Urokinase Versus VATS for Treatment of Empyema:
A Randomized Multicenter Clinical Trial
WHAT’S KNOWN ON THIS SUBJECT: There are discrepancies regarding which treatment is best in clinical practice for children with parapneumonic empyema, with some authors favoring video-assisted thoracoscopy and others favoring intrapleural
fibrinolytic agents.
WHAT THIS STUDY ADDS: This study is one of the few randomized clinical trials on this subject in children and thefirst multicenter trial. It exclusively included patients with septated empyema. Thoracoscopy andfibrinolysis with urokinase were equally effective for this condition.
abstract
BACKGROUND AND OBJECTIVE: Parapneumonic empyema (PPE) is a frequent complication of acute bacterial pneumonia in children. There is limited evidence regarding the optimal treatment of this con-dition. The aim of this study was to compare the efficacy of drainage plus urokinase versus video-assisted thoracoscopic surgery in the treatment of PPE in childhood.
METHODS:This prospective, randomized, multicenter clinical trial en-rolled patients aged,15 years and hospitalized with septated PPE. Study patients were randomized to receive urokinase or thoracoscopy. The main outcome variable was the length of hospital stay after treat-ment. The secondary outcomes were total length of hospital stay, number of days with the chest drain, number of days with fever, and treatment failures. The trial was approved by the ethics committees of all the participating hospitals.
RESULTS:A total of 103 patients were randomized to treatment and analyzed; 53 were treated with thoracoscopy and 50 with urokinase. There were no differences in demographic characteristics or in the main baseline characteristics between the 2 groups. No statistically significant differences were found between thoracoscopy and uroki-nase in the median postoperative stay (10 vs 9 days), median hospital stay (14 vs 13 days), or days febrile after treatment (4 vs 6 days). A second intervention was required in 15% of children in the thoracoscopy group versus 10% in the urokinase group (P= .47).
CONCLUSIONS:Drainage plus urokinase instillation is as effective as video-assisted thoracoscopic surgery as first-line treatment of septated PPE in children.Pediatrics2014;134:e1301–e1307
AUTHORS:Claudia Marhuenda, MD,aConcepció Barceló,
MD,aInmaculada Fuentes, MD,bGabriela Guillén, MD,a
Indalecio Cano, MD,cMaría López, MD,cFrancisco
Hernández, MD,dEduardo G. Pérez-Yarza, MD, PhD,e,f,g
José A. Matute, MD,hMaría A. García-Casillas, MD,hVíctor
Álvarez, MD, PhD,iand Antonio Moreno-Galdó, MD, PhDj
Departments ofaPediatric Surgery,bClinical Pharmacology, and jPediatric Pulmonology, Hospital Universitari Vall d’Hebron,
Universitat Autònoma de Barcelona. Barcelona, Spain;
cDepartment of Pediatric Surgery, Hospital 12 de Octubre,
Madrid, Spain;dDepartment of Pediatric Surgery, Hospital La Paz,
Madrid, Spain;eDepartment of Pediatrics, University of the
Basque Country, UPV/EHU, San Sebastian, Spain;fDivision of
Pediatric Respiratory Medicine, Hospital Universitario Donostia-Instituto Biodonostia, San Sebastián, España;gBiomedical
Research Centre Network for Respiratory Diseases (CIBERES), San Sebastián, Spain;hDepartment of Pediatric Surgery, Hospital
Gregorio Marañón, Madrid, Spain; andiDepartment of Pediatric
Surgery, Hospital Central de Asturias, Oviedo, Spain
KEY WORDS
children, empyema,fibrinolytics, parapneumonic effusions, randomized clinical trial, urokinase, VATS, video-assisted thoracoscopic surgery
ABBREVIATIONS IQR—interquartile range UK—urokinase
VATS—video-assisted thoracoscopic surgery
Drs Marhuenda, Fuentes, and Moreno-Galdó were responsible for the study concept and design, data acquisition, data analysis and interpretation, and drafting of the manuscript; Dr Barceló was responsible for the study concept and design and acquisition of data; Drs Guillén, López, and García-Casillas were responsible for data acquisition; Drs Cano, Hernández, Pérez-Yarza, Matute, and Álvarez were responsible for and coordinated data acquisition at 1 of the 6 sites. All authors agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved; they were all responsible for critical revision of the manuscript for important intellectual content; and all approved thefinal manuscript as submitted.
