Reduction of Severe Hyperbilirubinemia After
Institution of Predischarge Bilirubin Screening
WHAT’S KNOWN ON THIS SUBJECT: Universal predischarge bilirubin screening in a small, regional population is effective in reducing readmissions for moderate degrees of
hyperbilirubinemia.
WHAT THIS STUDY ADDS: Universal predischarge bilirubin screening in a very large, multistate national population is effective in eliminating readmissions for both moderate and severe degrees of hyperbilirubinemia in normal newborns whose parents are compliant with care instructions.
abstract
OBJECTIVE:The objective of this study was to demonstrate efficacy of universal predischarge neonatal bilirubin screening in reducing po-tentially dangerous hyperbilirubinemia in a large, diverse national population.
METHODS:This was a 5-year prospective study directed at neonates who were agedⱕ28 days and evaluated at facilities of the Hospital Corporation of America with a serum bilirubin level ofⱖ20.0 mg/dL. This time frame includes periods before, during, and after the initiation of systemwide institution of a program of universal predischarge neo-natal bilirubin screening. The primary outcome measures were serum bilirubin 25.0 to 29.9 and ⱖ30.0 mg/dL. Neonatal phototherapy use during these years was also analyzed.
RESULTS:Of the 1 028 817 infants who were born in 116 hospitals be-tween May 1, 2004, and December 31, 2008, 129 345 were delivered before implementation and 899 472 infants were delivered after imple-mentation of this screening program in their individual hospitals. With a program of universal screening, the incidence of infants with total bilirubin 25.0 to 29.9 mg/dL declined from 43 per 100 000 to 27 per 100 000, and the incidence of infants with total bilirubin ofⱖ30.0 mg/dL dropped from 9 per 100 000 to 3 per 100 000 (P⫽.0019 andP⫽.0051, respectively). This change was associated with a small but statistically significant increase in phototherapy use.
CONCLUSIONS:A comprehensive program of prevention, including universal predischarge neonatal bilirubin screening, significantly re-duces the subsequent development of bilirubin levels that are known to place newborns at risk for bilirubin encephalopathy.Pediatrics2010; 125:e1143–e1148
AUTHORS:Michael P. Mah, MD, Steven L. Clark, MD, Efe Akhigbe, MD, MPH, Jane Englebright, RN, PhD, Donna K. Frye, RN, Janet A. Meyers, RN, Jonathan B. Perlin, MD, PhD, Mitch Rodriguez, MD, and Arthur Shepard, MD
Hospital Corporation of America, Nashville, Tennessee
KEY WORDS
universal bilirubin screening, bilirubin encephalopathy, hyperbilirubinemia, kernicterus, neonate
ABBREVIATIONS
AAP—American Academy of Pediatrics HCA—Hospital Corporation of America
www.pediatrics.org/cgi/doi/10.1542/peds.2009-1412
doi:10.1542/peds.2009-1412
Accepted for publication Jan 7, 2010
Address correspondence to Steven L. Clark, MD, Hospital Corporation of America, Women and Newborn Clinical Services, PO Box 404, Twin Bridges, MT 59754. E-mail:
steven.clark@mountainstarhealth.com
PEDIATRICS (ISSN Numbers: Print, 0031-4005; Online, 1098-4275).
Copyright © 2010 by the American Academy of Pediatrics
FINANCIAL DISCLOSURE:The authors have indicated they have no financial relationships relevant to this article to disclose.
