PREMATURE TERMINATION OF TRANSCRIPTION
OF THE MURINE C-MFC GENE
SADIA ROBERTS
A thesis submitted for the degree
of Doctor of Philosophy
November 1992
University College,
Imperial Cancer Research Fund,
University of London.
44, Lincoln's Inn Fields
Gower Street,
London WC2A 3PX.
ProQuest Number: 10106819
All rights reserved
INFORMATION TO ALL USERS
The quality of this reproduction is dependent upon the quality of the copy submitted.
In the unlikely event that the author did not send a complete manuscript and there are missing pages, these will be noted. Also, if material had to be removed,
a note will indicate the deletion.
uest.
ProQuest 10106819
Published by ProQuest LLC(2016). Copyright of the Dissertation is held by the Author.
All rights reserved.
This work is protected against unauthorized copying under Title 17, United States Code. Microform Edition © ProQuest LLC.
ProQuest LLC
789 East Eisenhower Parkway P.O. Box 1346
A B ST R A C T
P re m a tu re te rm in a tio n or a tte n u a tio n o f RN A p o ly m e ra s e II tran scription has recently been found to regulate expression o f a num ber o f genes in c lu d in g the c - m y c o ncog ene, b u t its m ech an ism is not understood. By transcribing the c - m y c gene in injected X e n o p u s oocytes, various param eters w ere altered and their effects on the efficiency o f the c - m y c T2 attenuator w ere studied. The attenuator functioned dow nstream o f a variety o f prom oters. H owever polym erases situated further from the prom oter appear to have a low er ability to recognise the attenuator since term ination efficiency declined m arkedly when it was placed m ore than 400bp from the start site. Furtherm ore, only transcription w hich initiated at the c - m y c prom oter m ost proxim al to the atten u ato r (P2) term in ated prem aturely. T ranscription from the P I prom oter term inated at T2 only when the natural distance betw een P I and T2 was reduced. T ranscription reading through the c - m y c attenuator, but not that w hich term inates, was i n h i b i t e d by th e a d e n o s i n e a n a lo g 5 ,6 d i c h l o r o 1 B D -rib o fu ra n o s y lb e n z im id a z o le (D R B ). It is p ro p o sed th a t o n ly tho se elongation com plexes which are resistant to DRB are capable o f prem ature term ination, and that this subset exists only w ithin the first few hundred bases o f the transcription unit.
T ranscription initiated at the P I prom oter was found to term inate prematurely at two positions, T IA and T IB which flank the P2 TATA box and are thus closer to the P I promoter than T2. T IB is a T-rich sequence which resem b les p rev io u sly id e n tifie d a tten u atio n site s b ut T IA ap p ea rs to re p re se n t a d iffe re n t class o f te rm in atio n site. A 120bp seq u en ce c o n ta in in g T IA in d u c ed te rm in a tio n d o w n stream o f a h e te ro lo g o u s prom oter. T IA is located im m ediately upstream o f the P2 TA TA box. M utagenesis o f an elem ent overlapping this TATA box affected both the efficiency and the position o f term ination. A 28bp sequence including this elem ent bound a factor w hose sequence sp ecificity correlated w ith T IA te rm in a to r fu n c tio n .
ACKNOWLEDGEMENTS
I am grateful to Dr. D avid B entley fo r his su p erv isio n o f this research. I would also like to acknow ledge the rest o f the lab for th eir friendship and support; in p articu la r I am indebted to Sika R istevski for frequently feeding me at 6pm during the early years, and thereby keeping me alive. Sadly she has now found a substitute for me, apparently much m ore needy o f such support. I w ish to th an k B runo A m ati for read in g m a n u scrip ts and fo r n um erous discussions, and Nic Jones for his occasional words o f wisdom I also acknow ledge the friendship o f Roy P o llo ck and A lex E ccleston, as well as all other members o f the Jones, Treism an, G oodbourn and Land labs. Finally, I must not offend Gerrard Evan by forgetting to m ention his friendliness and good hum our.
TABLE OF CONTENTS
TITLE PAGE... i
ABSTRACT... ii
DEDICATION... ii i ACKNOWLEDGEMENTS... i v TABLE OF CONTENTS... v
LIST O FH G U R E S...ix
ABBREVIATIONS...XI C H A PTER 1 IN TR O D U C TIO N ...1
1.1 INTRODUCTION... 2
1.1 TERMINATION AND ATTENUATION OF PROKARYOTIC TRANSCRIPTION...3
1.1.1 RNA secondary structure in prokaryotic term in atio n ... 4
1.1.1.2 A ttenuation in bacterial opérons as a function o f alternative stem -lo o p s tru c tu re s ...6
1.1.2 R ho-dependent te rm in atio n ... 7
1.1.3 Factors which modify the elongation com petence of RNA p o l y m e r a s e ... 9
1.1.4 Proteins which bind to specific DNA sequences and block elongation by RNA po lym erase...10
1.2 TERMINATION OF EUKARYOTIC TRANSCRIPTION... 11
1.2.1. Factors required for accurate transcription initiation by RNA polym erases I, II and III...11
1.2.2 Elongation factors required for RNA pol II transcription... 12
1.2.3. The effect o f chrom atin on transcription elongation by prokaryotic and eukaryotic polym erases... 14
1.2.4. Intrinsic term ination by RNA polym erase... II... 15
1.2.5. The nuclear run-off assay... 17
1.2.6 RNA secondary structure and /or proteins may m odulate tran scriptional attenuation in som e eu karyotic v iru s e s ... 17
1.2.6.1. A denovirus... 17
1.2.6.2. SV40...19
1.2.6.3. The minute virus of mice... 20
1.2.6.4 Prom oter substitution experim ents the effects on SV40, Adenovirus and MVM attenuation...20 1.2.6.5. HIV-1 Tat protein binds to an RNA secondary structure and
1.2.6.6. Term ination in vaccinia virus involves a protein which I
recognises an RNA signal...|22
1.2.6.7. A DNA-binding factor which causes term ination in a ^ eu k a ry o tic v iru s ...J23 23 23 1.2.7 Term ination in eukaryotic cellular gen es... 1.2.7.1 DNA-binding factor-dependent term ination at 3' ends o f pol I genes prevents read-in into dow nstream genes... 1.2.7.2 Term ination in the m itochondrial genome requires a specific DNA binding p ro te in ...:25
1.2.7.3 Termination at the 3' ends of pol III genes is facilitated by an R N A -binding factor with ATPase activ ity ...26
1.2.7.4 Termination at the 3' ends of most pol II genes requires RNA p r o c e s s i n g ...27
1.2.7.5. Termination at a specific element at the 3' ends of pol II snRNA genes requires a signal in the p ro m o ter... 29
1.2.8. Prem ature term ination and pausing in pol II genes... 30
1.2.8.1. The c-myc oncogene... 30
1.2.8.1.1. A ttenuation o f c-m yc tran scription... 32
1.2.8.2. Prem ature term ination o f c-m yb tran scrip tio n ... 36
1.2.8.3. A ttenuation o f c-fos tran scrip tio n ...3 7 1.2.8.4. Prem ature term ination in adenosine deam inase g en es...38 1.2.8.5. Transcriptional pausing in the hsp 70 gene of D rosophila...J3 9
CHAPTER 2 ANALYSIS OF WHETHER ELEMENTS IN THE
PROMOTER SPECIFY TERMINATION...J41 2.1 Introduction, aims and summary of resu lts... 4 2 2.2 R e su lts...4 4
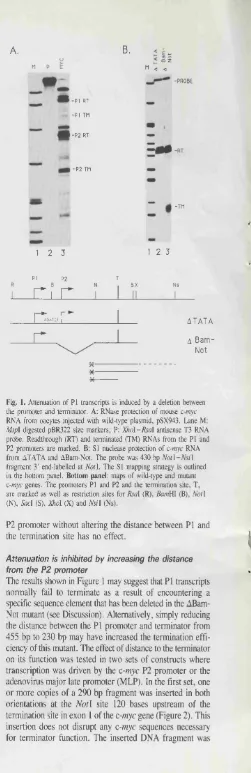
2.2.1 M apping o f prem aturely term inated c-m yc tra n sc rip tio n ... 2.2.2 Term ination efficiency in TK-myc hybrid g e n e s ... 2.2.3 The c-myc attenuator functioned downstream of the U1 snRNA p r o m o t e r ... 2.2.4 Effect of c-myc P2 prom oter mutations on term ination
efficiency at T 2 ... C H A PTER 3 E F F E C T O F DISTANCE AND DRB ON TERM IN A TIO N E F F IC IE N C Y AT T 2 ... 3.1 Introduction, aims and summary of resu lts... 3.2 R esu lts...
