Lung Function in Very Low Birth
Weight Adults
Heli-Kaisa Saarenpää, MDa, Marjaana Tikanmäki, MDa,b, Marika Sipola-Leppänen, MD, DMScia,b,c, Petteri Hovi, MD, DMScia,d, Karoliina Wehkalampi, MD, DMScia,d, Mirjami Siltanen, MD, DMScie, Marja Vääräsmäki, MD, DMScif,g,h, Anna-Liisa Järvenpää, MD, DMScid, Johan G. Eriksson, MD, DMScia,i,j,k,l, Sture Andersson, MD, DMScid, Eero Kajantie, MD, DMScia,d,f,h
abstract
BACKGROUND AND OBJECTIVES:Lung function attained in young adulthood is 1 of the strongestpredictors of obstructive airways disease in later life. Adults born preterm at very low birth
weight (VLBW;,1500 g) who have experienced bronchopulmonary dysplasia (BPD) have
reduced lung function. We studied the association of lung function in young adulthood with preterm birth at VLBW and with BPD and other prenatal and neonatal conditions.
METHODS:We performed spirometry for 160 VLBW subjects (29 with BPD according to
Northway criteria) aged 18 to 27 years and 162 term control subjects group-matched for
gender, age, and birth hospital. Lung function was expressed aszscores according to the
Global Lung Function Initiative standards.
RESULTS:Forced expiratory volume in 1 secondzscore was 1.41 units (95% confidence interval [CI]: 0.89 to 1.94) lower in BPD-VLBW subjects and 0.39 units (95% CI: 0.08 to 0.69) in
non–BPD VLBW subjects compared with control subjects. Corresponding differences for
forced expiratory volume in 1 second/forced vital capacity were 1.52 (95% CI: 0.99 to 2.05) and 0.51 (95% CI: 0.21 to 0.81), respectively. Maternal smoking in pregnancy predicted poorer
airflow in all groups; this finding was strongest in the BPD-VLBW group. Lung function was
unrelated to fetal or postnatal growth or to neonatal respiratory distress syndrome.
CONCLUSIONS:Young adults born at VLBW have reduced airflow. The outcome is stronger in those
who have a history of BPD but is present among those with no such history. Thisfinding
suggests an increased risk of later obstructive airways disease in adults born at VLBW.
WHAT’S KNOWN ON THIS SUBJECT:Children born preterm at very low birth weight have reduced lung function. Reduced lung function may extend to adult life, but to what extent this outcome is attributable to bronchopulmonary dysplasia and other prenatal and neonatal conditions is not known.
WHAT THIS STUDY ADDS:Young adults born preterm at very low birth weight had impaired airflow. Thisfinding suggests an increased risk of later obstructive airways disease and was observed also among those with no
bronchopulmonary dysplasia, regardless of other prenatal and neonatal complications.
a
National Institute for Health and Welfare, Diabetes Prevention Unit, Helsinki and Oulu, Finland;bInstitute of Health Sciences andhMedical Research Center Oulu, University of Oulu, Oulu, Finland;cDepartment of Pediatrics and Adolescence, MRC Oulu, andfDepartment of Obstetrics and Gynecology, MRC Oulu Oulu University Hospital and University of Oulu, Oulu, Finland;dChildren’s Hospital, Helsinki University Central Hospital and University of Helsinki, Helsinki, Finland;eHyvinkää Hospital, Hyvinkää, Finland;gDepartment of Children, Young People and Families, National Institute for Health and Welfare, Oulu, Finland;iFolkhälsan Research Centre, Helsinki, Finland; jDepartment of General Practice and Primary Health Care, University of Helsinki, Helsinki, Finland;kUnit of
General Practice, Helsinki University Central Hospital, Helsinki, Finland; andlVasa Central Hospital, Vasa, Finland
Dr Kajantie conceptualized and designed the study, conducted the initial analysis, drafted the manuscript for important intellectual content, and reviewed and revised the manuscript; Drs Järvenpää and Hovi conceptualized and designed the study and drafted the manuscript for important intellectual content; Drs Saarenpää, Tikanmäki, and Sipola-Leppänen conducted the initial analysis, drafted the manuscript for important intellectual content, and reviewed and revised the manuscript; and Drs Wehkalampi, Siltanen, Vääräsmäki, Eriksson, and Andersson drafted the manuscript for important intellectual content. All authors approved thefinal manuscript as submitted.
www.pediatrics.org/cgi/doi/10.1542/peds.2014-2651
In childhood, very low birth weight (VLBW) survivors have, on average, poorer lung function, particularly those with a history of bronchopulmonary dysplasia (BPD) but possibly also those without such a history.1–4Lung function in adults who were born as small preterm infants has not been extensively studied, even though this factor is, in addition to smoking, 1 of the strongest predictors of obstructive airways disease in later life.5Much of the existing evidence focuses on adults with a history of BPD, who do have more pulmonary symptoms and respiratory function abnormalities (including obstructed airflow) than those born at term.2,6–12Whether this outcome applies to adults born preterm at VLBW who have no history of BPD is less conclusive. Most studies may not include a sufficient number of subjects to distinguish between those with a history of BPD and those without.13–16Moreover, there are many other prenatal and neonatal conditions that could predispose VLBW infants to or protect them from later respiratory disease.17,18The long-term effects of these conditions could provide valuable insights regarding those mechanisms that link them with airway obstruction in later life.
The aim of the present study was to assess lung function in young adults born at VLBW, with or without a history of BPD. We also focused on other prenatal and neonatal factors that may predispose subjects to or protect them from adverse
pulmonary consequences in adult life.