The trial was registered with the European Clinical Trials Database (2007-003416-61) before enrolling patients in this study.
www.pediatrics.org/cgi/doi/10.1542/peds.2013-3935
doi:10.1542/peds.2013-3935
Accepted for publication Aug 5, 2014
(Continued on last page)
in childhood.1As a parapneumonic ef-fusion progresses, fibrin and cellular detritus accumulate, the purulentfluid becomes septated, and a thick peel forms over the visceral and parietal pleura. Parapneumonic pleural effu-sion is thus classified into 3 stages of progression: exudative (stage 1),fi bri-nopurulent (stage 2), and organiza-tional (stage 3).2
An estimated 0.6% to 2% of pneumonia cases in children are complicated by empyema.3 Since the 1990s, there has been a considerable worldwide increase in the incidence of empyema, with rates reaching 28.3% to 53% of all patients hospitalized for pneumonia.4–7In Spain, an increase from 1.7 to 8.5 cases/ 100 000 children has been documented.8
The 2 historical pillars of treatment of empyema are antibiotics and drainage of the purulentfluid by chest tube place-ment. However, in advanced stages of the condition, this approach is associated with lengthy hospital stays and high failure rates.9–11In addition to thoracot-omy,9–122 less invasive treatments have been examined: fibrinolytic agents in-stilled into the pleural space and video-assisted thoracoscopic surgery (VATS). The aim of both these approaches is to break up the septa, extract orfluidize thefibrin and cellular detritus, and thus restore normal function of the mecha-nisms of pleural reabsorption, making a quick recovery possible.13
Two randomized controlled pediatric clinical trials have been published comparing the effectiveness offi brino-lytic agents versus saline serum. Thomson et al14 found that administration of urokinase significantly reduced hospi-tal stay and was associated with a low failure rate (7%), whereas Singh et al15 found no clinical benefit. In several pediatric series of empyema, VATS has been successfully used as salvage therapy16–18 or as the initial
and shorter hospitalization than with chest tube drainage alone.23,24
The small numbers of pediatric studies comparing VATS andfibrinolytic agents as the initial therapy for parapneumonic empyema have concluded that the 2 treatments are equivalent.25–27 One of these studies, however, was a retrospec-tive review of a hospital experience,25 and the other 2 are clinical trials that included some patients with empyema without septations.26,27In another clinical trial, which included children with em-pyema originating from various causes, VATS was compared with streptokinase treatment.28The authors reported simi-lar success rates with the 2 methods and some advantages with the use of VATS.
To further evaluate the relative merits of these treatment modalities, the present trial was conducted to compare VATS versus urokinase instillation in a cohort of patients with septated empyema.
METHODS
The project was approved by the ethics committees of all the participating hos-pitals, as well as the Spanish Agency of Medicines and Medical Devices. Parental written informed consent, and consent of the children when applicable, was obtained from all participants.
Study Design and Sample Size
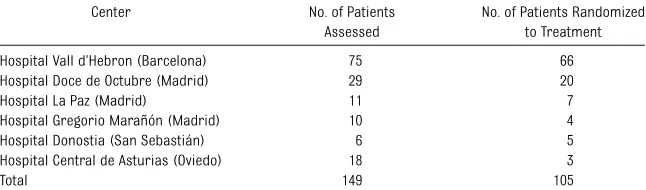
This study was a randomized, multi-center, open-label, parallel-group, pilot clinical trial, performed over 2 years at 6 Spanish university hospitals (Table 1).
Sample size wasfirst calculated based on preliminary results from a previous series of patients treated by the principle investigator in Hospital Universitari Vall d’Hebron (Barcelona, Spain) between 2001 and 2005. During that period, 58 patients with complicated effusion were treated: 33 with the use of VATS and 25 by using urokinase instillation. Mean postoperative hospital stay was 12.726 7.04 days in the VATS group and 14.166 7.19 days in the urokinase group. These data suggested that there would be no significant differences in the du-ration of hospitalization between the groups. However, an equivalence test of means by using 2 one-sided tests on data from a parallel-group design to achieve 80% power at a 5% signifi -cance level, and considering equiva-lence limits of –1.50 and 1.50, would have had to include 378 patients in each group. We considered it unfeasible to recruit such a large number of chil-dren; hence, a pilot clinical trial without formal calculation of the sample size was conducted. Also based on our pre-vious experience, the recruitment pe-riod was established at 2 years under the assumption that that period of time would suffice to enroll a larger number of patients than had been included in previous studies.