3.2.1. Increasing the distance to the T2 attenuator reduced the efficiency of term ination... 3.2.2 P I transcription was induced to term inate by a deletion which decreased the distance to the atten u ato r... 3.2.3 DRB altered the ratio o f term inated to read through
transcription... 3.2.4 DRB did not inhibit transcription initiation...
77
85
88 91 C H A PTE R 4 PREM A TU R E TERM IN A TIO N O F P I
T R A N S C R IP T IO N ... 97
4.1 Introduction, aims and summary o f resu lts...98
4.2 R esults...99
4.2.1 Term ination o f P I tran scription... .99
4.2.2 Term ination occurred in a P2 prom oter fragm ent placed dow nstream o f the a-globin p ro m oter... 104
4.2.3 Mutation of the E2F and M E la l sites had no effect on term ination at T 1 ... .109
4.2.4 M utations in the P2 TATA region reduced term ination efficiency at T IA or altered the site o f 3' end form ation... 114
4.2.5 Additional mutations in the P2 TATA region which inhibited term ination at T I A ... .119
4.2.6 An active initiation complex was insufficient to cause term ination at T I A ...122
4.2.7 A 28bp element containing the TATA region caused a low level of term ination... 125
4.2.8 A factor with DNA-binding specificity which correlates with ^ t e r m i n a t i o n ... 128
4.2.9. The oocyte factor has a different binding specificity to that o f TFIID ...128
I 4.2.10 A factor present in a HeLa extract has DNA-binding properties sim ilar to those o f the oocyte factor... 1131
CHAPTER 5 DISCUSSION... 5.1. Term ination of P2 transcription. 135 136 5.1.1 Location o f the c-myc T2 attenuation s ite ... jl3 6 5.1.2 Does the T2 site cause termination in m ammalian c e lls ? ...137
5.1.3. Effect o f the prom oter on term ination efficiency at T 2 ... 140
5.1.5. The effect o f transcription through tandem term inators !
ev id en ce for p olym erase h e te ro g e n e ity ...146
5.1.6. The effect o f DRB on transcription m ore evidence for polym erase heterogeneity, and the ! m echanism o f DRB in h ib itio n ...147
5.1.7. W hat are the biochem ical properties o f processive and non-processive elongation com plexes?... 153
5.2. Prem ature term ination o f transcription initiated at the PI p r o m o t e r ...il5 4 5.2.1. Chatacterization of term ination at T 1...154
5.2.2. Termination at T IA is inhibited by mutations in the P2 TATA r e g i o n ... .155
5.2.3. A factor present in oocyte nuclei binds to the T1 term inator and is im plicated in te rm in atio n ... i l5 7 5.2.4. P otential physiological significance of P I te rm in atio n ... ^160
5.3. F uture p e rsp e c tiv e s...164
C H A PTER 6 M ATERIA LS AND M E T H O D S ... 165
6.1. Chemicals and enzymes... 166
6.1. Chemicals and enzymes...j 166 6.2. General Buffers and S olutions...! 166
6.3. General procedures...! l6 8 6.4. Subcloning p ro ced u res...j 169 6.5. Preparation of single-stranded tem plates... 174
6 . 6 Site-directed m utagenesis...1176
6.7. DNA sequ encing...178
6.8. Oocyte injection and RNA extraction... 179
6.9. Radiolabelling o f DNA and RNA for use as probes... ,179
6.10. RNA mapping by S I, exonuclease VII and RNAase protection... |183
6.11. N orthern B lo ttin g ...[l8 4 6.12. P reparative in vitro tran scrip tio n ...1186
6.13. In vitro translation... 1186
6.14. Cell extracts used for gel retardation... 1187 6.15. Gel retardation assays...
6.16. Plasm id constructions R E F E R E N C E S ...
188 188 193
L IST O F FIG U R ES
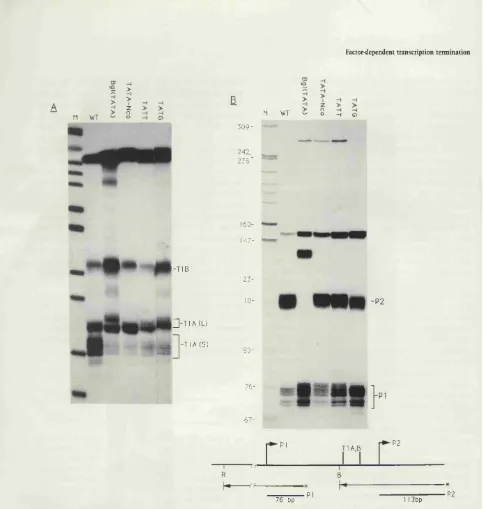
Figure 2.1. Termination occurs at the end o f exon 1 of the murine
c-myc gene in injected Xenopus leavis oocytes... 46 Figure 2.2. Truncated c-myc RNAs transcribed in oocytes detected by
RN A ase protection and by N orthern b lo ttin g ...48 Figure 2.3. Term ination at a T2-containing c-m yc fragm ent placed
downstream o f a series of linker scanning mutations o f the HSV TK
p r o m o t e r ... 52 Figure 2.4 Further analysis o f TK-myc hybrid genes... 54 Figure 2.5. Further analysis o f TK-myc hybrid genes and
com parison with T7 RNA control... 56 Figure 2.6. Term ination efficiency at the c-myc attenuator was
unaffected by upstream TK prom oter sequences... 59 Figure 2.7. Diagram o f U1 and TK/myc hybrid g e n e s... 62 Figure 2.8. Term ination occurs at the c-m yc attenuator placed
downstream o f the human U1 snRNA prom oter... 64 Figure 2.9. Term ination at the U1 3' box in attenuator-containing
plasm ids...67 Figure 2.10. Deletion of the M E la l site did not significantly affect
c-myc transcription in the oocyte... 70 Figure 2.11. A deletion of the P2 promoter reduces the ratio of
read th ro u g h to term in ated tra n s c rip tio n ... 72
Figure 3.1. Term ination at T2 is inhibited by increasing the distance to the attenuator
ex perim en t 1... 79 Figure 3.2. Term ination at T2 is inhibited by increasing the distance
to the attenuator
experim ents 2 and 3 ...81 Figure 3.3. Increased distance between the al-g lo b in prom oter and
the c-m yc attenuation site in h ib its term in atio n ...83 Figure 3.4. Attenuation of P I transcripts at T2 is induced by a
deletion betw een the prom oter and term inator...8 6
Figure 3.5. DRB specifically inhibits transcription which reads
Figure 4.1. Transcripts from the c-myc P I prom oter term inate
p r e m a t u r e l y ...1 0 0
Figure 4.2. Sequence o f P2 prom oter region and positions of term ination at T IA /B in the murine (M) and human (H) c-myc
genes...1 0 2
Figure 4.3. c-myc exon 1 RNAs are not post-transcriptionally cleaved
in the oocyte... 105 Figure 4.4. Term ination at T IA occurs downstream o f a heterologous
p r o m o t e r ... 107 Figure 4.5. Deletion of the M E la l factor binding site does not inhibit
term ination at T 1 ... 110 Figure 4.6. M utation o f the E2F site does not inhibit termination at
T 1 ... 112 Figure 4.7. Mutation of the P2 TATA box reduces termination at T IA
in the m urine c-m yc gene... 115 Figure 4.8. Mutation of the P2 TATA box also inhibits termination at
T IA in the human c-myc gene...117 Figure 4.9. Term ination at T IA shifts in response to an insertion
upstream of the P2 TATA box... 120 Figure 4.10. Effect of mutations of the P2 TATA region on initiation
and term ination...123 Figure 4.11. A 28bp c-myc element induces a low level of
term ination in the al-g lob in gene...126 Figure 4.12. A DNA binding factor in Xenopus oocyte nuclei with
specificity that correlates w ith te rm in atio n...129 Figure 4.13. A DNA-binding factor in HeLa extracts with properties
A B B R E V IA T IO N S
approx: ap p ro x im ate ly bp: base pairs
BSA: bovine serum album in A: adenine
C: cytosine G: guanine T: thym ine
ATP: adenosine 5' phosphate CTP: cytosine 5' phosphate OTP: guanosine 5' phosphate UTP: uridine 5' phosphate NTP: nucleoside 5' phosphate
