metal-organic papers
m1982
Jesionkaet al. [Ba(C12H27O3SSi)2(H2O)3] doi:10.1107/S1600536806028054 Acta Cryst.(2006). E62, m1982–m1984Acta Crystallographica Section E
Structure Reports
Online
ISSN 1600-5368
Triaquabis(tri-
tert
-butoxysilanethiolato)barium
Elzbieta Jesionka, Jaroslaw Chojnacki* and Wieslaw Wojnowski
Chemical Faculty, Gdansk University of Technology, Narutowicza 11/12, Gdansk PL-80952, Poland
Correspondence e-mail: jarekch@chem.pg.gda.pl
Key indicators
Single-crystal X-ray study
T= 100 K
Mean(C–C) = 0.013 A˚
Rfactor = 0.068
wRfactor = 0.174
Data-to-parameter ratio = 19.5
For details of how these key indicators were automatically derived from the article, see http://journals.iucr.org/e.
Received 13 July 2006 Accepted 19 July 2006
#2006 International Union of Crystallography All rights reserved
The reaction of metallic barium with tri-tert-butoxysilanethiol in the presence of traces of water gives the title compound, [Ba(C12H27O3SSi)2(H2O)3]. The Ba atom is coordinated by
two O,S-chelating silanethiolate ligands and three water O atoms, forming an intermolecular net of hydrogen bonds of the O—H O and O—H S types.
Comment
The chemistry of alkaline-earth metal chalcogenates has received little attention, despite their potential use in a number of technical applications such as the production of wide band gap semiconductors (Kondo et al., 1994) or two-colour IR optical windows (Kumta & Risbud, 1994; Lowe-Ma
et al., 1995). Only a few structures of heavier alkaline-earth thiolates, selenolates or tellurolates have been reported to date (Henke & Atwood, 1998; Henkeet al., 2001; Chadwicket al., 1998; Ruhlandt-Senge, 1997; Ruhlandt-Senge & Englich, 2000).
The structure and chemistry of silanethiolates have been a subject of research for about 20 years (Wojnowskiet al., 1985; Becker et al., 1990; Preuss et al., 1990; Peters et al., 1997; Kovacset al., 2000; Komuroet al., 2003). Most of the papers describe silanethiolates of transition metals (e.g. Becker, Dołe˛gaet al., 2001; Becker, Zalewskaet al., 2001) and of thep -block elements (Peters et al., 1998; Chojnacki et al., 2001). There are only a few structural reports of silanethiolates of the
s-block elements (Baranowska et al., 2002; Jesionka et al., 2005) and none to date of alkaline-earth silanethiolates. We present here the structure of the title compound, (I), the first silanethiolate of an alkaline-earth element.
Compound, (I) was obtained by the reaction of metallic barium with tri-tert-butoxysilanethiol under evolution of gaseous hydrogen. It should be emphasized that under rigorous anhydrous conditions no crystalline product was formed. Traces of water, which must have entered the system during the various manipulations, are necessary for crystal-lization, giving a hydrated product.
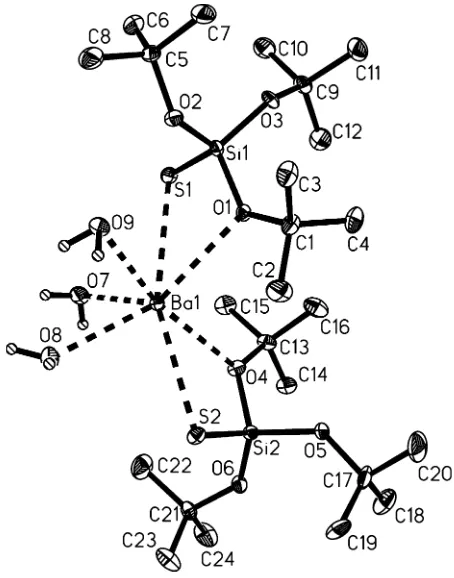
centre. The coordination polyhedron is formed by two S atoms, two O atoms from two chelating silanethiolate ligands, and three O atoms from coordinated water molecules. These silanethiolate ligands are oriented approximately opposite each other, the S—Ba—S valence angle being 158.51 (5). The
two Ba—S bond lengths are different [Ba1—S1 = 3.1576 (19)
and Ba1–S2 = 3.223 (2) A˚ ]. The departure from linearity of the S—Ba—S system is probably forced by the asymmetrical coordination of Ba by water molecules. The contacts between Ba and the chelating O atoms are 2.831 (5) and 2.923 (5) A˚ for atoms O1 and O4, respectively. The Ba—O coordination bond lengths to the water molecules vary from 2.697 (5) to 2.800 (5) A˚ . The Si—S bond lengths in (I), 2.063 (3) and 2.067 (3) A˚ , are considerably shorter than the relevant distances found in covalent silanethiolates and are comparable with Si—S bonds in ionic silanethiolates.
The presence of the three water molecules enables the formation of intermolecular hydrogen bonds, of the O— H O and O—H S types (Table 1, Fig. 2). These inter-molecular hydrogen bonds link the molecules of barium silanethiolate to give chains parallel to theaaxis (Fig. 3). This compound is an example of a macromolecular system in which monomeric units are linked by hydrogen bonds.