METHODS
Study Population
The subjects were derived from the Helsinki Study of Very Low Birth
Weight Adults.19The original VLBW
cohort consisted of 335 consecutive VLBW infants born between 1978 and 1985 who were discharged alive from the NICU of Helsinki University Central Hospital, the only tertiary neonatal center serving the province
of Uusimaa, Finland. For each VLBW subject, we chose as a control subject the next infant of the same gender who was born at term (gestational
age:$37 weeks) and was not small
for gestational age (birth weight SD score:–2.0 or higher20) at the same hospital. For the present study, 255 VLBW subjects and 314 control subjects who were living in greater Helsinki were invited to participate; of those, 166 (65.1%) and 172 (54.8%) accepted, respectively, and 160 VLBW subjects and 162 control subjects had lung function data available. The nonparticipants were unbiased except that those who had been diagnosed with cerebral palsy by 15 months of age were less likely to participate; a detailed
nonparticipation analysis has been published elsewhere.19
Prenatal and neonatal data were extracted from medical records. SD scores for weight, length, and head circumference at birth were based on Finnish standards,20and the Ponderal Index was calculated as weight/ length.3Preeclampsia was defined by using standard criteria,21and chorioamnionitis was diagnosed by a clinician on the basis of maternal fever, leukocytosis, and elevated C-reactive protein levels. BPD, based on the Northway criteria (respiratory distress, supplemental oxygen, and characteristic radiographicfindings at 1 month of postnatal age),22and respiratory distress syndrome (RDS), based on classic (clinical and radiograph) criteria,23,24 had both been diagnosed by 1 neonatologist (A.-L.J.). Ten infants had been exposed to antenatal glucocorticoids and 1 infant to postnatal
glucocorticoids, and 7 had received human surfactant (4 prophylactically at birth and 3 as rescue therapy for RDS symptoms) in a randomized trial. In addition, 12 infants had
participated in another randomized trial and been exposed to either antenatal glucocorticoids or placebo; this information was no longer available.
Clinical Measurements and Data Collection
The study protocol was approved by an ethics committee at the Helsinki University Central Hospital. Each participant signed an informed consent form. The participants were instructed not to use bronchodilators in the morning of the clinical visit and not to smoke during the visit before they had completed the lung function test.19 Medical history, use of medication, current smoking, physical activity, and parental educational attainment were assessed with questionnaires.19,25,26
Respiratory function was measured with spirometry testing (Medikro Windows, Spiro2000 version 1.3; Medikro, Kuopio, Finland). The device was calibrated daily before
measurements. Six VLBW subjects and 10 control subjects could not complete spirometry for various reasons. The graphs were inspected visually, and the measurement was repeated until 3 reproducible, technically acceptable spirometry curves were obtained. The largest value of each spirometry parameter was reported. The parameters measured were forced vital capacity (FVC) and the following parameters reflecting airflow: forced expiratory volume in 1 second (FEV1), the FEV1/FVC ratio, forced expiratory
flow when 75% of FVC has been
exhaled and when 50% of FVC have been exhaled (FEF50%), forced
expiratoryflow at 25% to 75%, and
peak expiratoryflow. Spirometry was
completed with the subject seated, wearing nose clips, and holding mouthpieces tightly between the lips and teeth. The lung volumes andflows were standardized to barometric pressure at sea level and body temperature. As main outcomes, these
findings were expressed aszscores for gender, age, and height (all subjects were of Finnish ancestry, and
as percent predicted based on Finnish reference values.27,28If the spirometry results suggested airflow obstruction according to Finnish guidelines (FVC
,80% of predicted value, FEV1
,90%, FEV1/FVC ratio,88%, peak
expiratoryflow,75%, or FEF50%
,63%), which are based on the
American Thoracic Society/European Respiratory Society standard criteria,29it was repeated 10 to 15 minutes after inhaling 200µg of salbutamol from a metered-dose inhaler through a spacer. The bronchodilation test was considered to indicate reversible airways obstruction if FEV1or FVC increased
$12% or FVC increased$200 mL
from baseline.30
Statistical Analysis
Data were analyzed with PASW Statistics 18.0 (IBM SPSS Statistics, IBM Corporation, Armonk, NY). Student’sttest was used to compare the differences of continuous variables between groups, and Pearson’sx2test was used for categorical variables. Multiple linear and logistic regression analyses were used to adjust for the potential confounders and other covariates. AllPvalues are 2-sided.
RESULTS
Characteristics of the VLBW and Control Subjects
The characteristics of the subjects are shown in Table 1. Because differences in respiratory function between the VLBW and control groups were similar
among women and men (Pvalues for
interaction gender*VLBW were not statistically significant), we report the results for both genders pooled.
Respiratory Function in the VLBW and Control Groups
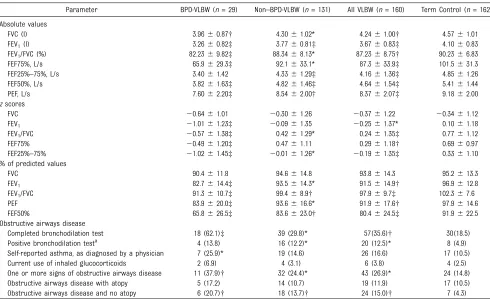
Lung function in all VLBW subjects versus lung function in control subjects was compared. Table 2 displays these measurements expressed in absolute units, inz scores according to Global Lung Function Initiative standards, and in
percent predicted according to Finnish standards.27,28VLBW adults had lower absolute FVC, reflecting lung volume, which was due to their smaller body size and was therefore not observed inzscores. By contrast, all variables reflecting airflow (FEV1, FEV1/FVC, forced expiratoryflow at 75% of FVC, FEF50%, and forced
expiratory flow at 25% to 75% of
FVC) were lower in VLBW adults also
when expressed aszscores or
percent predicted values.
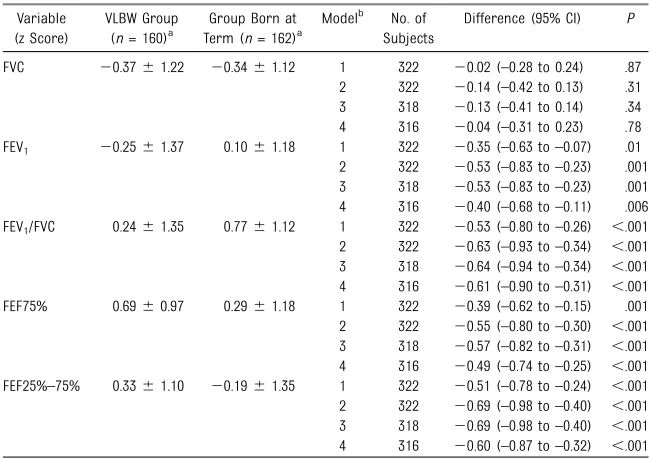
Further adjustments for age, gender, current height, BMI, parental education, maternal smoking during pregnancy, the subject’s current daily smoking, obstructive airways disease, atopy, and frequency of leisure time conditioning activity had little effect on the difference in the variables reflecting airflow (Table 3). Relationships between these covariates and outcomes are shown in Supplemental Table 4. To assess whether these differences could be attributed to manifest asthma, a secondary analysis was conducted excluding the subjects with$1 sign of obstructive airways disease (discussed in the paragraph entitled“Obstructive Airways Disease and Atopic Predisposition”). The results remained unchanged.