Inclusion and Exclusion Criteria
Previously healthy patients aged,15 years with community-acquired pneu-monia, a diagnosis of parapneumonic pleural effusion requiring chest tube
TABLE 1 Number of Patients Per Participating Center
Center No. of Patients Assessed
No. of Patients Randomized to Treatment
Hospital Vall d’Hebron (Barcelona) 75 66
Hospital Doce de Octubre (Madrid) 29 20
Hospital La Paz (Madrid) 11 7
Hospital Gregorio Marañón (Madrid) 10 4
Hospital Donostia (San Sebastián) 6 5
Hospital Central de Asturias (Oviedo) 18 3
drainage (Table 2), and sonographic features of complicated effusion were considered candidates for enrollment. Complicated effusion was defined as hyperechoicfluid withfibrinous septa (sonographic stage 2) or hyperechoic loculations with or without thick pari-etal peel (sonographic stage 3).10,28
Exclusion criteria are summarized in Table 3. Diagnostic thoracentesis with no attempt at evacuating the effusion was not a reason for exclusion.
Determinations
All patients underwent determination of hemoglobin, white blood cell count, platelets, and C-reactive protein in blood, and white blood cell count, pH, lactate dehydrogenase, and glucose in pleural fluid. Blood and pleural fluid cultures were performed, and Strepto-coccus pneumoniaewas investigated in pleuralfluid by using antigen detection or polymerase chain reaction assay.
Patients were followed up for 3 months after hospital discharge, with chest
radiographs at each visit. In each cen-ter, chest radiographs were assessed by a radiologist blinded to the outcome parameters. The radiographs were clas-sified as normal, showing small changes, or showing considerable pleural thick-ening.
Randomization
Patients were randomly allocated to receive 1 of the 2 treatments. The computer-generated randomization se-quence was stratified according to center and had varying block sizes to ensure balance in the number of pa-tients per group. Once the informed consent was signed, the attending phy-sician accessed a Web platform and obtained the treatment assigned to each new patient. Allocation was carried out at a 1:1 ratio. Treatment was not blinded.
Treatment Protocol
Patients were randomized to receive either VATS debridement or urokinase instillation. It was recommended that VATS be conducted by experienced surgeons or by a resident under the direct supervision of a senior surgeon. The aim of VATS was to break thefibrin septations, aspirate the purulentfluid, and abundantly irrigate the empyema cavity. After the procedure, 1 or 2 chest tubes were left in place.
In patients receiving urokinase treat-ment, 12F to 14F chest tubes were used, and the insertion site was previously selected by using sonography. The pleural fluid was first drained, and urokinase was then instilled into the pleural cavity through the chest tube. Ten milliliters of a 1000-IU/mL solution of urokinase in children aged,1 year and 40 mL in older children was adminis-tered every 12 hours for 3 days.14,26After instillation, the chest tube was clamped for 4 hours. It was then unclamped and connected to a suction system at–20 cm H2O for 8 hours, until the next dose of urokinase was administered. In both
treatment groups, chest tubes were removed when the drainage volume was,40 to 60 mL/24 h.
Antibiotic treatment recommendations were not included in the study protocol. It was assumed that patients would receive empiric treatment with antibiotics cov-ering the spectrum of the most common microorganisms in our setting, with subsequent treatment adjustment based on microbiology results. After removal of the chest tube, antibiotic treatment could be administered orally, provided that the patient had been afebrile (#37.5°C) for 24 hours. Patients could be discharged if they had been afebrile for at least 24 hours with oral treatment (suggested discharge criteria).
Persistent fever ($38°C) for.4 days after either of the study treatments, associated with persistent purulent pleural collections on ultrasound, was considered treatment failure. In cases of failure, salvage treatment was per-formed, which was individually de-termined by the attending physician according to the needs of each patient.