Experimental
The synthesis was carried out using a standard vacuum/N2line and
Schlenk techniques. (tBuO)
3SiSH was prepared according to the
literature (Pie˛kos´ & Wojnowski, 1962). The solvents were dried by standard methods and distilled under argon prior to use. Elemental analysis was performed on an Elemental Analyser EA 1108 (Carlo Erba Instruments).
For the preparation of triaquabis(tri-tert -butoxysilanethiolato)-barium, (I), an excess of metallic barium was added to tri-tert -butoxysilanethiol (5 ml, 16.5 mmol). The mixture was stirred and heated at 333 K for one week, yielding a white precipitate. The precipitate was washed with a small amount (ca2 ml) of hexane and dissolved in toluene (10 ml). The solution was separated from the excess of metal by filtration. The solvent was removed and the white precipitate was dried under vacuum. The final product, in the form of colourless crystals, was obtained by slow evaporation of a hexane solution. Elemental analysis (calculated for C24H60BaO9S2Si2): C 38.1
(38.4), H 7.9 (8.1), S 8.3 (8.5)%.
metal-organic papers
[image:2.610.312.565.69.261.2]Acta Cryst.(2006). E62, m1982–m1984 Jesionkaet al. [Ba(C12H27O3SSi)2(H2O)3]
m1983
Figure 1
A view of (I), with 50% probability displacement ellipsoids. Thick dashed lines denote coordination bonds.
Figure 2
[image:2.610.56.283.70.359.2]The hydrogen-bonding system in (I). Thick dashed lines denote coordination bonds, thin dashed lines hydrogen bonds. tert-Butoxy groups have been omitted. Primed atoms are at the symmetry position (x, 1 y,z), double primed atoms are at the symmetry position (1 +x,y,z) and triply primed atoms are at the symmetry position (1z, 1y,z).
Figure 3
[image:2.610.44.297.408.580.2]Crystal data
[Ba(C12H27O3SSi)2(H2O)3] Mr= 750.36
Triclinic,P1
a= 10.657 (2) A˚
b= 14.412 (3) A˚
c= 14.699 (3) A˚ = 62.88 (3) = 88.32 (3) = 72.02 (3)
V= 1894.6 (10) A˚3
Z= 2
Dx= 1.315 Mg m
3
MoKradiation = 1.26 mm1 T= 100 (2) K Needle, colourless 0.350.080.08 mm
Data collection
Kuma KM-4-CCD diffractometer !scans
Absorption correction: numerical [SCALE3inCrysAlis RED
(Oxford Diffraction, 2005), based on algorithms by Clark & Reid (1998)]
Tmin= 0.770,Tmax= 0.928
13908 measured reflections 7415 independent reflections 6598 reflections withI> 2(I)
Rint= 0.055 max= 26
Refinement
Refinement onF2 R[F2> 2(F2)] = 0.068 wR(F2) = 0.174
S= 1.11 7415 reflections 380 parameters
H atoms treated by a mixture of independent and constrained refinement
w= 1/[2
(Fo2) + (0.0623P)2 + 24.8951P]
whereP= (Fo2+ 2Fc2)/3 (/)max= 0.001
max= 4.35 e A˚ 3
min=1.14 e A˚ 3
Table 1
Hydrogen-bond geometry (A˚ ,).
D—H A D—H H A D A D—H A
O7—H7A O6 0.92 (5) 1.97 (6) 2.843 (7) 159 (10)
O7—H7B S1i
0.92 (5) 2.29 (5) 3.184 (5) 167 (10)
O8—H8A S2ii
0.91 (5) 2.41 (11) 3.309 (6) 170 (10)
O9—H9A S1i 0.91 (5) 2.44 (8) 3.226 (6) 144 (9)
O9—H9B S2ii
0.91 (5) 2.39 (7) 3.222 (6) 151 (10)
Symmetry codes: (i)xþ1;yþ1;z; (ii)x;yþ1;z.
H atoms attached to C atoms were positioned geometrically and refined as riding, with C—H = 0.98 A˚ andUiso(H) = 1.2Ueq(C). Water
H atoms were found in a difference Fourier synthesis and refined with O—H distances restrained to 0.91 (5) A˚ and withUiso(H) = 0.05 A˚
2.
The maximum electron-density peak is located 0.91 A˚ from the Ba atom and the deepest hole 0.82 A˚ from the same atom.
Data collection: CrysAlis CCD (Oxford Diffraction, 2005); cell refinement: CrysAlis RED(Oxford Diffraction, 2005); data
reduc-tion:CrysAlis RED; program(s) used to solve structure:SHELXS97 (Sheldrick, 1997); program(s) used to refine structure:SHELXL97 (Sheldrick, 1997); molecular graphics: ORTEP-3 (Farrugia, 1997); software used to prepare material for publication:WinGX(Farrugia, 1999).
Financial support for EJ from the Polish Ministry of Research and Information (project Nos. 3 T09A 150 27 and T09A 120 28) is gratefully acknowledged.
References
Baranowska, K., Chojnacki, J., Wojnowski, W. & Krossing, I. (2002).Acta Cryst.E58, m569–m570.
