1.1
Full text
Figure



Outline
Related documents
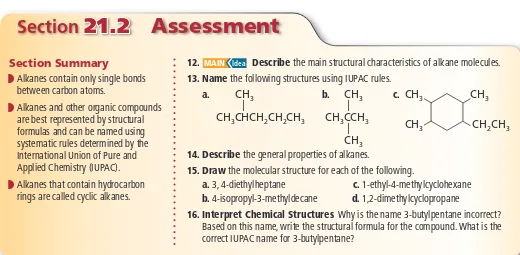
____________ are NOT connected to ____________ because one family of compounds contain only sin- gle bonds between the carbon atoms and the other family of compounds contain a pair
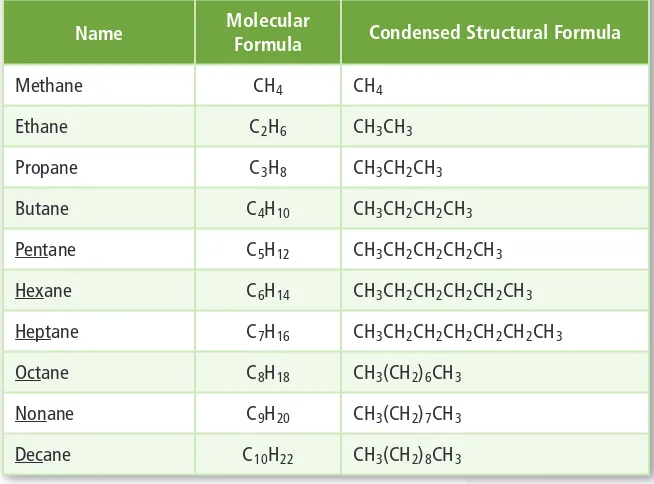
Carbon can form single bonds can form single bonds with hydrogen atoms, and both with hydrogen atoms, and both single and double bonds with single and double bonds with oxygen
(iv) A significant minority of candidates failed to realise that unsaturated hydrocarbons contain double bonds and gave unrelated incorrect tests, the commonest being the litmus
Material and Methods: The aim of this study is to assess and to compare upper trapezius and middle deltoid muscle activity in 2 traditional and improved design carpet
Answer: E Topic: Concept 4.2 Skill: Knowledge/Comprehension 14) Why are hydrocarbons insoluble in water? A)
main effects indicated that preferences for facial yellowness were stronger for judgments of male faces than female faces and stronger for judgments by male participants than
permanganate is an oxidizing agent that reacts with unsaturated aliphatic hydrocarbons, but does not react with alkanes or aromatic hydrocarbons.. The dilute KMnO 4 solution has
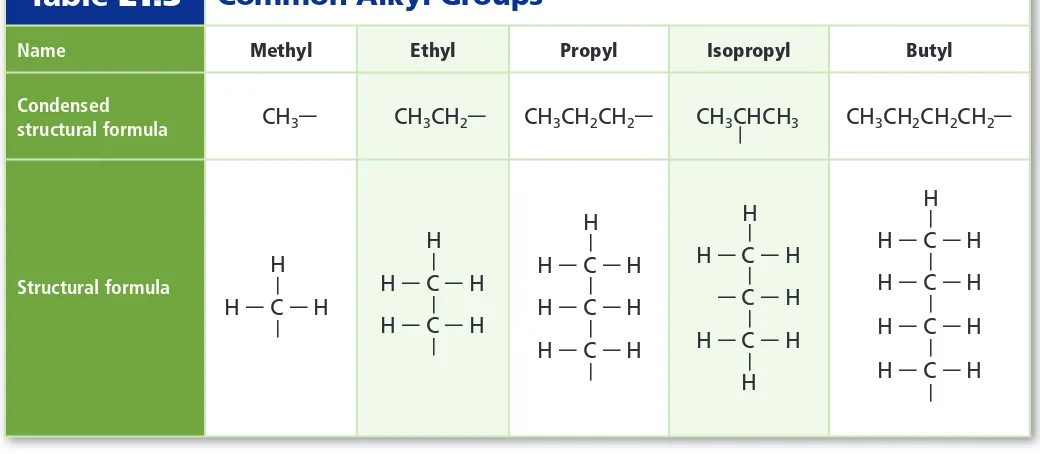
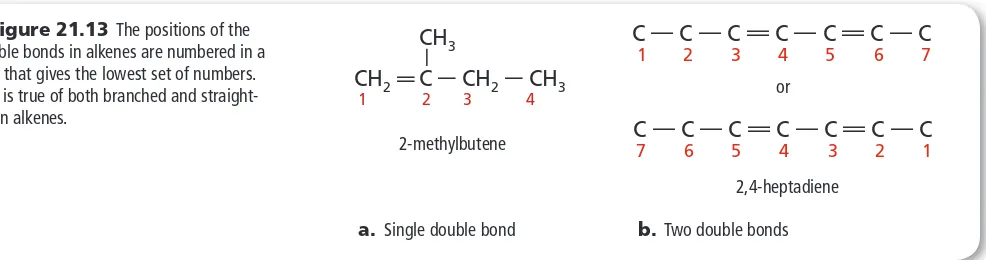
Naming is the same as used for alkanes, except that the parent structure is the longest continuous chain of carbon atoms that contains the carbon-carbon double bond or triple