Outcome Measures
The postoperative length of hospitali-zation was the primary outcome vari-able, defined as the number of days hospitalized, starting from the day of the intervention to discharge. The sec-ondary outcomes were the total num-ber of days hospitalized (including the days before initiating treatment), days the chest tube was in place, days the patient had fever after treatment, fail-ure rate of the assigned treatment, and treatment-related complications.
Statistical Analysis
Due to the multicenter nature of the study, demographic characteristics of the children in the 2 treatment groups were compared by using the Wilcoxon rank-sum stratified test (van Elteren test) for quantitative variables and the Cochran-Mantel-Haenszel test for categorical TABLE 2 Indications for Chest Tube
Drainage of Pleural Effusion
Fever and ultrasound-proven complicated effusion Effusion.1 cm and fever$38°C after 24 h of
appropriate intravenous antibiotic treatment Tachypnea
Oxygen requirement as a consequence of the effusion
Increase in the size of the effusion during the observation period
TABLE 3 Exclusion Criteria
Anechoic, nonseptated effusion Preexisting conditions
Cerebral paralysis Congenital cardiopathy
Previous cardiac or thoracic surgery in the affected hemithorax
Immunodeficiency
Significant thoracic trauma in the last 2 mo Thrombocytopenia or abnormal clotting Severe arterial hypertension Tuberculous empyema Pneumothorax before treatment
Patients treated with chest tube placement at the referring hospital
were calculated by using the Hodges-Lehmann method, adjusting for center clustering. Statistical significance was set at aPvalue of,.05. Data were an-alyzed by using SAS version 9.2 (SAS Institute, Inc, Cary, NC) and Stata version 13.1 (StataCorp, College Station, TX). An intention-to-treat analysis was applied, including all randomized patients who received the assigned treatment.
RESULTS
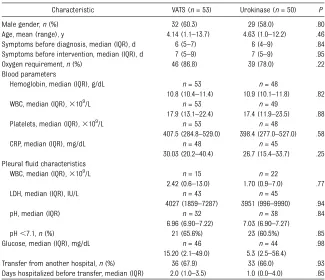
Over the study period (July 1, 2008–June 30, 2010), 149 patients were assessed as candidates for the trial. Forty-four patients were excluded before ran-domization for the following reasons: declined to participate (n= 20), did not meet selection criteria (n = 12), and other reasons (n= 12). Thus, 105 pa-tients were randomized to treatment: 53 in the VATS group and 52 in the uroki-nase group. All patients in the VATS group received the allocated interven-tion. One patient in the urokinase group was found to have pleural tuberculosis after randomization and did not receive the study treatment. Another patient was erroneously randomized twice and received the first treatment assigned (VATS). Thus, 53 patients in the VATS group and 50 patients in the urokinase group were analyzed. The 2 populations were comparable in terms of baseline characteristics (Table 4).
Primary End Point
The median length of postintervention hospitalization was 10 days in patients undergoing VATS (interquartile range [IQR]: 7–13) and 9 days in those un-dergoing urokinase instillation (IQR: 8– 12). There were no significant differ-ences between groups (Table 5).
Secondary End Points
No significant differences were found for total hospital stay, with a median of 14 days (IQR: 10–16) in the VATS group and
13 days (IQR: 10–18) in the urokinase group (P= .60), or duration of fever af-ter treatment (4 days [IQR: 2–7] with VATS versus 6 days [IQR: 3–7) with uro-kinase;P= .62) (Table 5). The chest tube was retained longer in patients re-ceiving urokinase (5 days) than in those undergoing VATS (4 days) (P,.001).
Eight (15.1%) patients treated with VATS and 5 (10%) treated with urokinase required another procedure to resolve empyema and were considered treat-ment failures; these differences were not significant. Analysis of potential factors predisposing to failure (gender,
age, duration of symptoms, and days of antibiotic treatment before the pro-cedure) yielded no predisposing fac-tors in either group. Reinterventions are shown in Table 6.
Microbiology Findings
In 40 (75%) patients from the VATS group and 34 (68%) from the urokinase group,S pneumoniaewas identified as the cause of pneumonia (P= .51).