tricians were urged to adopt a “kinder, gentler approach” to bilirubin man-agement.1 Other authors disagreed
with this approach, suggesting that a loss of concern about jaundice may re-sult in a reemergence of kernicterus.2
Managed care policies also resulted in a sharp decrease in newborn length of stay.3Beginning in the mid-1990s, a
se-ries of reports demonstrated the con-tinuing occurrence of kernicterus in healthy, term newborns.2,4–6
Further-more, the Pilot Kernicterus Registry suggested an increasing incidence of reported cases of kernicterus,7a
prob-lem that may be compounded by the increased incidence of late preterm births and the known effect of lower gestational age on hyperbiliru-binemia.8 Similar observations were
made in reports from Denmark and Brazil.9,10In 2001, statements issued by
the Joint Commission on Accreditation of Healthcare Organizations11and the
Centers for Disease Control and Pre-vention12warned practitioners of the
increasing threat of kernicterus and suggested that changes in practices were required to prevent kernicterus. The Joint Commission’s Sentinel Event Alert11 listed universal predischarge
bilirubin screening first in its list of risk-reduction strategies. In 2004, the American Academy of Pediatrics (AAP) revised its Clinical Practice Guideline13
on hyperbilirubinemia in the healthy newborn. The new guideline included recommendations for a systematic ap-proach to predischarge risk assess-ment for severe hyperbilirubinemia for all infants. While calling universal screening the “best documented method for assessing risk of subse-quent hyperbilirubinemia,” the AAP gave physicians a choice of 2 predis-charge risk assessment strategies: bil-irubin measurement incorporating hour-specific bilirubin nomograms for
Since the publication of the AAP Clini-cal Practice Guideline in 2004, the ef-ficacy of universal predischarge bili-rubin screening has remained in question. A 2006 report by Eggert et al16described the result of
implement-ing universal predischarge bilirubin screening in an 18-hospital regional health care system. They showed sig-nificant reductions in the incidence of bilirubin levels of ⱖ20.0 and ⱖ25.0 mg/dL (430.0 mol/L). For bilirubin levels ofⱖ30.0 mg/dL (510.0mol/L), the level defined by the National Quality Forum as a reportable event,17there
was a trend toward reduction, but the study was insufficiently powered for significance in these very rare out-comes. Our much larger study reports the results of instituting universal pre-discharge bilirubin screening in a na-tionwide hospital system that delivers ⬃5% of US births.
METHODS
The Hospital Corporation of America (HCA) is the largest hospital system in the United States, with facilities in 21 states. Previous publications sug-gested that the demographic makeup of this patient population is represen-tative of the United States as a whole.18,19In 2003, on the
recommen-dation of its Perinatal Clinical Work Group, the HCA committed to an enterprise-wide program of univer-sal predischarge neonatal bilirubin screening in an effort to eliminate bili-rubin levels of⬎30.0 mg/dL in normal, healthy, inborn neonates. Each HCA fa-cility was asked to endorse and imple-ment policies for universal predis-charge screening that included either serum or transcutaneous hour-specific bilirubin assessment, as well as provisions for neonatal follow-up guided by nomograms developed by Bhutani et al.14These guidelines
pro-neonatal follow-up and repeat biliru-bin assessment on the basis of an hour-specific predischarge bilirubin level. Such measurements augmented the adoption of protocols set forth in the revised Clinical Practice Guidelines of the AAP.13Of the 126 HCA hospitals
that delivered infants at the start of the project, 10 were excluded because they either ceased doing obstetrics or were divested from the company be-fore completion of the study. An official start date for universal bilirubin screening was obtained for each hos-pital. Monthly figures for live births and for infants with hyperbiliru-binemia were prospectively collected from each facility. These infants were assigned to either the unscreened or the screened group for analysis. For start dates that fell after the first of the month, the hospital’s births for the month were adjusted accordingly.
All neonates who were evaluated at an HCA facility within 28 days of birth with total serum bilirubin levels ofⱖ20.0 mg/dL between May 1, 2004, and De-cember 31, 2008, were identified from a common laboratory computer sys-tem. For patients with multiple results, only the highest value was used for the analysis. Neonates with bilirubin val-ues ofⱖ25.0 mg/dL were segregated into the unscreened or screened group on the basis of the screening start date at the hospital where they presented. Outborn infants were in-cluded in the study, because deidenti-fied patient data made it impossible to distinguish hospital of birth. Data were collected by 2 specified individuals (the perinatal nurse manager and the risk management director) in each fa-cility to ensure accuracy and submit-ted on a quarterly basis to our central data repository for this project.
predis-charge bilirubin screening would de-crease the incidence of subsequent bilirubin values of ⱖ25.0 mg/dL, and especially bilirubin values of ⱖ30.0 mg/dL. A second hypothesis was that this reduction would be accomplished without increasing the total number of bilirubin values ofⱖ20.0 mg/dL (used as a surrogate of increased resource use). The number and proportion of to-tal inborn infants who underwent pho-totherapy were also analyzed for each year of the study.