Becker, B., Dołe˛ga, A., Konitz, A., Swinder, L. & Wojnowski, W. (2001).Z. Anorg. Allg. Chem.627, 280–286.
Becker, B., Wojnowski, W., Peters, K., Peters, E. M. & von Schnering, H. G. (1990).Polyhedron,9, 1659–1666.
Becker, B., Zalewska, A., Konitz, A. & Wojnowski, W. (2001).Polyhedron,20, 2567–2576.
Chadwick, S., Englich, U. & Ruhlandt-Senge, K. (1998).Chem. Commun.pp. 2149–2150.
Chojnacki, J., Schnepf, A. & Wojnowski, W. (2001).Z. Kristallogr. New Cryst. Struct.216, 198–200.
Clark, R. C. & Reid, J. S. (1998).Comput. Phys. Commun.111, 243–258. Farrugia, L. J. (1997).J. Appl. Cryst.30, 565.
Farrugia, L. J. (1999).J. Appl. Cryst.32, 837–838.
Henke, K. & Atwood, D. A. (1998).Inorg. Chem.37, 224–227.
Henke, K., Hutchison, A. R., Krepps, M. K., Parkin, S. & Atwood, D. A. (2001).Inorg. Chem.40, 4443–4447.
Jesionka, E., Ciborska, A., Chojnacki, J. & Wojnowski, W. (2005).Acta Cryst.
C61, m321–m323.
Komuro, T., Matsuo, T., Kawaguchi, H. & Tatsumi, K. (2003).Inorg. Chem.42, 5340–5347.
Kondo, K., Ukita, M., Yshida, H., Kishita, Y., Okuyama, H., Ito, S., Ohata, T. & Nakano, K. (1994).J. Appl. Phys.76, 2621–2626.
Kovacs, I., Pearson, C. & Shaver, A. (2000).J. Organomet. Chem.596, 193–203. Kumta, P. N. & Risbud, S. H. (1994).J. Mater. Sci.29, 1135–1158.
Lowe-Ma, C. K., Vanderah, T. A. & Smith, T. E. (1995).J. Solid State Chem. 117, 363–372.
Oxford Diffraction (2005).CrysAlis CCDandCrysAlis RED. Versions 1.171. Oxford Diffraction Ltd, Abingdon, Oxford, England.
Peters, K., Peters, E. M., von Schnering, H. G., Wojnowski, W. & Tamulewicz, S. (1998).Z. Kristallogr. New Cryst. Struct.213, 347–348.
Peters, K., Peters, E. M., von Schnering, H. G., Wojnowski, W., Tamulewicz, S. & Radacki, K. (1997).Z. Kristallogr. New Cryst. Struct.212, 345–346. Pie˛kos´, R. & Wojnowski, W. (1962).Z. Anorg. Allg. Chem.318, 212–216. Preuss, F., Steidel, M. & Exner, R. (1990).Z. Naturforsch. Teil B,45, 1618–
1624.
Ruhlandt-Senge, K. (1997).Comments Inorg. Chem.19, 351–385. Ruhlandt-Senge, K. & Englich, U. (2000).Chem. Eur. J.6, 4063–4070. Sheldrick, G. M. (1997). SHELXS97 and SHELXL97. University of
Go¨ttingen, Germany.
Wojnowski, W., Wojnowski, M., Peters, K., Peters, E. M. & von Schnering, H. G. (1985).Z. Anorg. Allg. Chem.530, 79–88.
metal-organic papers
supporting information
sup-1
Acta Cryst. (2006). E62, m1982–m1984
supporting information
Acta Cryst. (2006). E62, m1982–m1984 [https://doi.org/10.1107/S1600536806028054]
Triaquabis(tri-
tert
-butoxysilanethiolato)barium
Elzbieta Jesionka, Jaroslaw Chojnacki and Wieslaw Wojnowski
Triaquabis(tri-tert-butoxysilanethiolato)barium
Crystal data
[Ba(C12H27O3SSi)2(H2O)2]
Mr = 750.36 Triclinic, P1 Hall symbol: -P 1
a = 10.657 (2) Å
b = 14.412 (3) Å
c = 14.699 (3) Å
α = 62.88 (3)°
β = 88.32 (3)°
γ = 72.02 (3)°
V = 1894.6 (10) Å3
Z = 2
F(000) = 784
Dx = 1.315 Mg m−3
Mo Kα radiation, λ = 0.71073 Å