Adverse Events
Forty-three (81.1%) patients in the VATS group and 41 (82%) in the urokinase
Male gender,n(%) 32 (60.3) 29 (58.0) .80
Age, mean (range), y 4.14 (1.1–13.7) 4.63 (1.0–12.2) .46 Symptoms before diagnosis, median (IQR), d 6 (5–7) 6 (4–9) .84 Symptoms before intervention, median (IQR), d 7 (5–9) 7 (5–9) .95 Oxygen requirement,n(%) 46 (86.8) 39 (78.0) .22 Blood parameters
Hemoglobin, median (IQR), g/dL n= 53 n= 48
10.8 (10.4–11.4) 10.9 (10.1–11.8) .82 WBC, median (IQR),3109/L n= 53 n= 49
17.9 (13.1–22.4) 17.4 (11.9–23.5) .88 Platelets, median (IQR),3109/L n= 53 n= 48
407.5 (284.8–529.0) 398.4 (277.0–527.0) .58
CRP, median (IQR), mg/dL n= 48 n= 45
30.03 (20.2–40.4) 26.7 (15.4–33.7) .25 Pleuralfluid characteristics
WBC, median (IQR),3109/L n= 15 n= 22
2.42 (0.6–13.0) 1.70 (0.9–7.0) .77
LDH, median (IQR), IU/L n= 43 n= 45
4027 (1859–7287) 3951 (996–9990) .94
pH, median (IQR) n =32 n= 38 .84
6.96 (6.90–7.22) 7.03 (6.90–7.27)
pH,7.1,n(%) 21 (65.6%) 23 (60.5%) .85
Glucose, median (IQR), mg/dL n= 46 n= 44 .98
15.20 (2.1–49.0) 5.3 (2.5–56.4)
Transfer from another hospital,n(%) 36 (67.9) 33 (66.0) .93 Days hospitalized before transfer, median (IQR) 2.0 (1.0–3.5) 1.0 (0.0–4.0) .85 CRP, C-reactive protein; LDH, lactate dehydrogenase; WBC, white blood cell count.
TABLE 5 Results for the Primary and Secondary Study Outcomes
Outcome VATS
(n= 53)
Urokinase (n= 50)
Median Difference (95% CI)
P
Primary outcome
PO hospital stay, median (IQR), d 10 (7–13) 9 (8–12) 0 (–1 to 1) .45 Secondary outcomes
Total hospital stay, median (IQR), d 14 (10–16) 13 (10–18) 0 (–2 to 2) .60 Chest tube in place, median (IQR), d 4 (3–5) 5 (4–6) 21 (–2 to–1) ,.001 PO fever, median (IQR), d 4 (2–7) 6 (3–7) 21 (–2 to 1) .62
Failure rate,n(%) 8 (15.1) 5 (10) .47
group had no treatment- or pneumonia-related complications.
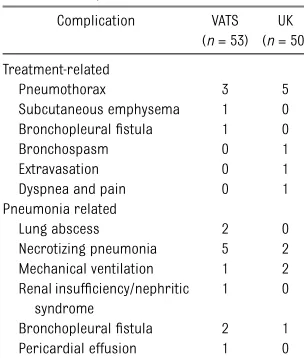
The treatment-related complications en-countered were minor, except for a severe bronchospasm requiring tracheal in-tubation and mechanical ventilation in 1 urokinase-treated patient and a broncho-pleuralfistula in 1 patient who underwent VATS. There were no bleeding complica-tions in the urokinase group. Complica-tions considered to be related to the patients’ pneumonia included 2 bron-chopleuralfistulas in the VATS group and 1 bronchopleuralfistula in the urokinase group, all occurring in patients who had necrotizing pneumonia or a pulmonary abscess (Table 7). There were no deaths.
Follow-up
Clinical and radiologic follow-up for 3 months after hospital discharge was possible in 36 (67.9%) patients in the VATS group and 42 (84%) in the uroki-nase group (P= .28). At the 3-month visit, radiologists considered chest radio-graphs normal or showing only small
changes in 66.7% (24 of 36) of VATS-treated patients and 59.5% (25 of 42) of those receiving urokinase (P= .40).