The 2 test with Yates correction
(InStat3 [GraphPad Software, La Jolla, CA]) was used to evaluate the signifi-cance of differences between the groups. Only the 2-tailedPvalues are reported.
This was a quality improvement project that used deidentified data for analy-sis. Exemption from institutional re-view board rere-view was obtained on the basis of 45CFR46.101(b) (2) and 46.102(f) as well as 45CFR164.514(a) to (c) of the Health Insurance Portability and Accountability Act.
RESULTS
A total of 1 028 817 infants were deliv-ered at the 116 study hospitals be-tween May 2004 and December 2008. On the basis of hospital-declared start dates, 129 345 births occurred be-fore implementation of universal bilirubin screening. The remaining 899 472 births occurred after routine predischarge bilirubin screenings were implemented at their birth hospitals. At the beginning of the study period, a small number of hospitals were al-ready practicing universal screening. There was a rapid shift in the latter part of 2004 such that by 2005 most infants were being screened. All cen-ters had implemented universal pre-discharge screening by January 1, 2006. Beyond this time, we observed a systemwide screening compliance
rate ofⱖ98% in each quarter of the study.
Infants with bilirubin levels ofⱖ25.0 mg/dL detected between May 2004 and December 2008 were assigned to ei-ther unscreened or screened status on the basis of the policy in effect at the presenting hospital. Table 1 demon-strates that institution of universal predischarge bilirubin screening was followed by a marked decrease in both levels of hyperbilirubinemia of 25.0 to 29.9 mg/dL and of bilirubin levels of
ⱖ30.0 mg/dL. The incidence of biliru-bin levels of 25.0 to 29.9 mg/dL de-clined by 38%, and bilirubin levels of
ⱖ30.0 mg/dL dropped by 65%. These
reductions were highly statistically significant with odds ratios and confi-dence intervals of 0.62 (0.46 – 0.83) and 0.35 (0.18 – 0.71), respectively.
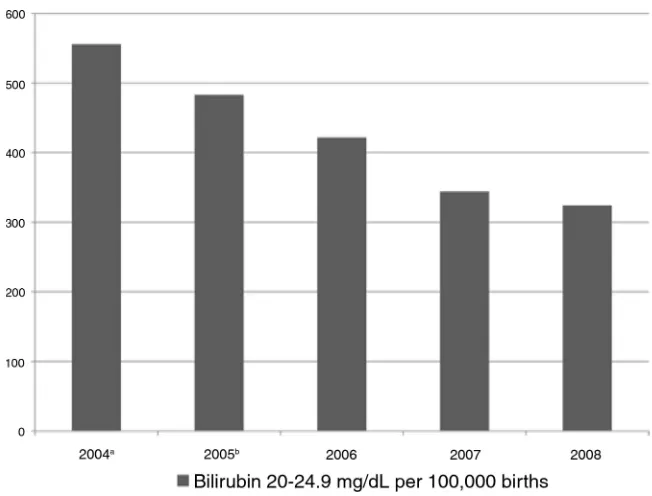
For infants with bilirubin levels of 20.0 to 24.9 mg/dL, there was small but highly significant (P⫽.0027) decrease in severe hyperbilirubinemia from 2004 to 2005 coincident with the imple-mentation of universal screening. Figure 1, showing the incidence of hyperbilirubinemia cases by year, demonstrates the magnitude of the reduction continuing to increase in subsequent years. We observed no adverse clinical effects of bilirubin screening.