dATP: 3'deoxyadenosine 5' triphosphate dCTP: 3’ deoxycytosine 5' triphosphate dOTP: 3' deoxyguanosine 5' triphosphate dTTP: 3' deoxythym idine 5’ triphosphate dNTP 3' deoxynucleotide 5' triphosphate ddNTP: 2'3' dideoxynucleotide 5’ triphosphate DNA: deoxyribonucleic acid
DRB: 5,6 d ich lo ro -1 -B -D -rib o fu ra n o sy lb en zim id az o le DTT: dithiothreitol
EDTA: ethylenediam ine tetraacetic acid HSV: Herpes Simplex virus
kb: kilobases kd: kilodaltons:
MLP: m ajor late prom oter
M OPS: 3 -N -m o rp h o lin o p ro p a n esu lp h o n ic acid PCR: polym erase chain reaction
PEG: polyethylene glycol
PIPES : piperazine-N , N '-bis (2-ethane sulfonic acid) PM SF : p h e n y lm e th y ls u lp h o n y lflu o rid e
nt: n u cleo tides
RNA: ribonucleic acid
SDS: sodium dodecyl sulphate TK: thym idine kinase
1.1 INTRODUCTION
There are three stages during RNA synthesis w hich could serve as targets for reg ulatory signals. These stages are tran scrip tio n al in itiatio n , elongation and term ination, o f w hich in itiatio n has undoubtedly been the most extensively studied. After RNA synthesis has initiated, the process of tra n s c rip t e lo n g a tio n p ro v id es fu rth e r o p p o rtu n itie s to re g u la te gene exp ression beyond those available during in itia tio n . Indeed, it is now ev id en t that this is a critical p o in t at w hich gene expression can be controlled. Prem ature term ination or pausing o f RNA polym erases during tran scrip tio n has been docum ented w ithin m any tran scrip tio n u n its, and its u se as a tra n s c rip tio n a l co n tro l m ech an ism is p a rtic u la rly w ell docum ented in prokaryotes. The im portance o f prem ature term ination or attenu ation in eukaryotic gene reg ulatio n is exem plified by its role in c o n tro llin g tra n sc rip tio n o f the c ~ m y c oncogene. By reg u latin g the effic ien cy o f a block to tran scrip tio n elo n g atio n , c e llu la r grow th and d if f e r e n tia tio n s ig n a ls can m o d u la te c - m y c mRNA levels. In the experim ents in this thesis, I analysed this c - m y c tran scrip tio n al block in an attem p t to gain in sig h t into the m echanism s g o vern in g eu k ary o tic a t te n u a t io n .
In addition to being a consequence o f reg u lato ry events during elongation, term ination occurs constitutively at the 3' ends o f m any genes w here it serves the basic and fundam ental purpose o f defining the extent o f a tran scrip tio n unit. This class o f term in atio n events w ill also be d iscu ssed h ere, sin ce the p ro cesses o f a tte n u a tio n and tra n s c rip tio n te rm in a tio n at th e end o f a gene m ay in v o lv e re la te d m o lecu la r m e c h a n is m s .
M uch o f our understanding o f attenuation has arisen from studying these events in prokaryotes and also in eukaryotic viruses. I will therefore discuss the m echanistic features which in these system s appear to specify attenuation. From the discussion in the final part o f this chapter, it will be ev iden t that transcription attenuation in euk aryotic cellu lar genes is less well understood than in prokaryotic systems. Although many of the details o f these processes have yet to be determ ined, com parison o f m echanism s im p lic a te d in te rm in a tio n /a tte n u a tio n in all o f th e se sy stem s rev ea ls certain recurrent themes.
below , it is as yet unclear w hether pausing versus tru e term ination is ta k in g p la c e d u rin g tra n s c rip tio n o f c e rta in g e n e s re g u la te d by attenuation). T ranscription term ination can be defined as an arrest o f elongation by RNA polym erase, followed by release o f the nascent RNA and polym erase from the tem plate DNA. Term inated RNAs can be isolated as free m olecules u n less u n stab le or ra p id ly p ro cessed . P au sin g is a tem porary cessatio n o f elo n g atio n , w here the p o ly m erase and n ascent tran scrip t rem ain associated with the tem plate; thus a paused polym erase retain s the cap acity to resum e tran scrip tio n . T he d istin c tio n betw een pau sin g and te rm in atio n can be m ade usin g p u lse -c h a se ex p erim en ts w hereby short transcripts are chased into longer products. In som e cases the separation o f tran scrip tio n com plexes on sucrose g rad ie n ts or gel filtration columns has been used to determ ine w hether short RNAs rem ain associated w ith the tem plate (M aderious and C hen, 1984; C am pbell and Setzer, 1992). In either case, the outcome o f pausing or term ination is that the am ount o f tran scrip tio n reading do w n stream o f the a rre st site is m o d u la te d .
1.1 T E R M IN A T IO N AND A T T E N U A T IO N OF P R O K A R Y O T IC TR AN SCR IPTION
B asal-level transcription in prokaryotes (review ed in von H ippel et al. (1984)) begins at the prom oter with recognition by RNA polym erase of sequences im m ediately 5' o f the tran scription start site. R ecognition of specific prom oter elem ents is a function o f the sigm a factor (review ed in H elm ann and C ham berlin (1988)) w hich w hen bound to the core RNA polym erase constitu tes the holoenzym e capable o f in itia tin g tran scriptio n. The freq uen cy o f in itia tio n events can be reg u la ted by tran scrip tio n al activators and repressors. S ubsequently, the tran scrip t is elongated with the c o n c u rre n t sp o n tan eo u s d is s o c ia tio n o f sig m a fa c to r fro m th e e lo n g a tio n co m p lex . T he e ffic ie n c y o f tra n s c rip tio n e lo n g a tio n is influenced by the intrinsic DNA or RNA sequence, and by term ination or an titerm in atio n factors. The final phase o f tran scrip tio n is term inatio n, w here the polym erase and nascen t RNA are released from the tem plate. Term ination and attenuation during transcription are discussed in separate sections throughout this chapter, as specified.
1 .1 .1 .1 S t e m - lo o p - m e d ia t e d in t r in s ic t e r m in a tio n
Term ination by RNA polym erase can be intrinsic to the transcribed sequence in that it is specified solely by nucleic acid signals in the absence o f accessory factors; alternatively it may be dependent on one or more proteins as in for instance rho-m ediated term ination (see below ). In their sim plest form s, intrinsic term inators at the 3' ends o f many bacterial genes con sist o f G C-rich regions o f interrupted dyad sym m etry w hich resu lt in the form ation o f RNA hairpins w hen tran scrib ed , follow ed by a short polyuridine stretch in the RNA (Platt, 1986). As described later, sim ilar m otifs are im p licated in term in ation in certain eu k ary o tic tran scrip tio n u n it s .