Cell parameters from 2130 reflections
θ = 2–30°
µ = 1.26 mm−1
T = 100 K
Needle, colourless 0.35 × 0.08 × 0.08 mm
Data collection
Kuma KM-4-CCD diffractometer 0.75° ω scans
Absorption correction: numerical
[SCALE3 in CrysAlisRED (Oxford Diffraction, 2005), based on algorithms by Clark & Reid (1998)]
Tmin = 0.770, Tmax = 0.928
13908 measured reflections 7415 independent reflections 6598 reflections with I > 2σ(I)
Rint = 0.055
θmax = 26°, θmin = 2.8°
h = −13→13
k = −17→12
l = −18→17
Refinement
Refinement on F2
Least-squares matrix: full
R[F2 > 2σ(F2)] = 0.068
wR(F2) = 0.174
S = 1.11
7415 reflections 380 parameters 6 restraints
H atoms treated by a mixture of independent and constrained refinement
w = 1/[σ2(F
o2) + (0.0623P)2 + 24.8951P] where P = (Fo2 + 2Fc2)/3
(Δ/σ)max = 0.001 Δρmax = 4.35 e Å−3 Δρmin = −1.14 e Å−3
Special details
supporting information
sup-2
Acta Cryst. (2006). E62, m1982–m1984
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
x y z Uiso*/Ueq
Ba1 0.24000 (4) 0.51271 (3) 0.08873 (3) 0.01222 (14)
S1 0.47139 (17) 0.61546 (14) 0.03647 (13) 0.0150 (3)
Si1 0.39404 (18) 0.69598 (15) 0.12171 (14) 0.0122 (4)
S2 0.02471 (16) 0.41090 (14) 0.22039 (13) 0.0147 (3)
Si2 0.18487 (18) 0.27103 (15) 0.30664 (14) 0.0118 (4)
O1 0.2566 (5) 0.6630 (4) 0.1579 (4) 0.0154 (10)
O2 0.3588 (5) 0.8306 (4) 0.0604 (4) 0.0148 (10)
O3 0.4821 (5) 0.6536 (4) 0.2311 (4) 0.0168 (10)
O4 0.3156 (5) 0.3147 (4) 0.2895 (4) 0.0133 (9)
O5 0.1763 (5) 0.1994 (4) 0.4293 (4) 0.0162 (10)
O6 0.2247 (5) 0.1810 (4) 0.2608 (4) 0.0149 (10)
O7 0.2778 (5) 0.3244 (4) 0.0650 (4) 0.0170 (10)
O8 −0.0007 (5) 0.6397 (4) −0.0246 (4) 0.0203 (11)
O9 0.2444 (5) 0.5798 (5) −0.1171 (4) 0.0213 (11)
C10 0.1742 (7) 0.6830 (6) 0.2333 (5) 0.0153 (14)
C11 0.0361 (7) 0.6887 (6) 0.2029 (6) 0.0209 (15)
H11A 0.0033 0.7473 0.1319 0.025*
H11B −0.0242 0.7049 0.2493 0.025*
H11C 0.0399 0.6177 0.2079 0.025*
C12 0.2331 (9) 0.5856 (7) 0.3398 (6) 0.0273 (17)
H12A 0.2399 0.5166 0.339 0.033*
H12B 0.1754 0.5939 0.3907 0.033*
H12C 0.3218 0.5836 0.3583 0.033*
C13 0.1668 (7) 0.7919 (6) 0.2298 (6) 0.0179 (14)
H13A 0.2565 0.7896 0.2466 0.021*
H13B 0.1104 0.8034 0.2802 0.021*
H13C 0.1289 0.8528 0.1606 0.021*
C20 0.3002 (7) 0.9135 (6) −0.0438 (5) 0.0171 (14)
C21 0.1859 (9) 0.8890 (7) −0.0800 (7) 0.0312 (19)
H21A 0.2208 0.818 −0.0807 0.037*
H21B 0.1456 0.9477 −0.1497 0.037*
H21C 0.1186 0.8858 −0.0328 0.037*
C22 0.4076 (9) 0.9162 (7) −0.1150 (6) 0.0277 (18)
H22A 0.4812 0.9293 −0.0897 0.033*
H22B 0.37 0.9757 −0.1848 0.033*
H22C 0.4406 0.8453 −0.116 0.033*
C23 0.2472 (9) 1.0232 (6) −0.0406 (7) 0.0301 (19)
H23A 0.1764 1.021 0.0036 0.036*
H23B 0.2116 1.0836 −0.1105 0.036*
H23C 0.3197 1.0354 −0.0129 0.036*
C30 0.5995 (8) 0.6720 (6) 0.2551 (6) 0.0213 (16)
C31 0.6735 (10) 0.5697 (8) 0.3543 (7) 0.040 (2)
H31A 0.6159 0.5601 0.4089 0.048*
H31B 0.754 0.5784 0.3749 0.048*
supporting information
sup-3