FIGURE 1
Incidence of severe hyperbilirubinemia by year.aData from May 1 to December 31, 2004, consisted of 832 cases of 149 727 births.bFor all of 2005, there were 1048 cases in 216 880 births, significantly different from the 2004 incidence atP⫽.0027. Additional declines were seen in each subsequent year.
TABLE 1 Incidence of Bilirubin Levels of 25.0 to 29.9 mg/dL andⱖ30 mg/dL in Unscreened Versus Screened Populations
Parameter Total Births Peak Bilirubin Level of 25.0–25.9 mg/dL (n/rate per 100 000)
Peak Bilirubin Level of⬎30.0 mg/dL (n/rate per 100 000)
Screened 899 472 238/26.5 27/3.0
Unscreened 129 345 55/42.5 11/8.5
OR (95% CI) NA 0.62 (0.46–0.83) 0.35 (0.18–0.71)
P NA .0019 .0051
OR indicates odds ratio; CI, confidence interval.
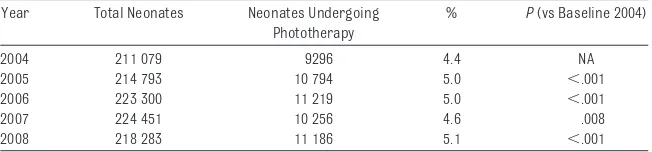
This reduction in severe hyperbiliru-binemia was associated with a small but highly significant increase in the use of neonatal phototherapy in each year after the initiation of universal screening (Table 2). The fraction of ne-onates who received phototherapy in-creased from 4.4% of all deliveries in 2004 to 5.1% of all deliveries in 2008, with a mean of 4.9% during years 2005–2008. Of the 11 186 infants who received phototherapy in 2008, 10 541 (94.2%) were treated during the birth admission only, 565 (5.1%) were not treated during the birth admission but required later hospitalization for totherapy, and 80 (0.7%) received pho-totherapy both during the birth admis-sion and subsequently.
As part of our quality improvement ef-fort, additional clinical data were ex-tracted for each of the 8 infants who presented in 2008 with bilirubin lev-els of ⱖ30.0 mg/dL. Two infants had glucose-6-phosphate dehydroge-nase deficiency, a congenital condition of special importance in select black infants at risk.20In addition, 1 infant
had hypothyroidism and 1 had multi-ple, ultimately lethal anomalies. Two infants were outborn at non-HCA cen-ters. In the remaining 2 cases, the par-ents had failed to keep scheduled follow-up appointments.
DISCUSSION
In this contemporary series encom-passing⬎1 million births, the imple-mentation of a program of universal predischarge bilirubin screening was associated with a dramatic and statis-tically significant decline in the
inci-dence of neonates with bilirubin 25.0 to 29.9 mg/dL and in those with biliru-bin levels ofⱖ30.0 mg/dL (Table 1). Al-though similar results for infants in the former group were previously demonstrated with universal screen-ing in a much smaller regional popula-tion and in pilot projects, these data are the first to demonstrate efficacy of such a program in a large, de-mographically heterogeneous popula-tion.16,21,22This report is also the first
to show a statistically significant drop in infants with bilirubin levels ofⱖ30.0 mg/dL.
The true incidence of hyperbiliru-binemia is not well documented. Bhu-tani et al23 estimated the incidence
of severe (ⱖ20.0 mg/dL), extreme (ⱖ25.0 mg/dL), and hazardous (ⱖ30.0 mg/dL) hyperbilirubinemia by combin-ing data from a number of disparate studies. Their approximate incidences per 100 000 births were 1400, 140, and 10, respectively. For bilirubin levels of
ⱖ25.0 mg/dL, our baseline incidence of 50 per 100 000 births was lower than the estimates of Bhutani et al. This may be related to differences in the demographics of these popula-tions or in practice patterns versus the older studies. For bilirubin levels of
ⱖ30.0 mg/dL, the preintervention inci-dence was comparable to the 10 per 100 000 estimate of Bhutani et al. The current incidence of clinical ker-nicterus and bilirubin encephalopathy in the United States is unknown; how-ever, estimates including data from Denmark and the United Kingdom sug-gesting an incidence in the range of 1
prevented in this population by our policy of universal screening.6,23,24
Ad-ditional research into the actual inci-dence of kernicterus and bilirubin encephalopathy and the ability of in-terventions to prevent new cases is needed.