The results were similar when the 19 subjects with neurosensory
impairments were excluded, as in previous publications.19
Bronchopulmonary Dysplasia
Of all VLBW subjects, 29 (18.1%) had a history of BPD (“BPD-VLBW”). They had lower gestational age at birth than VLBW subjects with no such history (Table 1). As adults, they were, on average, 3.1 cm shorter and were less likely to smoke. Figure 1 and
Supplemental Table 5 show that variables reflecting airflow were lower in BPD-VLBW subjects than in those with no such history. This difference remained statistically significant after adjusting for gestational age and birth weight SD score. Figure 1 and Supplemental Table 5 also show that
VLBW subjects with no history of BPD have lower airflow variables than control subjects.
Alternative criteria to define BPD were also used. Forty-nine subjects (30.6%) fulfilled the less strict criterion of supplemental oxygen at 28 days.31 The use of this definition attenuated the difference between the BPD-VLBW adults and the remaining VLBW adults (Supplemental Table 5). The criterion of supplemental oxygen at 36 postmenstrual weeks (moderate or severe BPD) was fulfilled by 13 subjects (8.1%). The use of this definition also seemed to attenuate the difference with the remaining VLBW adults, although the small numbers resulted in wide confidence intervals.
Other Prenatal and Neonatal Factors Among the VLBW Group
The 52 VLBW subjects born small for gestational age had respiratory function similar to the VLBW subjects born appropriate for gestational age (P values$.6). Birth weight SD scores and gestational age as continuous variables were unrelated to
respiratory function, whether entered separately or simultaneously in the regression model; there was also no interaction between these variables. The result was the same when the birth weight SD score was substituted with length or head circumference SD score or the Ponderal Index.
There were no differences in lung function between the 32 (20%) VLBW adults who had been exposed to maternal preeclampsia and the 128 who had not been exposed, between those 12 (7.5%) who had neonatal sepsis and those 145 who did not, or between those 77 (48.1%) who had neonatal RDS and the 81 who did not. The 14 (8.8%) VLBW adults who had been exposed to maternal
chorioamnionitis had a 0.59 units (–0.17 to 1.34 [adjusted for variables in model 3 in Table 3]) higherzscore
of FEV1/FVC ratio than the VLBW
a 0.65 units (0.14 to 1.16) lower FVC
zscore than singleton VLBW adults
but similar airflow variables.
To assess the effects of growth between birth and term among VLBW subjects, the associations of weights at 36 and 40 weeks of postmenstrual age with respiratory function were analyzed. Neither the weight SD
scores at 36 or 40 weeks nor their differences from birth were
associated with respiratory function.
Obstructive Airways Disease and Atopic Predisposition
Fifty-seven VLBW subjects and 30 control subjects (P= .0005) received short-acting salbutamol because their
spirometry suggested airflow obstruction according to standard criteria.27,28For 20 VLBW subjects (12.5%) and 8 control subjects (4.9%), spirometry repeated after inhaling salbutamol indicated reversible airflow obstruction (adjusted odds ratio [aOR (odds ratio adjusted as in model 3 of Table 3)]:
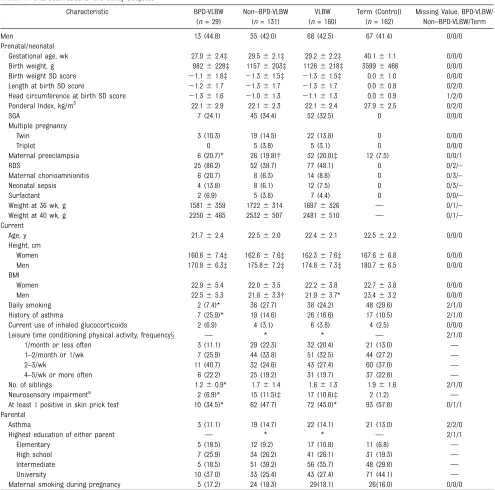
TABLE 1 Characteristics of the Study Subjects
Characteristic BPD-VLBW (n= 29)
Non–BPD-VLBW (n= 131)
VLBW (n= 160)
Term (Control) (n= 162)
Missing Value, BPD-VLBW/ Non–BPD-VLBW/Term
Men 13 (44.8) 55 (42.0) 68 (42.5) 67 (41.4) 0/0/0
Prenatal/neonatal
Gestational age, wk 27.962.4‡ 29.562.1‡ 29.262.2‡ 40.161.1 0/0/0 Birth weight, g 9826228‡ 11576203‡ 11266218‡ 35996466 0/0/0 Birth weight SD score 21.161.6‡ 21.361.5‡ 21.361.5‡ 0.061.0 0/0/0 Length at birth SD score 21.261.7 21.361.7 21.361.7 0.060.9 0/2/0 Head circumference at birth SD score 21.361.6 21.061.3 21.161.3 0.060.9 1/2/0 Ponderal Index, kg/m3 22.162.9 22.162.3 22.162.4 27.962.5 0/2/0
SGA 7 (24.1) 45 (34.4) 52 (32.5) 0 0/0/0
Multiple pregnancy
Twin 3 (10.3) 19 (14.5) 22 (13.8) 0 0/0/0
Triplet 0 5 (3.8) 5 (3.1) 0 0/0/0
Maternal preeclampsia 6 (20.7)* 26 (19.8)† 32 (20.0)‡ 12 (7.5) 0/0/1