Acta Cryst. (2006). E62, m1982–m1984
C32 0.6864 (8) 0.6910 (7) 0.1678 (7) 0.0258 (17)
H32A 0.7209 0.6236 0.1607 0.031*
H32B 0.7609 0.7098 0.1838 0.031*
H32C 0.633 0.7518 0.1031 0.031*
C33 0.5529 (9) 0.7759 (8) 0.2706 (7) 0.0317 (19)
H33A 0.5008 0.8396 0.2069 0.038*
H33B 0.6307 0.7901 0.2882 0.038*
H33C 0.4976 0.7639 0.3267 0.038*
C40 0.4529 (7) 0.2582 (6) 0.3403 (6) 0.0173 (14)
C41 0.5377 (8) 0.3180 (8) 0.2684 (7) 0.0315 (19)
H41A 0.5332 0.3125 0.2046 0.038*
H41B 0.6301 0.2844 0.3015 0.038*
H41C 0.5048 0.3959 0.2523 0.038*
C42 0.5003 (8) 0.1361 (6) 0.3649 (6) 0.0231 (16)
H42A 0.4452 0.0982 0.4126 0.028*
H42B 0.5934 0.101 0.3966 0.028*
H42C 0.4925 0.1313 0.301 0.028*
C43 0.4593 (8) 0.2652 (7) 0.4408 (6) 0.0234 (16)
H43A 0.4184 0.3422 0.4265 0.028*
H43B 0.5524 0.2379 0.4707 0.028*
H43C 0.4111 0.22 0.4894 0.028*
C50 0.0998 (8) 0.2247 (6) 0.5036 (5) 0.0184 (15)
C51 0.0993 (8) 0.3352 (7) 0.4928 (6) 0.0234 (16)
H51A 0.1911 0.3333 0.501 0.028*
H51B 0.052 0.3488 0.5461 0.028*
H51C 0.0545 0.3945 0.4245 0.028*
C52 0.1704 (8) 0.1297 (7) 0.6089 (6) 0.0265 (17)
H52A 0.1715 0.0596 0.6132 0.032*
H52B 0.1231 0.1406 0.6633 0.032*
H52C 0.262 0.1281 0.6176 0.032*
C53 −0.0420 (8) 0.2278 (7) 0.4851 (6) 0.0249 (17)
H53A −0.0838 0.2877 0.4162 0.03*
H53B −0.0934 0.2407 0.5371 0.03*
H53C −0.0399 0.1572 0.4901 0.03*
C60 0.1772 (8) 0.0902 (6) 0.2775 (6) 0.0195 (15)
C61 0.2548 (9) −0.0108 (6) 0.3771 (7) 0.0289 (18)
H61A 0.35 −0.0303 0.3707 0.035*
H61B 0.225 −0.073 0.3899 0.035*
H61C 0.2392 0.0062 0.4347 0.035*
C62 0.0282 (8) 0.1200 (7) 0.2835 (6) 0.0234 (16)
H62A 0.0086 0.1376 0.3405 0.028*
H62B −0.0001 0.0573 0.2952 0.028*
H62C −0.0198 0.1844 0.2186 0.028*
C63 0.2095 (9) 0.0682 (7) 0.1856 (7) 0.0290 (18)
H63A 0.1649 0.1354 0.1216 0.035*
H63B 0.1785 0.0084 0.1922 0.035*
H63C 0.306 0.0468 0.1842 0.035*
supporting information
sup-4
Acta Cryst. (2006). E62, m1982–m1984
H7B 0.352 (8) 0.332 (9) 0.034 (8) 0.05*
H8A −0.010 (11) 0.636 (9) −0.084 (6) 0.05*
H8B −0.074 (8) 0.630 (9) 0.008 (8) 0.05*
H9A 0.324 (7) 0.541 (8) −0.126 (9) 0.05*
H9B 0.187 (9) 0.585 (9) −0.166 (7) 0.05*
Atomic displacement parameters (Å2)
U11 U22 U33 U12 U13 U23
Ba1 0.0134 (2) 0.0110 (2) 0.0107 (2) −0.00529 (15) −0.00021 (14) −0.00297 (15)
S1 0.0125 (8) 0.0176 (8) 0.0179 (8) −0.0055 (6) 0.0039 (6) −0.0106 (7)
Si1 0.0115 (9) 0.0118 (9) 0.0117 (9) −0.0038 (7) 0.0010 (7) −0.0041 (7)
S2 0.0114 (8) 0.0144 (8) 0.0158 (8) −0.0035 (6) −0.0003 (6) −0.0054 (7)
Si2 0.0132 (9) 0.0128 (9) 0.0082 (8) −0.0041 (7) 0.0015 (7) −0.0040 (7)
O1 0.018 (2) 0.015 (2) 0.019 (3) −0.007 (2) 0.007 (2) −0.012 (2)
O2 0.020 (3) 0.011 (2) 0.012 (2) −0.0042 (19) −0.0011 (19) −0.0047 (19)
O3 0.018 (3) 0.018 (2) 0.013 (2) −0.008 (2) −0.0017 (19) −0.005 (2)
O4 0.012 (2) 0.016 (2) 0.010 (2) −0.0045 (19) 0.0002 (18) −0.0047 (19)
O5 0.018 (2) 0.019 (2) 0.010 (2) −0.007 (2) 0.0055 (19) −0.005 (2)