The study of Eggert et al16excluded
out-born infants from the analysis. The combination of losing inborn infants to other centers and excluding outborn infants would result in an artificially low incidence figure. Our analysis in-cluded outborn patients, allowing us to approximate better the actual inci-dence of varying levels of hyperbiliru-binemia in the population. Inclusion of such infants would not materially af-fect our primary results for 2 reasons. In many regions, HCA has a vertically integrated system whereby patients would be referred for specialty care to other HCA facilities, limiting loss of pa-tients to outside centers. More impor-tant, this was a comparative study; we have no reason to think that the in-gress and efflux of hyperbilirubinemia cases differed before and after the in-stitution of universal bilirubin screen-ing. Furthermore, the inclusion of such infants would, if anything, result in an apparent attenuation rather than ex-aggeration of the effects of our screen-ing program.
The measurement of predischarge se-rum bilirubin level could not have re-sulted in a decrease in the number of infants with severe hyperbiliru-binemia in the absence of increased phototherapy use. Our demonstration (Table 2) of a modest but statistically significant increase in neonatal photo-therapy use would therefore be ex-pected. This finding is an additional im-portant validation that the decrease in infants with severe hyperbiliru-binemia was linked causally to the in-troduction of universal screening and Phototherapy
2004 211 079 9296 4.4 NA
2005 214 793 10 794 5.0 ⬍.001
2006 223 300 11 219 5.0 ⬍.001
2007 224 451 10 256 4.6 .008
subsequent treatment of appropriate infants rather than any change in pa-tient demographics or disease fre-quency over time.
Quantification of the cost-effectiveness of universal bilirubin screening pro-grams is beyond the scope of this anal-ysis. We measured the incidence of hy-perbilirubinemia levels of 20.0 to 24.9 mg/dL—a level generally associated with readmission—and found a small but significant decrease in the 2004 – 2005 data and larger decreases in sub-sequent years (Fig 1). This suggests that universal screening does not in-crease overall rehospitalization rates, potentially generating cost savings. Conversely, such savings must be weighed against the cost of photother-apy and a potential increase in length of stay in an additional 0.5% of infants. In a similar manner, calculations place the cost per case of kernicterus pre-vented by universal screening in the range of millions of dollars per case, balanced by lifetime costs of care for patients with kernicterus in the same
range.25,26It should be noted that
uni-versal predischarge bilirubin screen-ing was recently endorsed by the Canadian Paediatric Society27in an
en-vironment where health care is large-ly government owned and operated and cost-effectiveness is of para-mount concern.
Although gratifying in demonstrating the sustainable impact of the interven-tion, the data presented in Fig 1 sug-gest that universal bilirubin screening in isolation was not responsible for all of the observed improvement in out-comes. With publication of the 2004 AAP Clinical Practice Guideline,13 the
Perinatal Clinical Workgroup believed that it could not endorse predischarge bilirubin screening without concur-rently implementing the rest of the AAP recommendations. Moreover, chang-ing the practices and perceptions of the thousands of nurses and physi-cians involved in care of infants is a lengthier process than implementing a laboratory test.28It is likely that
fac-tors such as additional staff
educa-tion, improved physician and paren-tal awareness of hyperbilirubinemia and kernicterus, and enhanced lac-tation support all contributed to the ongoing reduction in hyperbiliru-binemia; however, universal predis-charge bilirubin measurement was the lynchpin of a larger systematic program for prevention of hyperbil-irubinemia, the full effects of which are still being revealed. This pro-gram was well accepted by the over-whelming majority of nurses, physi-cians, and parents.
