (C–H 0.95 Å), and were included in the refinement in the riding model approximation with U (H) set to 1.2 U eq (C).