RDS 25 (86.2) 52 (39.7) 77 (48.1) 0 0/2/–
Maternal chorioamnionitis 6 (20.7) 8 (6.3) 14 (8.8) 0 0/3/–
Neonatal sepsis 4 (13.8) 8 (6.1) 12 (7.5) 0 0/3/–
Surfactant 2 (6.9) 5 (3.8) 7 (4.4) 0 0/0/–
Weight at 36 wk, g 15816359 17226314 16976326 — 0/1/– Weight at 40 wk, g 22506465 25326507 24816510 — 0/1/– Current
Age, y 21.762.4 22.562.0 22.462.1 22.562.2 0/0/0 Height, cm
Women 160.667.4‡ 162.667.6‡ 162.367.6‡ 167.666.8 0/0/0 Men 170.966.3‡ 175.867.2‡ 174.867.3‡ 180.766.5 0/0/0 BMI
Women 22.965.4 22.063.5 22.263.8 22.763.8 0/0/0 Men 22.565.3 21.863.3† 21.963.7* 23.463.2 0/0/0 Daily smoking 2 (7.4)* 36 (27.7) 38 (24.2) 48 (29.6) 2/1/0 History of asthma 7 (25.9)* 19 (14.6) 26 (16.6) 17 (10.5) 2/1/0 Current use of inhaled glucocorticoids 2 (6.9) 4 (3.1) 6 (3.8) 4 (2.5) 0/0/0 Leisure time conditioning physical activity, frequencyx — * * — 2/1/0 1/month or less often 3 (11.1) 29 (22.3) 32 (20.4) 21 (13.0) — 1–2/month or 1/wk 7 (25.9) 44 (33.8) 51 (32.5) 44 (27.2) —
2–3/wk 11 (40.7) 32 (24.6) 43 (27.4) 60 (37.0) —
4–5/wk or more often 6 (22.2) 25 (19.2) 31 (19.7) 37 (22.8) — No. of siblings 1.260.9* 1.761.4 1.661.3 1.961.6 2/1/0 Neurosensory impairmenta 2 (6.9)* 15 (11.5)‡ 17 (10.6)‡ 2 (1.2) — At least 1 positive in skin prick test 10 (34.5)* 62 (47.7) 72 (45.0)* 93 (57.8) 0/1/1 Parental
Asthma 3 (11.1) 19 (14.7) 22 (14.1) 21 (13.0) 2/2/0
Highest education of either parent — * * — 2/1/1
Elementary 5 (18.5) 12 (9.2) 17 (10.8) 11 (6.8) —
High school 7 (25.9) 34 (26.2) 41 (26.1) 31 (19.3) — Intermediate 5 (18.5) 51 (39.2) 56 (35.7) 48 (29.8) — University 10 (37.0) 33 (25.4) 43 (27.4) 71 (44.1) — Maternal smoking during pregnancy 5 (17.2) 24 (18.3) 29(18.1) 26(16.0) 0/0/0
Data are presented asn(%) or mean6SD. VLBW defined as,1500 g. Thettest was used for continuous variables, and thex2test was used for categorical variables. SGA, small for
gestational age (birth weight SD score–2.0 or lower). *Pvalue for the difference with term,.05.†Pvalue for the difference with term,.01.‡Pvalue for the difference with term,.001.
xPvalue for linear trend.
2.75 [95% confidence interval (CI): 1.17 to 6.44]). Six VLBW subjects and 4 control subjects currently used inhaled glucocorticoids. Of all the VLBW subjects, 26 (16.6%) reported that they had been diagnosed with asthma by a physician compared with 17 (10.4%) of the control subjects (aOR: 1.81 [95% CI: 0.88 to 3.75]). One or more of the signs (reversible airflow obstruction, current use of inhaled glucocorticoids, and previous diagnosis of asthma) suggestive of obstructive airways disease was present in 43 (26.9%) VLBW subjects and 24 (14.8%) control subjects (aOR: 2.01 [95% CI: 1.08 to 3.74]). Of these 43 VLBW subjects, 11 had a history of BPD (37.9% of BPD-VLBW subjects; aOR compared with control subjects: 3.63 [95% CI: 1.24 to 10.62]), and 32 (24.4%) had no such history (aOR: 1.81 [95% CI: 0.94 to 3.50]).
As previously reported,3272 VLBW
subjects and 93 control subjects had atopic predisposition as defined by skin-prick positivity to at least 1 of the tested common aeroallergens (crude odds ratio: 0.61 [95% CI: 0.39 to 0.94]). When comparisons of respiratory function were adjusted for the presence of signs suggesting obstructive airways disease and atopic predisposition, the results remained similar (model 4, Table 3). Obstructive airways disease with atopy (positive skin prick test result
and $1 sign suggesting obstructive
airways disease) was found in 19 (11.9%) VLBW subjects and in 17 (10.5%) control subjects (aOR: 1.09 [95% CI: 0.50 to 2.39]).
Maternal Smoking During Pregnancy and Smoking of the Subject
Subjects whose mothers smoked during pregnancy had reduced
airflow (Supplemental Table 4). The
subjects’own smoking habits were
not associated with airflow regardless of whether adjusted or not adjusted for maternal smoking. The association of maternal or subjects’own smoking with respiratory function was similar among the VLBW and control groups (Pfor interaction..1). However, maternal smoking was a stronger predictor of poor respiratory function in the BPD-VLBW group than in control subjects. This interaction is illustrated in Supplemental Table 6.