CONCLUSIONS
Examination of these data, including the circumstances of the 8 infants who had bilirubin levels of ⱖ30.0 mg/dL and presented in 2008, suggests that we have largely succeeded in eliminat-ing levels of neonatal bilirubin as-sociated with the development of bilirubin encephalopathy for healthy infants who are born in HCA hospi-tals and receive the recommended follow-up.
REFERENCES
1. Newman TB, Maisels MJ. Evaluation and treatment of jaundice in the term newborn: a kinder, gentler approach.Pediatrics.
1992;89(5 pt 1):809 – 818
2. Brown AK, Johnson L. Loss of concern about jaundice and the reemergence of kernicterus in full-term infants in the era of managed care. In: Fanaroff AA, Klaus MH, eds.The Year Book of Neonatal and Perinatal Medicine. Philadelphia, PA: Mosby Yearbook; 1996:17–28
3. Braveman P, Egerter S, Pearl M, Marchi K, Miller C. Problems associated with early discharge of newborn infants: early dis-charge of newborns and mothers—a criti-cal review of the literature. Pediatrics.
1995;96(4 pt 1):716 –726
4. Penn AA, Enzmann DR, Hahn JS, Stevenson DK. Kernicterus in a full term infant. Pediat-rics.1994;93(6 pt 1):1003–1006
5. Maisels MJ, Newman TB. Kernicterus in otherwise healthy, breast-fed term newborns. Pediatrics. 1995;96(4 pt 1): 730 –733
6. Ebbesen F. Recurrence of kernicterus in
term and near-term infants in Denmark.
Acta Paediatr.2000;89(10):1213–1217 7. Bhutani VK, Johnson L. Synopsis report
from the pilot USA Kernicterus Registry.J Perinatol.2009;29(suppl 1):S4 –S7 8. Keren R, Tremont K, Luan X, et al: Visual
as-sessment of jaundice in term and late pre-term infants.Arch Dis Child Fetal Neonatal Ed.2009;94(5):F317–F322
9. Ebbesen F, Andersson C, Verder H, et al. Ex-treme hyperbilirubinemia in term and near-term infants in Denmark.Acta Paedi-atr.2005;94(1):59 – 64
10. Facchini FP, Mezzacappa MA, Rosa IR, Mez-zacappa Filho F, Aranha-Netto A, Marba ST. Follow-up of neonatal jaundice in term and late premature newborns[in Portu-guese]. J Pediatr (Rio J). 2007;83(4): 313–322
11. Joint Commission on Accreditation of Healthcare Organizations. Kernicterus threatens healthy newborns.Sentinel Event Alert.2001;18(18):1– 4
12. Centers for Disease Control and Prevention.
Kernicterus in full-term infants: United States, 1994 –1998. MMWR Morb Mortal Wkly Rep.2001;50(23):491– 494
13. American Academy of Pediatrics Subcom-mittee on Hyperbilirubinemia. Manage-ment of hyperbilirubinemia in the new-born infant 35 or more weeks of gestation [published correction appears inPediatrics.
2004;114(4):1138]. Pediatrics.2004;114(1): 297–316
14. Bhutani VK, Johnson L, Sivieri EM. Pre-dictive ability of a predischarge hour-specific serum bilirubin for subsequent significant hyperbilirubinemia in healthy term and near-term newborns. Pediat-rics.1999;103(1):6 –14
15. Johnson LH, Bhutani VK, Brown AK. System-based approach to management of neona-tal jaundice and prevention of kernicterus.
