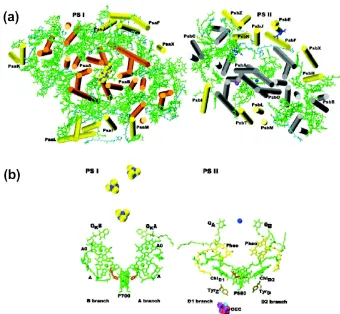
Harnessing Solar Energy for the Production of Clean Fuel
Full text
Figure



Outline
Related documents
MC/9: Mouse mast cell line; HMC-1: Human mast cell line; THP-1: Human acute monocytic leukemia-1 cell line; HMEC-1: Human dermal microvascular endothelial cell line; PC-3:
U.Belsare and coworkers studied the thermal degradation of terpolymer derived from 2-aminothiophenol, hexamethylenediamine with formaldehyde [16].Synthesis and thermal
When persons with MS alone were compared to persons without MS or any other chronic conditions, differences in overall and single attribute utility scores were similar to those
To be able to capture these objectives the study uses the 2004 tax reforms to establish a dummy period and ascertain the buoyancy, while using the logged coefficient of GDP
' This question is located in the last subpart of defendant Cicinelli's proposed question no. ^ This question is located in the second-to-last subpart of defendant Cicinelli's
Self-confidence of the respondents was mea- sured by using five statements, allocating scores of 1 to 2. Total scores below 8 were categorized as low, scores between 8 and 10
There are several advantages of Socket Cloning in cluster-based web servers: 1.) The cloned socket sends packets directly to the client and does not need to be for- warded to
We present a general-purpose design framework for build- ing highly concurrent systems, based on three design com- ponents — tasks , queues , and thread pools — which encapsu- late