DISCUSSION
Our mainfinding was that young
adults born at VLBW had, on average,
reduced airflow compared with their
peers born at term. Thefinding was
stronger in those with a history of BPD but was also present in the majority of VLBW adults with no such
TABLE 2 Lung Function Test Results (Absolute and % of Predicted) in Study Groups
Parameter BPD-VLBW (n= 29) Non–BPD-VLBW (n= 131) All VLBW (n= 160) Term Control (n= 162) Absolute values
FVC (l) 3.9660.87† 4.3061.02* 4.2461.00† 4.5761.01 FEV1(l) 3.2660.82‡ 3.7760.81‡ 3.6760.83‡ 4.1060.83
FEV1/FVC (%) 82.2369.82‡ 88.3468.13* 87.2368.75† 90.2366.83
FEF75%, L/s 65.9629.3‡ 92.1633.1* 87.3633.9‡ 101.5631.3 FEF25%–75%, L/s 3.4061.42 4.3361.29‡ 4.1661.36‡ 4.8561.26 FEF50%, L/s 3.8261.63‡ 4.8261.46‡ 4.6461.54‡ 5.4161.44 PEF, L/s 7.6062.20‡ 8.5462.00† 8.3762.07‡ 9.1862.00
zscores
FVC 20.6461.01 20.3061.26 20.3761.22 20.3461.12 FEV1 21.0161.23‡ 20.0961.35 20.2561.37* 0.1061.18
FEV1/FVC 20.5761.38‡ 0.4261.29* 0.2461.35‡ 0.7761.12
FEF75% 20.4961.20‡ 0.4761.11 0.2961.18† 0.6960.97 FEF25%–75% 21.0261.45‡ 20.0161.26* 20.1961.35‡ 0.3361.10 % of predicted values
FVC 90.4611.8 94.6614.8 93.8614.3 95.2613.3
FEV1 82.7614.4‡ 93.5614.3* 91.5614.9† 96.9612.8
FEV1/FVC 91.3610.7‡ 99.468.9† 97.969.7‡ 102.367.6
PEF 83.9620.0‡ 93.6616.6* 91.9617.6† 97.9614.6 FEF50% 65.8626.5‡ 83.6623.0† 80.4624.5‡ 91.9622.5 Obstructive airways disease
Completed bronchodilation test 18 (62.1)‡ 39 (29.8)* 57(35.6)† 30(18.5) Positive bronchodilation testa 4 (13.8) 16 (12.2)* 20 (12.5)* 8 (4.9) Self-reported asthma, as diagnosed by a physician 7 (25.9)* 19 (14.6) 26 (16.6) 17 (10.5) Current use of inhaled glucocorticoids 2 (6.9) 4 (3.1) 6 (3.8) 4 (2.5) One or more signs of obstructive airways disease 11 (37.9)† 32 (24.4)* 43 (26.9)* 24 (14.8) Obstructive airways disease with atopy 5 (17.2) 14 (10.7) 19 (11.9) 17 (10.5) Obstructive airways disease and no atopy 6 (20.7)† 18 (13.7)† 24 (15.0)† 7 (4.3)
Data are presented as mean6SD orn(%). Thezscores are based on Global Lung Function Initiative standards.56Values are also presented as percent predicted by Finnish
standards27,28,30to allow comparison with previous studies. VLBW was defined as,1500 g. One or more signs of obstructive airways disease: reversible obstruction in bronchodilator
test, asthma previously diagnosed by a physician or inhaled glucocorticoid treatment. No missing values. FEF75%, forced expiratoryflow at 75% of FVC; FEF25%–75%, forced expiratory
flow at 25% to 75% of FVC; PEF, peak expiratoryflow. *Pvalue for the difference with term,.05.†Pvalue for the difference with term,.01.‡Pvalue for the difference with term,.001.
history. The difference was not explained by their smaller body size, family socioeconomic status, smoking, or maternal smoking during
pregnancy. Another keyfinding was
that there were few additional risk factors other than BPD and maternal smoking during pregnancy.
Other than the study in 26 VLBW survivor adolescents by Northway et al,10only a few studies have assessed spirometry in adults born very preterm. No reduction in
airflow was found among 35 VLBW
subjects and 48 control subjects in
a study in London, England.14 By
contrast, a study comparing 42 VLBW young adults from the Dutch POPS (Project on Preterm and Small for Gestational Age Infants) cohort with 48 control subjects15and another study in Trondheim, Norway, with 37 VLBW subjects and 63 control participants,16 found
a lower percent predicted FEV1of
14% and 13%, respectively. The reason for the difference between these 3 studies is not clear. All had a similar proportion of VLBW subjects with a history of BPD (∼20%); this size did not allow for statistically meaningful comparisons regarding BPD. Another study included 83 subjects (63 with BPD),
born at a birth weight,1000 g or
before 28 weeks of gestation, who exhibited reducing airflow with increasing severity of the early neonatal respiratory history.33 The largest studies published before the present study include 1 in Victoria, Australia, with 147 VLBW survivors (33 with BPD) and 37 normal birth weight control subjects, studied at age 19 years and a partly
overlapping group reassessed at 26 years.2,34Another large study was published from Belfast, Northern Ireland, with 96 small preterm survivors (56 with BPD) and 55 control subjects.12Consistent with
ourfindings and those previously
reported in children,4these studies
showed reduced airflow also in
VLBW adults without BPD, although this outcome was strongest in the BPD-VLBW groups. Our results
extend thesefindings by
demonstrating that the effects of VLBW birth per se and BPD seem to override the effects of most other clinical conditions, including intrauterine growth restriction, growth restriction during postnatal care, and neonatal RDS, which had very little additional value in predicting lung function in young adult life.
We also found that maternal smoking during pregnancy predicted reduced airflow of the offspring. This
TABLE 3 Mean Differences in Lung Function in a Multiple Regression Analysis in Young Adults Born at VLBW and at Term
Variable (z Score)
VLBW Group (n= 160)a
Group Born at Term (n= 162)a
Modelb No. of
Subjects
Difference (95% CI) P
FVC 20.3761.22 20.3461.12 1 322 20.02 (–0.28 to 0.24) .87 2 322 20.14 (–0.42 to 0.13) .31 3 318 20.13 (–0.41 to 0.14) .34 4 316 20.04 (–0.31 to 0.23) .78 FEV1 20.2561.37 0.1061.18 1 322 20.35 (–0.63 to–0.07) .01
2 322 20.53 (–0.83 to–0.23) .001 3 318 20.53 (–0.83 to–0.23) .001 4 316 20.40 (–0.68 to–0.11) .006 FEV1/FVC 0.2461.35 0.7761.12 1 322 20.53 (–0.80 to–0.26) ,.001
2 322 20.63 (–0.93 to–0.34) ,.001 3 318 20.64 (–0.94 to–0.34) ,.001 4 316 20.61 (–0.90 to–0.31) ,.001 FEF75% 0.6960.97 0.2961.18 1 322 20.39 (–0.62 to–0.15) .001 2 322 20.55 (–0.80 to–0.30) ,.001 3 318 20.57 (–0.82 to–0.31) ,.001 4 316 20.49 (–0.74 to–0.25) ,.001 FEF25%–75% 0.3361.10 20.1961.35 1 322 20.51 (–0.78 to–0.24) ,.001 2 322 20.69 (–0.98 to–0.40) ,.001 3 318 20.69 (–0.98 to–0.40) ,.001 4 316 20.60 (–0.87 to–0.32) ,.001
VLBW defined as,1500 g. FEF75%, forced expiratoryflow at 75% of FVC; FEF25%–75%, forced expiratoryflow at 25% to 75% of FVC.
aMean6SD.
bModel 1 adjusted for age and gender; model 2, model 1 + height and BMI; model 3, model 2 + parental educational
attainment, maternal smoking during pregnancy, current daily smoking of the subject, and frequency of leisure time conditioning physical activity; model 4, model 3 + subjects with history of asthma or having positive bronchodilation test or using currently inhaled glucocorticoids and subjects having atopy.