The m echanism w hich specifies this form o f in trin sic term ination rem ains som ew hat controversial. It seem s clear how ever that the RNA hairpin first induces the polym erase to pause at the U -rich tract (Farnham and Platt, 1981), (although not all transcriptional pauses are caused by RNA h airp in s (L aF lam m e et al., 1985)). A ltho ug h p o ly m erase p au sin g is in s u ffic ie n t to cause te rm in atio n , a p au sin g step appears alw ays to com prise one o f the events leading to intrinsic term ination (Farnham and Platt, 1981; Platt, 1986). Since a transcription bubble of about 17bp forms in DNA which is being transcribed (Gamper and Hearst, 1982), it was thought that these strands o f DNA are norm ally held apart during transcription by a putative hybrid o f 10-12bp between the DNA and nascent RNA. Formation o f an RNA stem -loop w eakens this h ybrid, cau sin g the tran scrip t and paused polym erase to be released from the tem plate at the uridine residues, sin ce h y d ro g en bond s b etw een dA -U b a s e -p a irs are re la tiv e ly w eak (M artin and Tinoco, 1980). The DNA strands can then reanneal.
However, the results o f several studies have challenged this model. A nalysis o f artificial hybrid E. Coli term inators revealed that sequences flanking the stem -loop and the polyuridine stretch are also im portant for term in atio n , and certain term in atio n sites la ck in g the U resid u es are efficient (Reynolds and Cham berlin, 1992). M oreover, there is controversy co ncerning the num ber o f b ase-p airs in the R N A -D N A hy brid during transcription. I f significant DNA-RNA base-pairing does not occur during elongation, the stem -loop could not cause term ination through w eakening this base-pairing. A lthough approxim ately 12 bases have been found in some studies to be associated with the DNA tem plate (see Y ager and von H ippel (1991), another study involving RN Aase footprinting o f m am m alian RNA polym erase com plexes suggested that only three bases or less were hybridized to DNA (Rice et al., 1991). A recent report also challenged the sim plicity o f the existing model, since it was found that not only stability but also the sequence o f the stem was im portant for the function o f the bacteriophage lam bda tR2 term inator. In this study, a num ber o f mutations w hich w eak en ed the stem s tru c tu re elim in a te d te rm in a tio n ; how ev er certain m utations o f the stem w ith eq uiv alent stab ilities po ssessed very different term ination capabilities (Cheng et al., 1991). To account for these o b serv atio n s, a m odel w hich invokes a se q u en ce -d e p en d en t in teractio n between RNA or DNA and polym erase was suggested (Cheng, et al., 1991). T hus, RNA p o ly m erase m ay co n tain a b in d in g site fo r the n ascen t tran scrip t, an in teractio n w hich could be d isru p ted by RNA secondary structure. In this model the polym erase rather than a nucleic acid hybrid w ould hold the tran scrip tio n bubble open and sta b iliz e the elo n g atio n com plex. A rela ted m odel invoking a re la tiv e ly seq u en ce n o n -sp ecific p o ly m e ra s e -n u c le ic acid in te ra c tio n w as also su g g e s te d (A rn d t and Cham berlin, 1990; Rice, et al., 1991), although this latter m odel does not easily account for the sequ ence-dep en den ce o b served by C heng et al. Hence although RNA hairpins are clearly involved in term ination (see also below), the mechanism by which they operate has yet to be resolved.
1 .1 .1 .2 A t t e n u a t i o n in b a c t e r ia l o p é r o n s as a f u n c t io n of a l t e r n a t i v e s t e m - lo o p s t r u c t u r e s .
gen etics. Som e o f the m echanistic features w hich govern atten u atio n resem b le th ose described above for in trin sic te rm in atio n , in p a rtic u la r there is a central role for RNA secondary structure. H owever attenuation invokes a requirem ent for regulatory factors; these may be ribosom es or o th e r p ro tein s.
A ttenuation in amino acid b io synthetic opérons generally occurs in a transcribed leader region o f an operon w hich con tain s a clu ster o f codons for the encoded amino acid (Zurawski et ai., 1978; Carter et al., 1986; Lynn et al., 1987). A hairp in-io op in this lead er region induces RNA polym erase to pause during transcription. A ribosom e can then bind to the leader RNA and initiate synthesis o f a leader peptide. As deduced from the effe c t on po lym erase pau sin g o f m utation s in the lead er as w ell as inhib itors o f tran slatio n, binding o f the ribosom e and tran slatio n o f the leader peptide releases RNA polym erase from its pause (L andick et al., 1985). The polym erase and ribosom e are believed to then move through the leader sequences in unison. A deficiency in the supply o f the encoded amino acid and hence the appropriate charged tRNA causes the ribosom e to stall at the cluster o f codons for the encoded amino acid. This stalling prom otes the form ation o f an antiterm in ator stem -loop in the tran scrip t w hich RNA polym erase can traverse (this structure is not follow ed by a p o ly u rid in e tra c t); in the absence o f th is stem -lo o p an a lte rn a tiv e , term inato r stem -loop w ill autom atically form (S troynow ski and Y anofsky, 1982). T herefore the presence o f adequate supplies o f the charged tRNA dictates that the ribosom e does not stall and the term inator stem -loop causes transcription to prem aturely term inate in the leader region o f the operon.
(Kuroda et al., 1986; Shimotsu et al., 1986). In the p u r operon o f B. sub ti l is , the co ncentration o f guanine nucleotides appears to control tran scrip tio n through a term inatio n-an ti term ination m echanism . The lead er region of this operon is very sim ilar to the tryptophan operon o f B. subtilis', the fact that it does not encode a leader peptide suggests that a regulatory molecule analogous to m t r may control elongation (Ebbole and Zalkin, 1987). A sim ilar m echanism also operates in the bgl operon o f E. coli which is requ ired for catabolism o f beta-gluco sid es. The Bgl G gene p ro du ct encoded by this operon, is required in t r a n s for expression o f the operon, since it prev en ts p rem atu re tran scrip tio n term in atio n w ith in the leader region (M ahadevan and W right, 1987). Bgl G is an RN A-binding protein; in the presence o f inducing beta-glucosides, it appears to bind to a stem -loop in the nascent RNA, w hich prevents form ation o f a term in ato r hairpin thereby p erm itting polym erases to transcrib e the operon (H oum an et al., 1990). Bgl G itself is regulated in the absence of inducer by the Bgl F protein; Bgl F which is also encoded by the b g l operon, is present at low levels in uninduced cells w here it inactivates Bgl G by phosphorylation (A m ster-Choder et al., 1989).
1.1.2 R h o -d e p e n d e n t te r m in a tio n
T erm ination in E. coli is usually subdivided into categories o f the in trin sic, facto r-in d ep en d en t term ination described above, and th a t w hich is d e p e n d e n t on the rho p ro te in (R o b erts, 1969). R h o -d e p e n d e n t term in ato rs are p resen t in b acterio p h ag e lam bda and in a num ber of b a c te ria l o p éro n s. In c o n tra st to in trin s ic te rm in a tio n , rh o -d e p e n d e n t term ination does not require stretches o f uridine residues; instead, rho is an RNA binding protein w hich interacts as a hexam er with nascent RNA lacking RNA secondary structure (Finger and R ichardson, 1982; Chen et al., 1986; R ichardson, 1990). A consensus RNA sequence for rho has been difficult to ascertain, but appears to consist o f a C-rich and G-poor region o f particular length and location (Alifano et al., 1991; Hart and Roberts, 1991).
(G alluppi and R ichardson, 1980). P olym erase pausing can occur at rho- dependent term ination sites in the absence o f rho and is unaffected by rho; pausing is probably necessary to perm it sufficient tim e for release o f the transcrip t and polym erase from the tem plate (Lau et al., 1983). Indeed, efficient term ination requires a kinetic coupling betw een RNA polym erase and rho. Polym erase m utants w hich term inate w ith a higher than wild-type e ffic ie n c y e lo n g a te m ore slow ly th an u su al, w h ile te rm in atio n - defective m utants elongate m ore rapidly; m oreover, a slow -acting m utant o f rho is suppressed by a mutant RNA polym erase that elongates slowly (Jin et al., 1992). As suggested by these authors, the elongation properties of p o ly m erase could be altered by te rm in atio n or an titerm in atio n facto rs which increase or decrease the efficiency o f polym erase pausing.