O6 0.016 (2) 0.014 (2) 0.017 (2) −0.0071 (19) 0.0040 (19) −0.008 (2)
O7 0.016 (2) 0.022 (3) 0.016 (3) −0.009 (2) 0.010 (2) −0.010 (2)
O8 0.016 (3) 0.025 (3) 0.017 (3) −0.008 (2) 0.004 (2) −0.007 (2)
O9 0.016 (3) 0.029 (3) 0.018 (3) −0.003 (2) 0.001 (2) −0.013 (2)
C10 0.022 (4) 0.013 (3) 0.015 (3) −0.006 (3) 0.008 (3) −0.009 (3)
C11 0.016 (4) 0.020 (4) 0.027 (4) −0.004 (3) 0.009 (3) −0.012 (3)
C12 0.032 (4) 0.022 (4) 0.021 (4) −0.009 (3) 0.011 (3) −0.005 (3)
C13 0.020 (4) 0.018 (3) 0.022 (4) −0.007 (3) 0.008 (3) −0.014 (3)
C20 0.021 (4) 0.011 (3) 0.012 (3) −0.001 (3) −0.001 (3) −0.002 (3)
C21 0.028 (4) 0.025 (4) 0.032 (5) −0.004 (3) −0.009 (4) −0.008 (4)
C22 0.031 (4) 0.021 (4) 0.019 (4) −0.004 (3) 0.008 (3) −0.004 (3)
C23 0.043 (5) 0.011 (4) 0.027 (4) −0.004 (3) 0.003 (4) −0.004 (3)
C30 0.022 (4) 0.023 (4) 0.017 (4) −0.006 (3) −0.008 (3) −0.009 (3)
C31 0.040 (5) 0.034 (5) 0.029 (5) −0.004 (4) −0.020 (4) −0.004 (4)
C32 0.018 (4) 0.032 (4) 0.036 (5) −0.014 (3) 0.007 (3) −0.020 (4)
C33 0.032 (5) 0.038 (5) 0.041 (5) −0.015 (4) 0.002 (4) −0.029 (4)
C40 0.013 (3) 0.014 (3) 0.023 (4) −0.001 (3) −0.005 (3) −0.009 (3)
C41 0.018 (4) 0.037 (5) 0.028 (4) −0.012 (4) 0.001 (3) −0.005 (4)
C42 0.020 (4) 0.019 (4) 0.025 (4) 0.002 (3) −0.005 (3) −0.011 (3)
C43 0.016 (4) 0.025 (4) 0.028 (4) −0.001 (3) −0.006 (3) −0.014 (3)
C50 0.028 (4) 0.019 (4) 0.011 (3) −0.011 (3) 0.008 (3) −0.007 (3)
C51 0.024 (4) 0.031 (4) 0.022 (4) −0.011 (3) 0.008 (3) −0.017 (3)
C52 0.028 (4) 0.035 (5) 0.016 (4) −0.013 (4) 0.007 (3) −0.012 (3)
C53 0.024 (4) 0.032 (4) 0.021 (4) −0.014 (3) 0.012 (3) −0.012 (3)
C60 0.027 (4) 0.012 (3) 0.019 (4) −0.009 (3) 0.000 (3) −0.005 (3)
C61 0.036 (5) 0.012 (4) 0.029 (4) −0.011 (3) −0.005 (4) 0.000 (3)
C62 0.026 (4) 0.028 (4) 0.022 (4) −0.017 (3) 0.005 (3) −0.011 (3)
supporting information
sup-5
Acta Cryst. (2006). E62, m1982–m1984
Geometric parameters (Å, º)
Ba1—O8 2.698 (5) C23—H23A 0.98
Ba1—O9 2.729 (5) C23—H23B 0.98
Ba1—O7 2.798 (5) C23—H23C 0.98
Ba1—O1 2.831 (5) C30—C31 1.521 (11)
Ba1—O4 2.923 (5) C30—C32 1.532 (11)
Ba1—S1 3.1576 (19) C30—C33 1.545 (11)
Ba1—S2 3.224 (2) C31—H31A 0.98
Ba1—Si2 3.704 (2) C31—H31B 0.98
Ba1—Si1 3.715 (2) C31—H31C 0.98
Ba1—H7B 2.97 (11) C32—H32A 0.98
S1—Si1 2.067 (3) C32—H32B 0.98
Si1—O3 1.637 (5) C32—H32C 0.98
Si1—O2 1.641 (5) C33—H33A 0.98
Si1—O1 1.676 (5) C33—H33B 0.98
S2—Si2 2.063 (3) C33—H33C 0.98
Si2—O5 1.636 (5) C40—C41 1.506 (11)
Si2—O6 1.661 (5) C40—C43 1.530 (10)
Si2—O4 1.666 (5) C40—C42 1.538 (10)
O1—C10 1.469 (8) C41—H41A 0.98
O2—C20 1.445 (8) C41—H41B 0.98
O3—C30 1.441 (9) C41—H41C 0.98
O4—C40 1.462 (8) C42—H42A 0.98
O5—C50 1.455 (8) C42—H42B 0.98
O6—C60 1.462 (8) C42—H42C 0.98
O7—H7A 0.92 (5) C43—H43A 0.98
O7—H7B 0.92 (5) C43—H43B 0.98
O8—H8A 0.91 (5) C43—H43C 0.98
O8—H8B 0.92 (5) C50—C53 1.526 (11)
O9—H9A 0.91 (5) C50—C51 1.526 (10)
O9—H9B 0.91 (5) C50—C52 1.529 (11)