FIGURE 1
finding was strongest among VLBW subjects with a history of BPD. No data were available to distinguish the effects of maternal smoking from exposure to parental smoking after birth. Regardless of whether the critical smoking exposure is prenatal or postnatal, thefinding reinforces previous suggestions of the joint harmful effects of smoking exposure and lung injury.35
Paradoxically, asthma in childhood or early adult life36up to 31 years of age37is predicted by high birth weight. This outcome is more likely to reflect atopic asthma, which is more common in children and young adults.38Low birth weight, by contrast, is associated with late adulthood outcomes such as death from chronic obstructive airways disease39and higher rates of asthma
medication use40and premorbid
outcomes, including impaired airflow.41These birth weight studies are paralleled by studies linking preterm birth with manifest disease, including hospital-treated
obstructive airways disease among older adult women42and“asthma”in children and young adults.43–45The use of the term“asthma”for obstructive airways disease in those born preterm has been criticized, and the effect of standard asthma treatment in these subjects is uncertain.46Importantly, most of these studies included all infants born before 37 weeks of gestation, suggesting that ourfindings of
obstructed airflow in the VLBW
group (comprising the smallest
0.9%–1.5% of all newborns) are
relevant to all people born preterm.47
Together with smoking, lung function in early adulthood is 1 of the
strongest predictors of chronic obstructive pulmonary disease.5It also predicts cardiovascular mortality.48To prevent chronic obstructive pulmonary disease, abstinence from smoking is obviously important. Moreover, adults born at VLBW undertake less physical activity49and have a less healthy diet50than their peers born at term. Promotion of physical activity and a healthy diet have other benefits, including prevention of
cardiovascular disease.
A major strength of the present study was its sufficient size and detailed perinatal and neonatal data, which allowed us to assess the effects of potential confounders and clinical conditions that underlie or follow preterm birth at VLBW. In terms of its limitations, our study may not be representative of all VLBW infants in the original cohort. However, a previous detailed nonparticipant analysis19raised little concern over participation bias. To assess reversible airflow
obstruction, we performed
bronchodilator testing according to Finnish clinical guidelines, which warrant testing only in subjects whose baseline spirometry indicates airflow obstruction. These selection criteria may miss some subjects who would have experienced improved airflow after bronchodilator use, and the frequency of reversible airflow obstruction thus represents a minimum estimate. We did not have data on lung function in childhood, which precludes
discussion on whether alterations in lung function tend to improve, deteriorate, or remain similar over time.2,14,33,51–53 Our subjects represent treatment during
a presurfactant period, and only a few received antenatal or postnatal glucocorticoids. The study is thus directly relevant to VLBW survivors born in the 1970s or 1980s (who are now in their 30s and 40s) who total
∼500 000 people in the United States only.54,55
CONCLUSIONS
Young adults born at VLBW have
reduced airflow compared with their
peers born at term. The reduction is stronger among those with a history of BPD but is also present among those without. Of the many potential prenatal and postnatal risk factors assessed, only maternal smoking in pregnancy predicted reduced airflow. It may be particularly harmful for VLBW subjects who develop BPD. Our results offer a strong example of programming of respiratory function early in life. They also underscore the importance of following up the VLBW cohorts to assess whether the reduced airflow translates into increased rates of chronic
obstructive pulmonary disease later in life.
ABBREVIATIONS
aOR: adjusted odds ratio
BPD: bronchopulmonary dysplasia CI: confidence interval
FEF50%: forced expiratoryflow
when 50% of forced vital capacity has been exhaled
FEV1: forced expiratory volume in 1 second
FVC: forced vital capacity RDS: respiratory distress
syndrome
VLBW: very low birth weight
Address correspondence to Eero Kajantie, National Institute for Health and Welfare, PL 30, 00271 Helsinki, Finland. E-mail: eero.kajantie@helsinki.fi PEDIATRICS (ISSN Numbers: Print, 0031-4005; Online, 1098-4275).
Copyright © 2015 by the American Academy of Pediatrics
FUNDING:Supported by grants from the Academy of Finland, the Emil Aaltonen Foundation, the Finnish Foundation for Pediatrics Research, the Finnish Special Governmental Subsidary for Health Sciences (EVO), the Jalmari and Rauha Ahokas Foundation, the Juho Vainio Foundation, Medical Societies of Finland (Duodecim and Finska Läkaresällskapet), the Novo Nordisk Foundation, the Päivikki and Sakari Sohlberg Foundation, the Signe and Ane Gyllenberg Foundation, the Sigrid Jusélius Foundation, and the Yrjö Jahnsson Foundation.
POTENTIAL CONFLICT OF INTEREST:The authors have indicated they have no potential conflicts of interest to disclose.