J Pediatr.2002;140(4):396 – 403
16. Eggert LD, Wiedmeier SE, Wilson J, Chris-tensen RD. The effect of instituting a prehospital-discharge newborn bilirubin screening program in an 18-hospital health system. Pediatrics.2006;117(5). Available
17. National Quality Forum. Serious reportable events in healthcare, 2005–2006 update. Avail-able at: www.qualityforum.org. Accessed Feb-ruary 15, 2010
18. Clark S, Belfort M, Saade G, et al. Implemen-tation of a conservative checklist-based protocol for oxytocin administration: ma-ternal and newborn outcomes.Am J Obstet Gynecol.2007;197(5):480.e1– 480.e5 19. Clark SL, Miller DD, Belfort MA, Dildy GA, Frye
DK, Meyers JA. Neonatal and maternal out-comes associated with elective term deliv-ery. Am J Obstet Gynecol. 2009;200(2): 156.e1–156.e4
20. Kaplan M, Herschel M, Hammerman C, et al. Neonatal hyperbilirubinemia in African American males: the importance of
glucose-6-21. Stark AR, Lannon CM. Systems changes to prevent severe hyperbilirubinemia and pro-mote breastfeeding: pilot approaches.J Perinatol.2009;29(suppl 1):S53–S57 22. Lazarus C, Avchen RN. Neonatal
hyperbiliru-binemia management: a model for change.
J Perinatol.2009;29(suppl 1):S58 –S60 23. Bhutani VK, Johnson LH, Maisels MJ, et al.
Kernicterus: epidemiological strategies for its prevention through systems-based approaches. J Perinatol. 2004;24(10): 650 – 662
24. Manning D, Todd P, Maxwell M, et al. Pro-spective surveillance study of severe hyper-bilirubinemia in the newborn in the UK and Ireland.Arch Dis Child Fetal Neonatal Ed.
2007;92(5):F243–F246
2004;114(4):917–924
26. Centers for Disease Control and Preven-tion. Kernicterus/jaundice–video tran-script. Available at: www.cdc.gov/ncbddd/ d d / k e r n i c t e r u s / k e r n i c t e r u s v i d e o s / kernicterustransc.html Accessed Feb-ruary 15, 2010
27. Canadian Paediatric Society. Guidelines for detection, management and prevention of hyperbilirubinemia in term and late pre-term newborn infants (35 or more weeks’ gestation): summary.Paediatr Child Health.
2007;12(5):401– 407
DOI: 10.1542/peds.2009-1412 originally published online April 5, 2010;
2010;125;e1143
Pediatrics
Janet A. Meyers, Jonathan B. Perlin, Mitch Rodriguez and Arthur Shepard
Michael P. Mah, Steven L. Clark, Efe Akhigbe, Jane Englebright, Donna K. Frye,
Bilirubin Screening
Reduction of Severe Hyperbilirubinemia After Institution of Predischarge
Services
Updated Information &
http://pediatrics.aappublications.org/content/125/5/e1143
including high resolution figures, can be found at:
References
http://pediatrics.aappublications.org/content/125/5/e1143#BIBL
This article cites 24 articles, 8 of which you can access for free at:
Subspecialty Collections
b
http://www.aappublications.org/cgi/collection/hyperbilirubinemia_su
Hyperbilirubinemia
sub
http://www.aappublications.org/cgi/collection/fetus:newborn_infant_
Fetus/Newborn Infant
following collection(s):
This article, along with others on similar topics, appears in the
Permissions & Licensing
http://www.aappublications.org/site/misc/Permissions.xhtml
in its entirety can be found online at:
Information about reproducing this article in parts (figures, tables) or
Reprints
http://www.aappublications.org/site/misc/reprints.xhtml
DOI: 10.1542/peds.2009-1412 originally published online April 5, 2010;
2010;125;e1143
Pediatrics
Janet A. Meyers, Jonathan B. Perlin, Mitch Rodriguez and Arthur Shepard
Michael P. Mah, Steven L. Clark, Efe Akhigbe, Jane Englebright, Donna K. Frye,
http://pediatrics.aappublications.org/content/125/5/e1143
located on the World Wide Web at:
The online version of this article, along with updated information and services, is
by the American Academy of Pediatrics. All rights reserved. Print ISSN: 1073-0397.