U pon en co u n terin g a paused p o ly m erase, rho p ro b ab ly m ediates release o f the nascent transcript from the elongation com plex. Indeed, rho was found to be an R N A -dependent, NTP h y d rolysis-depen dent helicase, suggesting that it may effect term ination by unw inding the RNA-D N A duplex (Brennan, et al., 1987). It is not clear whether the ATPase activity of rho is required for translocation of the protein as well as for its helicase activity.
E ffic ien t rho-m ediated term ination involves at least one additional cellular factor, the E. coli NusG protein, as dem onstrated using E. coli cells depleted o f Nus G by a conditional lethal m utant (Sullivan and Gottesman, 1992). The role o f Nus G in termination is not known, but was postulated to involve a direct interaction between Nus G and rho, possibly assisting the attachm ent o f rho to RNA (Sullivan and Gottesman, 1992).
A e u k a ry o tic p ro te in w hich ca u se s te rm in a tio n in a m ann er analogous to rho has yet to be described. H owever as discussed later, a eukaryotic factor im plicated in RNA pol III term ination displays ATPase-dependent h elicase activ ity in the presence o f D NA/RNA and RNA/RNA hybrids (Bachmann et al., 1990).
1.1.3 Factors w hich m odify the elo n g a tio n co m p ete n c e of RNA p o l y m e r a s e
p a rtic u la rly w here the te rm in atio n c a p a b ility o f RN A p o ly m erase is determ ined some distance from the actual term ination site. This idea will also be discussed later, w ith regard to experim ents in this thesis which d e m o n stra te d d is ta n c e -d e p e n d e n t te rm in a tio n o f c - m y c tr a n s c rip tio n (Chapter 3 and Discussion)
In th e w e ll-c h a ra c te ris e d a n tite rm in a to r s y ste m o f E. col i bacteriophage lam bda, lytic grow th o f the phage is dependent on phage- encoded transcriptional activator proteins, N and Q. N and Q w hich are required for early and late expression o f the phage genom e respectively, perm it transcrip tion by im pairing the function o f term inators (A dhya et al., 1974; Franklin, 1974; Grayhack and Roberts, 1982). These proteins which modify E. coli RNA polym erase to a term ination resistant form, require ci s-acting sequences to perm it antiterm ination in an opero n-sp ecific m anner.
1.1.4 P r o te in s w h ich b in d to sp ecific DNA se q u e n c e s a n d blo ck e lo n g a tio n by RN A p o ly m e ra s e
In the experim ents presented in C hapter 4 o f this thesis, a DNA-binding factor is im plicated in term ination. The m echanism by w hich this occurs is not known. From the discussion so far, it is apparent that in most p ro k a ry o tic fa c to r-d e p e n d e n t term in atio n and a n tite rm in a tio n , rib o so m es or proteins interact with RNA rather than DNA. H owever a role for DNA- binding factors in blocking the m ovem ent o f a polym erase has also been stud ied , not only w ith regard to the p ro p erties o f sp ecific term ination factors, but also in terms o f how RNA polym erase deals with nucleoprotein com plexes w hich it en counters as it trav erses a gene. F or instance, bacteriophage SP6 RNA polym erase can transcribe through a transcription com pex assem bled onto the internal control region o f a X enopus 5S RNA gene w ithout disrupting it (W olffe et al., 1986). Furtherm ore, transcription by SP6 polym erase in vitro is not inhibited by the presence on the template o f histones; the histones, possibly transiently, are displaced from the DNA during transcription (Lorch et al., 1987) (see section 1.2.3. for a discussion o f the effects o f chrom atin on elongation).
repressor can cause term ination through a D N A -binding activity, by which it may also regulate transcription. Furtherm ore, when its operator site is engineered into a pol II gene, the lac repressor can block elongation by eukaryotic RNA polym erase II and cause term ination (D euschle et al., 1990). M utants of the E. coli EcoRI endonuclease which bind to their cognate sites bu t are defective in DNA cleavage, also block elongation by E. coli RNA p o ly m erase in vitro (Pavco and Steege, 1990). The m echanism by which blockage occurs is not always clear. It may be a result o f steric hindrance to the polym erase by some but not all proteins, or alternatively due to a sp ecific in teractio n betw een the tran scrib in g po ly m erase and the DNA- bo u n d p ro tein . As d iscu ssed la ter, in eu k ary o tes the D N A -b inding te rm in a tio n fa c to r TT FI app ears to s p e c ific a lly in te ra c t w ith RNA polym erase I to cause termination (Kuhn et al., 1990).
1.2 TERMINATION OF EUKARYOTIC TRANSCRIPTION
Follow ing a brief description o f the initial assembly on the prom oter o f a transcription com plex, this section w ill focus on eukaryotic elongation and te rm in a tio n . V iral and c e llu la r tr a n s c r ip tio n a tte n u a tio n or term ination mediated by RNA polymerases I, II and III will be described. 1 .2 .1 . F a cto r s req u ir ed for a c c u r a te t r a n s c r ip t io n in it ia t io n by RNA polymerases I, II and III
T ran scrip tio n in itia tio n in eu k ary o tes re q u ire s a co m p lex ity o f tran scription factors w hich in itially assem ble into a p rein itiatio n com plex near the transcription start site. T ranscription o f pol I, II and III genes re q u ire s th e p o ly m erase enzym e in c o n ju n c tio n w ith a n um ber o f accessory factors. Pol II requires the general factors TFIIA -TFIIJ, of which TFIID binds in a sequence-specific manner to the TATA elem ent (reviewed in Saw adogo and Sentenac (1990); Zaw el and R einberg (1992)). These factors assemble onto the TFIID/TATA com plex to form a stable preinitiation com plex. A num ber o f these factors are com posed o f m ore than one polypeptide; TFIID is particularly com plex and com prises the TA TA -binding protein TBP and a num ber o f associated proteins (TAFs) which function at least in part as m ediators o f transcriptional activator function (D ynlacht et al., 1991; Tanese et al., 1991; Timmers and Sharp, 1991). Many o f the basal factors have recently been cloned, w hich has led to the rev elatio n o f significant evolutionary conservation o f at least part o f the pol II com plex
from yeast to humans (G uarente and B erm ingham -M cD onogh, 1992). Pol I utilizes at least two factors, UBF and S L l (review ed in Sollner-W ebb and Mougey (1991)). Pol II requires the general factors TFIIIB and TFIIIC with an additional factor TFIIIA which is required for transcription o f 5S rRNA genes (reviewed in G abrielson (1991)). TFIIIC and TFIIIA w hich bind to intragenic sequences function as assem bly factors for TFIIIB and pol III (Kassavetis et al., 1990).
A lthough o rig in ally b elieved to be d istin ct from one another, a rela tio n sh ip betw een tran scrip tio n by the three po ly m erase system s has recently been established. RNA pols I, II and III share a num ber o f subunits (W oychik et al., 1990) and at least one general factor, the TATA- binding protein TBP, w hich is required for transcription from both TATA- less and TATA-containing prom oters (M argottin et al., 1991; Pugh and Tjian, 1991; Comai et al., 1992; Corm ack and Struhl, 1992; Schultz et al., 1992). D espite these common features, it will be evident from the discussion below that transcription term ination at the 3' ends o f pol I, II and III genes involves sig n ifican tly d isp arate m echanism s. H ow ever, pols II and III in trin sically recognise sim ilar sequences w hich cause term ination in vitro (see below).