REFERENCES
1. Doyle LW, Cheung MM, Ford GW, Olinsky A, Davis NM, Callanan C. Birth weight
,1501 g and respiratory health at age 14.Arch Dis Child. 2001;84(1):40–44
2. Doyle LW, Faber B, Callanan C, Freezer N, Ford GW, Davis NM. Bronchopulmonary dysplasia in very low birth weight subjects and lung function in late adolescence.Pediatrics. 2006;118(1): 108–113
3. Hakulinen AL, Järvenpää AL, Turpeinen M, Sovijärvi A. Diffusing capacity of the lung in school-aged children born very preterm, with and without
bronchopulmonary dysplasia.Pediatr Pulmonol. 1996;21(6):353–360
4. Kotecha SJ, Edwards MO, Watkins WJ, et al. Effect of preterm birth on later FEV1: a systematic review and meta-analysis.Thorax. 2013;68(8):760–766
5. Kalhan R, Arynchyn A, Colangelo LA, Dransfield MT, Gerald LB, Smith LJ. Lung function in young adults predicts airflow obstruction 20 years later.Am J Med. 2010;123(5):468.e1–468.e7
6. Gough A, Spence D, Linden M, Halliday HL, McGarvey LP. General and respiratory health outcomes in adult survivors of bronchopulmonary dysplasia: a systematic review.Chest. 2012;141(6):1554–1567
7. Halvorsen T, Skadberg BT, Eide GE, Røksund O, Aksnes L, Øymar K. Characteristics of asthma and airway hyper-responsiveness after premature birth.Pediatr Allergy Immunol. 2005; 16(6):487–494
8. Halvorsen T, Skadberg BT, Eide GE, Røksund OD, Carlsen KH, Bakke P. Pulmonary outcome in adolescents of extreme preterm birth: a regional cohort study.Acta Paediatr. 2004;93(10): 1294–1300
9. Korhonen P, Laitinen J, Hyödynmaa E, Tammela O. Respiratory outcome in school-aged, very-low-birth-weight children in the surfactant era.Acta Paediatr. 2004;93(3):316–321
10. Northway WH Jr, Moss RB, Carlisle KB, et al. Late pulmonary sequelae of bronchopulmonary dysplasia.N Engl J Med. 1990;323(26):1793–1799
11. Wong PM, Lees AN, Louw J, et al. Emphysema in young adult survivors of moderate-to-severe bronchopulmonary dysplasia.Eur Respir J. 2008;32(2): 321–328
12. Gough A, Linden M, Spence D, Patterson CC, Halliday HL, McGarvey LP. Impaired lung function and health status in adult survivors of bronchopulmonary dysplasia. Eur Respir J. 2014;43(3):808–816
13. Anand D, Stevenson CJ, West CR, Pharoah PO. Lung function and respiratory health in adolescents of very low birth weight. Arch Dis Child. 2003;88(2):135–138
14. Narang I, Rosenthal M, Cremonesini D, Silverman M, Bush A. Longitudinal evaluation of airway function 21 years after preterm birth.Am J Respir Crit Care Med. 2008;178(1):74–80
15. Vrijlandt EJ, Gerritsen J, Boezen HM, Grevink RG, Duiverman EJ. Lung function and exercise capacity in young adults born prematurely.Am J Respir Crit Care Med. 2006;173(8):890–896
16. Evensen KA, Steinshamn S, Tjønna AE, et al. Effects of preterm birth and fetal growth retardation on cardiovascular risk factors in young adulthood.Early Hum Dev. 2009;85(4):239–245
17. Bose C, Van Marter LJ, Laughon M, et al; Extremely Low Gestational Age Newborn Study Investigators. Fetal growth restriction and chronic lung disease among infants born before the 28th week of gestation.Pediatrics. 2009; 124(3). Available at: www.pediatrics.org/ cgi/content/full/124/3/e450
18. Kramer BW, Kallapur S, Newnham J, Jobe AH. Prenatal inflammation and lung development.Semin Fetal Neonatal Med. 2009;14(1):2–7
19. Hovi P, Andersson S, Eriksson JG, et al. Glucose regulation in young adults with
very low birth weight.N Engl J Med. 2007;356(20):2053–2063
20. Pihkala J, Hakala T, Voutilainen P, Raivio K. Characteristic of recent fetal growth curves in Finland [in Finnish].Duodecim. 1989;105(18):1540–1546
21. National High Blood Pressure Education Program Working Group on High Blood Pressure in Pregnancy. Report of the national high blood pressure education program working group on high blood pressure in pregnancy.Am J Obstet Gynecol. 2000;183(1):S1–S22
22. Northway WH Jr, Rosan RC, Porter DY. Pulmonary disease following respiratory therapy of hyaline-membrane disease. Bronchopulmonary dysplasia.N Engl J Med. 1967;276(7):357–368
23. Reynolds EO. Hyaline membrane disease. Am J Obstet Gynecol. 1970;106(5): 780–797
24. Usher R. The respiratory distress syndrome of prematurity. Clinical and therapeutic aspects.Pediatr Clin North Am. 1961;8:525–538
25. Kajantie E, Strang-Karlsson S, Hovi P, et al. Adults born at very low birth weight exercise less than their peers born at term.J Pediatr. 2010;157(4): 610–616, 616.e1
26. Strang-Karlsson S, Räikkönen K, Pesonen AK, et al. Very low birth weight and behavioral symptoms of attention deficit hyperactivity disorder in young adulthood: the Helsinki study of very-low-birth-weight adults.Am J Psychiatry. 2008;165(10):1345–1353
27. Sovijärvi A, Piirilä P, Korhonen O, Louhiluoto E, Pekkanen L. Performing and evaluating spirometry and PEF measurements. recommendation by the Finnish Society of Clinical Physiology and Finnish Society of Pulmonary Medicine [in Finnish].Moodi.2003;4:113
non-smoking, healthy adults.Scand J Clin Lab Invest Suppl. 1982;159:5–20
29. Pellegrino R, Viegi G, Brusasco V, et al. Interpretative strategies for lung function tests.Eur Respir J. 2005;26(5): 948–968
30. Sovijärvi A, Kainu A, Malmberg P, Pekkanen L, Piirilä P. Current care guidelines. working group set up by the Finnish Medical Society Duodecim the Finnish Respiratory Society, the Finnish Pediatric Society and the Finnish society of Clinical Physiology. Helsinki: The Finnish Medical Society Duodecim, 2014. Available at: Www.kaypahoito.fi. Suositus spirometria- ja PEF-mittauksen
suorittamisesta. Accessed March 3, 2014
31. Jobe AH, Bancalari E. Bronchopulmonary dysplasia.Am J Respir Crit Care Med. 2001;163(7):1723–1729
32. Siltanen M, Wehkalampi K, Hovi P, et al. Preterm birth reduces the incidence of atopy in adulthood.J Allergy Clin Immunol. 2011;127(4):935–942