C10—C11 1.517 (10) C51—H51A 0.98
C10—C12 1.526 (10) C51—H51B 0.98
C10—C13 1.526 (9) C51—H51C 0.98
C11—H11A 0.98 C52—H52A 0.98
C11—H11B 0.98 C52—H52B 0.98
C11—H11C 0.98 C52—H52C 0.98
C12—H12A 0.98 C53—H53A 0.98
C12—H12B 0.98 C53—H53B 0.98
C12—H12C 0.98 C53—H53C 0.98
C13—H13A 0.98 C60—C62 1.522 (11)
C13—H13B 0.98 C60—C61 1.527 (10)
C13—H13C 0.98 C60—C63 1.529 (11)
C20—C22 1.525 (10) C61—H61A 0.98
C20—C23 1.528 (10) C61—H61B 0.98
C20—C21 1.538 (11) C61—H61C 0.98
supporting information
sup-6
Acta Cryst. (2006). E62, m1982–m1984
C21—H21B 0.98 C62—H62B 0.98
C21—H21C 0.98 C62—H62C 0.98
C22—H22A 0.98 C63—H63A 0.98
C22—H22B 0.98 C63—H63B 0.98
C22—H22C 0.98 C63—H63C 0.98
O8—Ba1—O9 66.66 (16) C23—C20—C21 110.2 (7)
O8—Ba1—O7 99.64 (16) C20—C21—H21A 109.5
O9—Ba1—O7 74.16 (16) C20—C21—H21B 109.5
O8—Ba1—O1 95.11 (16) H21A—C21—H21B 109.5
O9—Ba1—O1 116.47 (16) C20—C21—H21C 109.5
O7—Ba1—O1 164.54 (15) H21A—C21—H21C 109.5
O8—Ba1—O4 130.50 (15) H21B—C21—H21C 109.5
O9—Ba1—O4 141.97 (15) C20—C22—H22A 109.5
O7—Ba1—O4 69.68 (14) C20—C22—H22B 109.5
O1—Ba1—O4 97.13 (14) H22A—C22—H22B 109.5
O8—Ba1—S1 117.59 (12) C20—C22—H22C 109.5
O9—Ba1—S1 76.38 (12) H22A—C22—H22C 109.5
O7—Ba1—S1 116.62 (11) H22B—C22—H22C 109.5
O1—Ba1—S1 59.11 (10) C20—C23—H23A 109.5
O4—Ba1—S1 109.79 (10) C20—C23—H23B 109.5
O8—Ba1—S2 73.02 (12) H23A—C23—H23B 109.5
O9—Ba1—S2 124.69 (12) C20—C23—H23C 109.5
O7—Ba1—S2 77.14 (10) H23A—C23—H23C 109.5
O1—Ba1—S2 103.02 (10) H23B—C23—H23C 109.5
O4—Ba1—S2 57.50 (10) O3—C30—C31 106.0 (6)
S1—Ba1—S2 158.51 (5) O3—C30—C32 111.6 (6)
O8—Ba1—Si2 105.50 (12) C31—C30—C32 111.3 (7)
O9—Ba1—Si2 134.17 (12) O3—C30—C33 107.4 (6)
O7—Ba1—Si2 62.46 (10) C31—C30—C33 111.2 (7)
O1—Ba1—Si2 109.07 (10) C32—C30—C33 109.2 (7)
O4—Ba1—Si2 25.84 (9) C30—C31—H31A 109.5
S1—Ba1—Si2 135.63 (5) C30—C31—H31B 109.5
S2—Ba1—Si2 33.76 (5) H31A—C31—H31B 109.5
O8—Ba1—Si1 107.83 (12) C30—C31—H31C 109.5
O9—Ba1—Si1 100.69 (12) H31A—C31—H31C 109.5
O7—Ba1—Si1 147.38 (11) H31B—C31—H31C 109.5
O1—Ba1—Si1 25.37 (10) C30—C32—H32A 109.5
O4—Ba1—Si1 103.31 (10) C30—C32—H32B 109.5
S1—Ba1—Si1 33.78 (4) H32A—C32—H32B 109.5
S2—Ba1—Si1 127.27 (5) C30—C32—H32C 109.5
Si2—Ba1—Si1 123.65 (5) H32A—C32—H32C 109.5
O8—Ba1—H7B 108 (2) H32B—C32—H32C 109.5
O9—Ba1—H7B 65 (2) C30—C33—H33A 109.5
O7—Ba1—H7B 18.0 (11) C30—C33—H33B 109.5
O1—Ba1—H7B 154.2 (16) H33A—C33—H33B 109.5
O4—Ba1—H7B 77 (2) C30—C33—H33C 109.5
supporting information
sup-7
Acta Cryst. (2006). E62, m1982–m1984
S2—Ba1—H7B 94.9 (12) H33B—C33—H33C 109.5
Si2—Ba1—H7B 76.4 (16) O4—C40—C41 107.3 (6)
Si1—Ba1—H7B 130.9 (13) O4—C40—C43 108.3 (6)
Si1—S1—Ba1 88.05 (8) C41—C40—C43 110.3 (6)
O3—Si1—O2 105.6 (3) O4—C40—C42 111.2 (6)
O3—Si1—O1 103.5 (3) C41—C40—C42 110.6 (7)
O2—Si1—O1 110.7 (3) C43—C40—C42 109.2 (6)
O3—Si1—S1 116.7 (2) C40—C41—H41A 109.5
O2—Si1—S1 115.2 (2) C40—C41—H41B 109.5
O1—Si1—S1 104.42 (18) H41A—C41—H41B 109.5
O3—Si1—Ba1 120.14 (19) C40—C41—H41C 109.5