1.2.2 E longation factors req u ired for RNA pol II tran scrip tion
It rem ains to be seen how many o f the initiation factors described above rem ain a sso c ia te d w ith RN A p o ly m erase d u rin g tra n s c rip tio n elo n g atio n . N ev erth eless d uring the elo n g atio n p h ase, pol II u tiliz es factors with w hich it can asso ciate prior or subsequent to tran scription initiation. For instance, a factor term ed TFIIF (also known as RAP30/74) was o riginally identified by pol II affinity chrom atography (S opta et al., 1985); review ed in G reenblatt (1991); TFIIF which is essential for initiation, also affects the elongation step. A second factor termed SII (also known as TFIIS or DmS-II, according to its source o f purification) also interacts with pol II (Rappaport et al., 1988; P rice et al., 1989) and affects elongation; how ever SII is not required for initiation. An additional activity term ed TFIIX has been identified, w hich like SII also affects elongation efficiency but is not required at the initiation step (Reinberg et al., 1987; Bengal et al.,
1991). It is not known w hether TFIIX also interacts with pol II.
assay (described in section 1.2.4), to which the effects of added elongation facto rs could be determ ined (P rice, et al., 1989; R eines et al., 1989; SivaRam an et al., 1990). A lternatively, prom oter-dependent assays included assem bly o f stab le p re in itia tio n com plexes using a n u clear ex tra c t or p u rified fractions; these w ere then treated w ith heparin to perm it one round o f transcription (Reinberg and Roeder, 1987) or washed (W iest et al.,
1992) or subject to gel filtration (Bengal, et al., 1991) to rem ove elongation factors. A ddition o f p u rified SII, T F IIF or TFIIX to these p u rified transcription com plexes has defined a role for these factors in elongation. All o f these methods have dem onstrated that SII increases the efficiency of elongation through pause or attenuation sites.
SII, T F IIF and TFIIX appear to affect elo n g atio n by d iffere n t m echanism s. U nlike SII and TFIIX , T FIIF first enters the transcrip tio n com plex at the preinitiation stage. Secondly, an effect o f TFIIF and TFIIX but not o f SII, is to increase in the rate o f elongation (Bengal, et al., 1991; W iest, et al., 1992). However SII, TFIIF and TFIIX all suppressed pausing in one study (Bengal, et al., 1991), although the antipausing activity o f TFIIF was not observed in the experiments o f (W iest, et al., 1992), possibly due to the particu lar conditions used in the latter experim ents. SII or TFIIX decreased pausing w hether added at the b eginning o f the tran scrip tio n reac tio n or w hile the po ly m erase was arreste d du ring elo n g atio n ; by c o n tra st T F IIF had no e ffe c t on polym erases w hich had reached the attenuation site (Bengal, et al., 1991). T FIIF also appears to bind more tightly to RNA polym erase than SII (Bengal, et al., 1991; W iest, et al., 1992). Taken together, the interpretations o f these and other observations can be summarised as (1) TFIIF and TFIIX affect the overall rate o f elongation; and (2) when the polym erase reaches an attenuation site, it exists in a pausing con form ation that SII or T FIIX can som ehow alter into an elongation-com petent conformation (Price, et al., 1989; Bengal, et al., 1991; W iest, et al., 1992).
It is conceivable that at the prom oter or during elon gation , these p o ly m e r a s e - e lo n g a tio n fa c to r in te r a c tio n s p r o v id e o p p o r tu n itie s to regulate attenuation or term ination. For exam ple, it is p ossible that the prom oter sp ecificity observed in the function o f certain term in ato rs and attenuators (see below) could be accounted for by this type o f regulation. It is also possible that additional factors affect the elongation step o f pol II tra n s c rip tio n in vivo. G ene-specific elongation factors analogous to the phage lambda N and Q proteins described above, have yet to be identified in eukaryotes. In a sim ilar vein, phosphorylation of pol II occurs at some
po in t during the transitio n from in itiatio n to elon gation (P ayne et al., 1989). T his ev en t could also p o te n tia lly p ro v id e a ta rg e t step for m echanism s w hich m odulate the processivity of RNA polym erase through attenuation sites (discussed in S pencer (1990); see also D iscussion). By contrast, as intragenic pause or attenuation sites have not been identified in genes transcribed by pol I or III, it is possible that these polym erases do not use accessory factors or m odifications to stabilize elongation com plexes.
1 .2 .3 . The e ffe c t o f ch r o m a tin on t r a n s c r ip t io n e lo n g a t io n by p r o k a r y o t ic a n d e u k a r y o t ic p o ly m e r a s e s
How do polym erases transcribe through chrom atin? It is known that chrom atin is p resen t on tem plates w hich are tran scrip tio n a lly active i n v i v o y altho ug h co n ceiv ab ly in a form w hich d iffers from th a t on untranscribed genes. T here is general agreem ent that nu cleosom es can in h ib it tran scrip tio n in itiatio n if assem bled prior to in itiatio n factors i n v i tr o (e.g. (Lorch, et al., 1987; Felts et al., 1990). In order that transcription can occur in vivo, it is p o ssib le that som e kind o f com petition occurs b e tw e e n in itia tio n f a c to rs and h is to n e s , p e rh a p s in f lu e n c e d by tra n sc rip tio n a l activ ato rs (rev iew ed in F e lse n fe ld (199 2)). P o ssib ly , re p lic a tio n o f som e genes co u ld be a p re re q u isite fo r tra n sc rip tio n a l a c tiv a tio n .
the p o sitio n in g o f nu cleoso m es by activ ato rs or rep resso rs has been suggested from studies in yeast (reviewed in Felsenfeld, 1992). It is not clear how ever that the inhibition o f elongation by chrom atin observed i n v i t r o occurs in vivo. For instance, it was found th at although polym erase III could tran scrib e through a tem plate co n tain in g a sm all num ber of histones in vitro, it was inhibited by extended nucleosom al arrays (Felts, et al., 1990). This inhibition was not reproduced in vivo, since the tem plates in w hich ch ro m a tin in h ib ite d e lo n g a tio n in vitro w ere tra n s c rib e d efficien tly in vivo (Felts, et al., 1990). Thus, it is possible that factors exist in vivo which are absent from purified system s, w hich alleviate inhibition o f elongation by nucleosom es. W hether such pu tativ e factors could also play a role in regulation of elongation rem ains to be seen.
1.2.4. Intrinsic term ination by RNA p olym erase II
Since a stable elongation com plex requires accessory factors, it is p erh ap s not su rp risin g th a t p u rifie d RN A p o ly m erase II in trin s ic a lly term in ates tran scrip tio n at sp ecific sites in vitro. The fact o f intrinsic term ination by pol II endorses the idea that the transcribing polym erase could po ten tially be reg ulated at attenuation sites by elon gation factors. P o s t-in itia tio n even ts have been stu d ied in vitro using only p u rified polym erase in the absence o f any accessory factors. Purified pol II alone in itia te s tra n s c rip tio n e ffic ie n tly n ear the s in g le -s tra n d /d o u b le stran d ju n c tio n o f linear prom oterless tem plates con tain in g sin gle-stran ded poly-dC tails (Kadesch and C ham berlin, 1982). This technique w hich has been used in analyses of transcription elongation by both pol II (D edrick et al.,
1987; Reinberg and Roeder, 1987; Reines et al., 1987; Price, et al., 1989; R eines, et al., 1989) and pol III (Cam pbell and Setzer, 1992) perm its an assessm en t o f the elo n g atio n p ro p erties o f p o ly m erase (and selected purified factors, like SII described above). Purified pol II in the absence of accesso ry facto rs elicited te rm in atio n at som e b u t not all p ro k ary o tic term inator sequences tested, w hich consisted o f stretches o f T residues on the non-coding strand (D edrick, et al., 1987). In trin sic term ination sites for pol II w ere also identified in the human c - m y c gene (K erpp ola and Kane, 1988)) and in the human histone H3.3 gene in which the term ination sites corresponded to 5-8 Ts on the non-tem plate strand (R eines, et al., 1987). However, the length o f the T stretch in these in vitro studies did not correlate w ith the strength o f the term ination site, and som e T -stretches did no t fu n ctio n as te rm in ato rs. N ev erth e less in each case w here
term ination occurred, the term ination site corresponded to a run o f Ts. These observations suggest that the Ts are necessary but not sufficient for term ination, and additional features contribute to the efficiency o f pol II term ination (Dedrick, et al., 1987; Reines, et al., 1987). In this regard, it is in terestin g that T -stretches are used as term in atio n sites even w hen a term ination factor is known to be involved (see vaccinia virus term ination b e lo w ).