33. Vollsæter M, Røksund OD, Eide GE, Markestad T, Halvorsen T. Lung function after preterm birth: development from mid-childhood to adulthood.Thorax. 2013;68(8):767–776
34. Gibson AM, Reddington C, McBride L, Callanan C, Robertson C, Doyle LW. Lung function in adult survivors of very low birth weight, with and without bronchopulmonary dysplasia [published online ahead of print September 5, 2014].Pediatr Pulmonol. doi: 10.1002/ ppul.23093
35. O’Reilly M, Thébaud B. Using cell-based strategies to break the link between bronchopulmonary dysplasia and the development of chronic lung disease in later life.Pulm Med. 2013;2013:874161
36. Flaherman V, Rutherford GW. A meta-analysis of the effect of high weight on asthma.Arch Dis Child. 2006;91(4): 334–339
37. Xu B, Pekkanen J, Laitinen J, Järvelin MR. Body build from birth to adulthood and
risk of asthma.Eur J Public Health. 2002; 12(3):166–170
38. Remes ST, Patel SP, Hartikainen AL, Järvelin MR, Pekkanen J. High birth weight, asthma and atopy at the age of 16 yr.Pediatr Allergy Immunol. 2008; 19(6):541–543
39. Barker DJ, Godfrey KM, Fall C, Osmond C, Winter PD, Shaheen SO. Relation of birth weight and childhood respiratory infection to adult lung function and death from chronic obstructive airways disease.BMJ. 1991;303(6804):671–675
40. Barker DJ, Osmond C, Forsén TJ, Thornburg KL, Kajantie E, Eriksson JG. Foetal and childhood growth and asthma in adult life.Acta Paediatr. 2013;102(7): 732–738
41. Lawlor DA, Ebrahim S, Davey Smith G. Association of birth weight with adult lung function:findings from the British Women’s Heart and Health Study and a meta-analysis.Thorax. 2005;60(10):851–858
42. Broström EB, Akre O, Katz-Salamon M, Jaraj D, Kaijser M. Obstructive pulmonary disease in old age among individuals born preterm.Eur J Epidemiol. 2013;28(1):79–85
43. Jaakkola JJ, Ahmed P, Ieromnimon A, et al. Preterm delivery and asthma: a systematic review and meta-analysis.J Allergy Clin Immunol. 2006;118(4):823–830
44. Crump C, Winkleby MA, Sundquist J, Sundquist K. Risk of asthma in young adults who were born preterm: a Swedish national cohort study. Pediatrics. 2011;127(4). Available at: www.pediatrics.org/cgi/content/full/127/ 4/e913
45. Been JV, Lugtenberg MJ, Smets E, et al. Preterm birth and childhood wheezing disorders: a systematic review and meta-analysis.PLoS Med. 2014;11(1): e1001596
46. Filippone M, Carraro S, Baraldi E. The term“asthma”should be avoided in describing the chronic pulmonary disease of prematurity.Eur Respir J. 2013;42(5):1430–1431
47. Blencowe H, Cousens S, Oestergaard MZ, et al. National, regional, and worldwide estimates of preterm birth rates in the year 2010 with time trends since 1990 for selected countries: a systematic analysis and implications.Lancet. 2012; 379(9832):2162–2172
48. Sin DD, Wu L, Man SF. The relationship between reduced lung function and cardiovascular mortality: a population-based study and a systematic review of the literature.Chest. 2005;127(6): 1952–1959
49. Kaseva N, Wehkalampi K, Strang-Karlsson S, et al. Lower conditioning leisure-time physical activity in young adults born preterm at very low birth weight.PLoS One. 2012;7(2):e32430
50. Kaseva N, Wehkalampi K, Hemiö K, et al. Diet and nutrient intake in young adults born preterm at very low birth weight.J Pediatr. 2013;163(1):43–48
51. Blayney M, Kerem E, Whyte H, O’Brodovich H. Bronchopulmonary dysplasia: improvement in lung function between 7 and 10 years of age.J Pediatr. 1991;118(2):201–206
52. Baraldi E, Carraro S, Filippone M. Bronchopulmonary dysplasia: definitions and long-term respiratory outcome. Early Hum Dev. 2009;85(suppl 10):S1–S3
53. Chambers DC. Lung function in ex-preterm adults.Am J Respir Crit Care Med. 2009;179(6):517–, author reply 517
54. Lee KS, Kim BI, Khoshnood B, et al. Outcome of very low birth weight infants in industrialized countries: 1947-1987.Am J Epidemiol. 1995;141(12): 1188–1193
55. Taffel SM. Trends in low birth weight: United States, 1975-85.Vital Health Stat 21. 1989;(48):1–30
DOI: 10.1542/peds.2014-2651 originally published online September 7, 2015;
2015;136;642
Pediatrics
Johan G. Eriksson, Sture Andersson and Eero Kajantie
Karoliina Wehkalampi, Mirjami Siltanen, Marja Vääräsmäki, Anna-Liisa Järvenpää,
Heli-Kaisa Saarenpää, Marjaana Tikanmäki, Marika Sipola-Leppänen, Petteri Hovi,
Lung Function in Very Low Birth Weight Adults
Services
Updated Information &
http://pediatrics.aappublications.org/content/136/4/642 including high resolution figures, can be found at:
References
http://pediatrics.aappublications.org/content/136/4/642#BIBL This article cites 51 articles, 14 of which you can access for free at:
Subspecialty Collections
http://www.aappublications.org/cgi/collection/pulmonology_sub Pulmonology
sub
http://www.aappublications.org/cgi/collection/fetus:newborn_infant_ Fetus/Newborn Infant
following collection(s):
This article, along with others on similar topics, appears in the
Permissions & Licensing
http://www.aappublications.org/site/misc/Permissions.xhtml in its entirety can be found online at:
Information about reproducing this article in parts (figures, tables) or
Reprints
DOI: 10.1542/peds.2014-2651 originally published online September 7, 2015;
2015;136;642
Pediatrics
Johan G. Eriksson, Sture Andersson and Eero Kajantie
Karoliina Wehkalampi, Mirjami Siltanen, Marja Vääräsmäki, Anna-Liisa Järvenpää,
Heli-Kaisa Saarenpää, Marjaana Tikanmäki, Marika Sipola-Leppänen, Petteri Hovi,
Lung Function in Very Low Birth Weight Adults
http://pediatrics.aappublications.org/content/136/4/642
located on the World Wide Web at:
The online version of this article, along with updated information and services, is
http://pediatrics.aappublications.org/content/suppl/2015/09/01/peds.2014-2651.DCSupplemental Data Supplement at:
by the American Academy of Pediatrics. All rights reserved. Print ISSN: 1073-0397.