O2—Si1—Ba1 131.53 (19) H41A—C41—H41C 109.5
O1—Si1—Ba1 46.36 (16) H41B—C41—H41C 109.5
S1—Si1—Ba1 58.17 (6) C40—C42—H42A 109.5
Si2—S2—Ba1 85.99 (8) C40—C42—H42B 109.5
O5—Si2—O6 105.8 (3) H42A—C42—H42B 109.5
O5—Si2—O4 111.2 (3) C40—C42—H42C 109.5
O6—Si2—O4 102.6 (2) H42A—C42—H42C 109.5
O5—Si2—S2 117.5 (2) H42B—C42—H42C 109.5
O6—Si2—S2 113.9 (2) C40—C43—H43A 109.5
O4—Si2—S2 104.99 (19) C40—C43—H43B 109.5
O5—Si2—Ba1 151.01 (19) H43A—C43—H43B 109.5
O6—Si2—Ba1 100.48 (18) C40—C43—H43C 109.5
O4—Si2—Ba1 49.89 (17) H43A—C43—H43C 109.5
S2—Si2—Ba1 60.25 (7) H43B—C43—H43C 109.5
C10—O1—Si1 127.7 (4) O5—C50—C53 108.6 (6)
C10—O1—Ba1 122.4 (4) O5—C50—C51 110.6 (6)
Si1—O1—Ba1 108.3 (2) C53—C50—C51 110.4 (6)
C20—O2—Si1 132.7 (4) O5—C50—C52 104.9 (6)
C30—O3—Si1 132.2 (5) C53—C50—C52 111.2 (6)
C40—O4—Si2 131.1 (4) C51—C50—C52 111.0 (6)
C40—O4—Ba1 122.4 (4) C50—C51—H51A 109.5
Si2—O4—Ba1 104.3 (2) C50—C51—H51B 109.5
C50—O5—Si2 134.1 (5) H51A—C51—H51B 109.5
C60—O6—Si2 131.8 (5) C50—C51—H51C 109.5
Ba1—O7—H7A 113 (7) H51A—C51—H51C 109.5
Ba1—O7—H7B 92 (7) H51B—C51—H51C 109.5
H7A—O7—H7B 126 (10) C50—C52—H52A 109.5
Ba1—O8—H8A 114 (7) C50—C52—H52B 109.5
Ba1—O8—H8B 117 (7) H52A—C52—H52B 109.5
H8A—O8—H8B 108 (10) C50—C52—H52C 109.5
Ba1—O9—H9A 108 (7) H52A—C52—H52C 109.5
Ba1—O9—H9B 132 (7) H52B—C52—H52C 109.5
H9A—O9—H9B 102 (10) C50—C53—H53A 109.5
O1—C10—C11 106.1 (5) C50—C53—H53B 109.5
O1—C10—C12 108.0 (6) H53A—C53—H53B 109.5
C11—C10—C12 110.7 (6) C50—C53—H53C 109.5
supporting information
sup-8
Acta Cryst. (2006). E62, m1982–m1984
C11—C10—C13 109.3 (6) H53B—C53—H53C 109.5
C12—C10—C13 111.3 (6) O6—C60—C62 111.6 (6)
C10—C11—H11A 109.5 O6—C60—C61 107.9 (6)
C10—C11—H11B 109.5 C62—C60—C61 110.8 (6)
H11A—C11—H11B 109.5 O6—C60—C63 105.9 (6)
C10—C11—H11C 109.5 C62—C60—C63 110.5 (6)
H11A—C11—H11C 109.5 C61—C60—C63 110.0 (7)
H11B—C11—H11C 109.5 C60—C61—H61A 109.5
C10—C12—H12A 109.5 C60—C61—H61B 109.5
C10—C12—H12B 109.5 H61A—C61—H61B 109.5
H12A—C12—H12B 109.5 C60—C61—H61C 109.5
C10—C12—H12C 109.5 H61A—C61—H61C 109.5
H12A—C12—H12C 109.5 H61B—C61—H61C 109.5
H12B—C12—H12C 109.5 C60—C62—H62A 109.5
C10—C13—H13A 109.5 C60—C62—H62B 109.5
C10—C13—H13B 109.5 H62A—C62—H62B 109.5
H13A—C13—H13B 109.5 C60—C62—H62C 109.5
C10—C13—H13C 109.5 H62A—C62—H62C 109.5
H13A—C13—H13C 109.5 H62B—C62—H62C 109.5
H13B—C13—H13C 109.5 C60—C63—H63A 109.5
O2—C20—C22 109.0 (6) C60—C63—H63B 109.5
O2—C20—C23 105.9 (6) H63A—C63—H63B 109.5
C22—C20—C23 110.5 (6) C60—C63—H63C 109.5
O2—C20—C21 110.9 (6) H63A—C63—H63C 109.5
C22—C20—C21 110.2 (7) H63B—C63—H63C 109.5
Hydrogen-bond geometry (Å, º)
D—H···A D—H H···A D···A D—H···A
O7—H7A···O6 0.92 (5) 1.97 (6) 2.843 (7) 159 (10)
O7—H7B···S1i 0.92 (5) 2.29 (5) 3.184 (5) 167 (10)
O8—H8A···S2ii 0.91 (5) 2.41 (11) 3.309 (6) 170 (10)
O9—H9A···S1i 0.91 (5) 2.44 (8) 3.226 (6) 144 (9)
O9—H9B···S2ii 0.91 (5) 2.39 (7) 3.222 (6) 151 (10)