The m echanism o f intrinsic term ination by pol II appears to differ from that by E. coli polym erase because not all bacterial term inators w ere recognised by pol II. Furtherm ore as described above, bacterial intrinsic term ination requires RNA secondary structure; in contrast the use o f tailed tem plates to study eukaryotic term ination appears to rule out involvem ent o f sig n ifican t RNA stru ctu re in pol II in trin sic term ination , since one characteristic o f this technique is that for som e reason the nascent RNA rem ains hybridised to the tem plate during tran scrip tio n (D edrick, et al.,
1987). H ow ever, it rem ains p o ssib le th a t RNA stru c tu re s in flu en ce term ination at these attenuators in vivo. An alternative hypothesis is that a structure in the DNA tem plate rather than the nascent RNA m ediates in trin s ic te rm in a tio n by e u k a ry o tic p o ly m e ra se : In te re s tin g ly , th e e lectro p h o retic m o b ilities o f re stric tio n fragm ents co n tain in g term in atio n sites suggested that a T -stretch in the H3.3 gene causes the DNA to bend (K erppola and Kane, 1990).
1 .2 .5 . T h e n u c le a r r u n - o f f a ssa y
term ination and term ination at the 3' ends o f genes. In the form er case, truncated RNAs appear frequently to be unstable and so cannot be mapped from the pool o f steady state RNA from cells, and in the latter case primary tran scripts are rapidly processed.
1. 2.6 RNA s e co n d a r y s t ru c t u r e and / o r p ro t ei ns m ay m o d ul a te t r a n s c r i p t i o n a l a t t e n u a t i o n in s o m e e u k a r y o t i c v i r u s e s
1. 2.6. x. A de no vi r us
In eukaryotes, attenuation was first docum ented in adenovirus type 2 (Ad 2) by a study o f nascent transcript synthesis in isolated nuclei (Evans et al., 1979). Late in infection by Ad 2, a proportion of transcription from the m ajor late prom oter (M LP) pauses or prem aturely term inates at sites approxim ately 120 and ISOnts dow nstream . These short RNAs w ere stable enough to be isolated from infected cells (M aderious and Chen, 1984). The tem poral occurrence o f adenovirus attenuation suggests that it may have a regulatory function. Also, attenuation may be responsible for a decrease in ML prom oter-proxim al tran scrip tio n in abo rtive viral in fectio ns (Johnston et al., 1985).
180nt R N A s co rre sp o n d in g to th o s e id e n tifie d in vivo w ere transcribed in H eLa extracts. The accum ulation in vitro o f this short RNA in advance o f readthrough transcrip ts suggested it to be a p ro d u ct o f pausing or term ination rather than processing (H aw ley and R oeder, 1985). A distinction betw een pausing and term ination was also made: the inability o f the short RNA to be chased into a longer product, at least under the p articu lar experim ental conditions em ployed, suggested that it arose from term in atio n rath er than pausing (W iest and H aw ley, 1990). H ow ever, pausing is believed to constitute part o f the term ination m echanism , since th e c o n c e n tra tio n o f a p a r tic u la r n u c le o tid e in c o rp o ra te d at th e term ination site in vitro modulated attenuation (W iest, et al., 1992).
The site at +180 resem bles prokaryotic rho -ind epend en t term inators in that it consists o f a uridine-rich tract w hich is preceded by potential RNA stem -loop structures between +110 and +180 (Seiberg et al., 1987). RNA secondary structure has been im plicated in this block to elongation in a num ber o f studies. Term ination was disrupted in vitro by transcriptio n in the p resen ce o f ITP in stead o f GTP w hich d e sta b iliz e s secondary stru ctu re (S eiberg, et al., 1987). A nalysis o f term in ation efficien cies of synthetic stem -loops o f varying stab ilities follow ed by d iffere n t num bers
o f U residues downstream o f the MLP, suggested both features are required for efficien t term ination (Bengal and A ioni, 1989). W hen the proposed natu ral secondary structure was m utated how ever, this had no effect on term in atio n and a num ber o f alternative RNA stem -loops in this region were suggested (K essler et al., 1989). However, it was subsequently found th a t a 65 base region from +133 to +198 was su fficie n t to program term ination in vitro (W iest and Hawley, 1990). Since this 65bp region does n o t co n tain all the sequences req u ired for th e se p ro po sed secon dary s tru c tu re s , th is c a sts d o u b t on w h e th e r se c o n d a ry s tru c tu re is a p re re q u isite for adenovirus term inatio n.
S e v e ra l s tu d ie s h a v e s u g g e s te d th a t a d e n o v iru s te rm in a tio n efficiency is m odified by elongation factors. W hen transcribed in vitro, the anionic detergent sarkosyl was required to detect term ination, possibly by rem oving elon gation factor(s) from the tem p late or the polym erase (H aw ley and R oeder, 1985). R e la tiv e ly h ig h re a c tio n te m p eratu res enhanced the block to elongation in vitro, consistent with the presence of anti term ination factors in a HeLa extract (K essler, et al., 1989). M oreover, e f fic ie n t a rre s t o ccu rred in th e ab sen ce o f sark o sy l w hen p u rifie d p o ly m e ra se w as used to tra n sc rib e p ro m o te r-le ss te m p lates, or w hen in itiation com plexes w ere washed prior to elongation (W iest, et al., 1992). T h is in h ib itio n o f te rm in atio n im p lied from th e se e x p e rim e n ts was p artially but not w holly attributed to the elongation factor SII described above (Wiest, et al., 1992).
I .2 .6 .2 . SV40
Short nascent simian virus 40 (SV40) RNAs were initially observed by sedim entation analysis o f pulse-labelled RNA from infected cells; these short transcripts mapped to the prom oter-proxim al region o f the viral DNA. A 95bp attenuated RNA initiated at the SV40 m ajor late prom oter can be isolated in vitro (Hay et al., 1982) but due to presumed instability has not yet b een d etecte d in vivo (R esnekov et al., 1989). A ccording to kinetic experim ents perform ed in vitro, this truncated RNA appears to arise from a te rm in a tio n ev ent, ra th e r than p au sin g (H ay and A lo n i, 1984). The functional significance o f this attenuation is not know n, but occurs in a tem porally regulated manner (Hay, et al., 1982).
that the m echanism s o f prokaryotic term ination and SV40 attenuation are related. It was postulated that this region could fold into an alternative h a irp in -lo o p n ot follow ed by a run o f u rid in es, and th ereb y p erm it transcription readthrough under appropriate conditions (H ay, et al., 1982). That RNA secondary structure is a com ponent o f the attenuation process is su p p o rte d by the in h ib ito ry e ffe c t on te rm in a tio n r e s u ltin g fro m tre a tm e n t o f in fec ted cells w ith p ro fla v in e (H ay, et al., 1982), an in tercalatin g agent w hich is believed to in terfere w ith RNA seco ndary structure, and by in vitro transcription reactions using IM P instead o f GMP which destabilizes secondary structure (Hay and A loni, 1984). The stability o f the p ro p o sed h airp in stru ctu re s app ears to be o f im p o rtan ce in te rm in atio n also as ju d g e d by m u tatio n al an aly ses o f th ese p u ta tiv e structures (Resnekov, et al., 1989).
The involvem ent o f viral and cellular factors in SV40 attenuation has also been postulated. A salt-soluble cellular factor was im plicated in te rm in atio n in vitro (Hay and A loni, 1984). A lthough viral p roteins are clearly not required for term ination to occur in vitro w here u n in fec ted H eLa cell extracts are em ployed, it is possible that viral (or host) factors reg u la te atten u atio n in vivo. It was suggested but not yet proven that a sm all S V 4 0 -e n co d ed p ro te in term ed th e a g n o p ro te in m ay re g u la te term ination through a direct m odulation o f the stab ility o f the proposed stem-loops (Hay, et al., 1982). A second candidate which has been suggested (Resnekov, et al., 1989) is SV40 T antigen, since it has R N A -binding and helicase activities (Carroll et al., 1988) through w hich it could potentially disrupt RNA secondary structure. H owever the tem poral appearance o f T antigen does not correlate w ith that o f the attenuated RNA (Hay, et al., 1982).
1.2 .6 .3. T h e m in u te v iru s o f m ice
A ttenuation has also been docum ented in the m inute virus of mice, a 5149b p lin ear sin g le -stran d ed p arv o v iru s (R esn eko v and A loni, 1989). V iral transcription initiates from two prom oters, P4 and P39 which are 1800 bp ap art; tra n s c rip tio n from th e u p stream P4 p ro m o te r p au se s or term inates 142-147 bp from the start site. The truncated RNAs accum ulated in infected cells from which they could be isolated. C ertain features bear sim ilarity to SV40 and adenovirus attenuation. Term ination or pausing was also observed in vitro in a HeLa nuclear extract in the presence o f sarkosyl. In k in etic experim ents the sh ort RNA appeared b efo re the fu ll-le n g th
prod uct, dem onstrating th at the atten uated RNA w as not g enerated by processing. Since treatm ent o f infected cells w ith the in tercalating agent proflav in e decreased term ination, RNA secondary stru ctu re was im plicated in this process. In addition a salt soluble factor appeared to be required for attenuation (Resnekov and A loni, 1989).
1. 2.6. 4 P r o m o t e r s u bs t i t u t i o n e x p e r i m e n t s : the e f f ec ts on SV40, A d e n o v i r u s and MV M at t en u at io n
The question o f w hether the prom oter plays any role in specifying viral atten u atio n has been stu d ied in vitro by p ro m o te r su b stitu tio n exp erim ents. Both SV40 and MVM term inato rs fun ction ed w hen their prom oters w ere exchanged w ith the adenovirus M LP; this suggests either th a t th e se p ro m o te rs are c o m p a tib le w ith each o th e r to sp e c ify term ination, or that SV40 and MVM term ination is not influenced by the prom oter (Resnekov and A loni, 1989; R esnekov, et al., 1989). However, term in atio n at the adenovirus site did not o ccu r effic ie n tly w hen the ad en o v iru s M LP w as su b stitu te d w ith the m ouse 6-g lo b in p ro m o ter, sug gestin g som e p rom oter sp ecificity for ad eno virus term in atio n (W iest and Hawley, 1990). The latter result suggests the possibility that different p ro m o ters m ay sp ecify d iffe re n c e s in th e e lo n g a tio n co m p eten c e o f tra n s c rip tio n co m p lex es.
1.2.6.5. HIV-1 Tat protein binds to an RNA s ec on d ar y st ruct ure and p r o m o t e s a n t i t e r m i n a t i o n o f t r a n s c r i p t i o n
In H IV -1 , th e v ir a lly - e n c o d e d T a t p r o te in tr a n s a c tiv a te s transcription via an element situated dow nstream of the cap site in the LTR. T his 60bp elem en t w hich is know n as TA R (fo r tra n s a c tiv a to r o f transcription) is believed to function at the level o f RNA. TAR contains a p o te n tia l RNA stem -lo op stru c tu re , and m u tatio n s w hich d is ru p t the secondary stru ctu re in the stem or the p rim ary sequence o f the loop prevent transactivation by Tat (Berkhout et al., 1989; Selby et al., 1989).
transcription was not increased by Tat, suggesting that Tat does not affect in itia tio n o f tra n sc rip tio n , b u t ra th e r its elo n g a tio n . In ce lls not co n ta in in g T at, sh o rt ap p ro x im ate ly 60bp tra n s c rip ts w ere fou nd to accum ulate. The 3' ends o f the p rem aturely term in ated RN As m apped im m ediately dow nstream o f the proposed stem -loop stru cture in the TAR elem ent. These experim ents suggested that interaction o f T at with the TAR elem ent abrogated a d iscrete prem atu re term inatio n event in the HIV-1 LTR. H ow ever the prem ise that th e TAR elem en t is a tran scrip tio n term inator is inaccurate. Deletions in the TAR elements o f HIV-1 (Laspia et al., 1989) and HIV-2 (Toohey and Jones, 1989) did not increase the basal level o f transcription .
In one study, transcriptional po larity was d etected by nuclear run off, such that there was a gradual decline in transcription tow ards the 3' end o f the transcription unit in the absence o f Tat (Laspia, et al., 1989). A grad ien t o f polym erases along the HIV-1 tran scrip tio n u nit is co nsistent with the idea that term ination occurs gradually along the gene rather than at one specific site. An elem ent w hich may cause this transcriptio nal polarity in the absence o f Tat has been identified (see Discussion). It is now b elieved that these hetero gen eo usly term inated p ro ducts are d egraded in the 3' to 5' direction back to the stem-loop which stabilizes the RNA from further degradation and allows detection o f short 60nt RNAs.
In the experim ents o f L aspia et al. (1989) in w hich a gradual transcription al p olarity was observed, coexpression o f adenovirus/H IV /C A T tem plates with either Tat or adenovirus E l A resulted in transactivation of p ro m o ter-p ro x im al tran scrip tio n . This e ffe c t o f T at on in itia tio n o f transcription rem ains the subject o f som e controversy. H ow ever whereas the transcriptional polarity was not altered by E lA , a decrease in polarity was caused by T at, such that a g reater pro po rtion o f tran scrip tio n was capable o f reading through into dow nstream CAT sequences (Laspia, et al., 1989). It therefore appears that the m echanism by w hich Tat exerts its effect on elongation is to increase the processivity o f elongation com plexes (see Discussion). This was also dem onstrated by in vitro experim ents which show ed that Tat increased the am ount o f tran scrip tio n at p rom oter-distal site s, w ith o u t affectin g th e am ount o f p ro m o ter-p ro x im a l tra n sc rip tio n (M arciniak and Sharp, 1991). It is possible that Tat disrupts the function of a te rm in a tio n fa c to r or h elp s to re c ru it e lo n g a tio n fa c to rs to the transcription com plex and/or somehow increases th eir activity. It has been speculated that T at-m ed iated anti term ination may conceivably be related to a known prokaryotic mode o f anti term ination. Tat is rem iniscent o f phage
lam bda N protein. Like N, Tat is a sm all, arginine-rich protein, which fu n ctio n s throu gh an RNA stem -loo p stru c tu re to cau se tra n sc rip tio n anti term ination. This basic region is both necessary and sufficient for TAR binding (Lazinski, et al., 1989). H ow ever the m echanism by w hich Tat increases the processivity o f polym erases, and w hether it is sim ilar to that of N or Q remains to be determined.
1. 2.6. 6. Te rm in at io n in va cc i ni a virus in vo lv e s a p rot ei n which rec o gni se s an RNA signal
V accinia virus which is a poxvirus, replicates in the cytoplasm and has its own transcription system . This system includes a D N A -dependent RNA polym erase w hich exhibits extensive sequence hom ology w ith RNA polym erase II (Broyles and Moss, 1986). Term ination at the 3' end o f the vaccinia grow th factor (VGF) gene was analysed in extracts from vaccinia virions; term ination was found to be dependent at least in part on a T-rich s e q u e n c e (T ^ N T) lo c ated 50bp u p stream from the te rm in a tio n site (Rohrm ann et al., 1986). This signal is recognised as RNA, according to base analog substitution experim ents (Shuman and M oss, 1988); how ever it seems unlikely that RNA secondary structure is involved since term ination was not sen sitiv e to d eletio n o f sequen ces flank ing the T5NT site (R ohrm ann, et al., 1986). A u rid in e rich te rm in atio n sig nal on the n o n te m p late strand is rem in iscen t o f p ro k ary o tic in trin sic te rm in ato rs. H ow ever the vaccinia sequence induces term ination to occur at a distance 50bp dow nstream , and secondary structure appears not to constitute part of th e te rm in a tio n sig n a l; m o ro ev e r v a c c in ia te rm in a tio n re q u ire s an accesso ry facto r.