DISCUSSION
The present study is the third randomized clinical trial in children that compared VATS and fibrinolytic agents as alter-native treatments for parapneumonic empyema. In addition, it is the first multicenter study and thefirst to ex-clusively include patients with septated empyema.
The results show that urokinase in-stillation is as effective as VATS as the initial therapy for treating septated pleural empyema. There were no sig-nificant differences between the 2 groups in the length of postoperative hospitalization (ie, the main outcome measure). Furthermore, the only dif-ference found among all the variables studied was the fact that the chest tube was in place longer in the urokinase group. The study design, which required urokinase instillation through the tube for 3 days, may have resulted in bias regarding this variable. Nonetheless, this required use did not lead to lengthier postoperative hospitalization in the uro-kinase group. The treatment failure rates and requirements for additional proce-dures were also found to be similar in the 2 treatment groups.
Our results support the notion that initial treatment of purulent pleural effusion in children with VATS or urokinase in-stillation speeds pus drainage and re-covery and, therefore, shortens hospital stay and duration of chest tube place-ment. In a meta-analysis by Avansino et al,29the mean hospital stay of 3418 children undergoing chest tube drain-age alone was 2068.3 days; patients required drainage a mean of 10.663.4 days; and treatment failure occurred in 23.6% of patients. These results are very unfavorable compared with those from studies in which the initial treatment was VATS or urokinase.
Two previous randomized clinical trials involving fibrinolytic agents and VATS have been conducted in children. The first of these, reported by Sonnappa et al in 2006, included 60 patients and com-pared VATS with urokinase instillation,26 whereas the second, reported by St Pe-ter in 2009 and including 36 patients,27 compared VATS with instillation of alte-plase. Both studies were performed in a single center and had similar designs. Our results are in keeping with the findings from these previous studies in thatfibrinolytic instillation and VATS are equally effective for treating patients with more severe empyema. The results of a protocol usingfibrinolysis to treat empyema have recently replicated the findings of these previous trials.30 Although the design of the present study is very similar to the study of Sonnappa et al,26 there are 2 main differences. First, our study included patients with parapneumonic pleural effusion in sono-graphic stages 2 and 3 but not those with anechoic, nonseptated effusion (stage 1), who accounted for 16 patients in the study of Sonnappa et al. The second dif-ference was that our trial was a multi-center study. As noted by Sonnappa et al, the results of multicenter studies can be more reliably extrapolated to the general population.
The duration of postoperative hospital stay was longer in both our patient groups than in the earlier studies: 10 days for VATS and 9 days for urokinase versus 6 and 6 days in the study by Sonnappa et al26and 6.9 and 6.8 days in the study by St Peter et al,27respectively. These differences are not attributable to a delay in transferring our patients for treatment, as the interval was similar to that reported in the study by Sonnappa et al. The lengthier hospitalization in our study may be due, in part, to the fact that children with anechoic, non-septated effusions were not included in our series, but we believe the main reason is possible differences in pediatric TABLE 6 Reinterventions According to
Treatment Group
Reintervention VATS Group Urokinase Group
VATS 2 2
Urokinase 4 1
Chest drain 1 2
Thoracotomy 1 0
Total 8 5
TABLE 7 Treatment and Pneumonia-Related Complications
Complication VATS (n= 53)
UK (n= 50)
Treatment-related
Pneumothorax 3 5
Subcutaneous emphysema 1 0 Bronchopleuralfistula 1 0
Bronchospasm 0 1
Extravasation 0 1
Dyspnea and pain 0 1 Pneumonia related
Lung abscess 2 0
Necrotizing pneumonia 5 2 Mechanical ventilation 1 2 Renal insufficiency/nephritic
syndrome
1 0
Bronchopleuralfistula 2 1 Pericardial effusion 1 0
until drainage volume was minimal (,40–60 mL/24 h), and patients were not discharged until they had been afebrile for 24 hours. This practice may have differed in the previous studies reporting a shorter hospital stay.
The treatment failure rateswere similar in the 2 groups (15% for VATS and 10% for urokinase) and were comparable to the values reported by the studies of Sonnappa et al and St Peter et al (16.6% for fibrinolysis).26,27 Among the 13 pa-tients in whom the initial treatment failed, only 5 subsequently underwent surgery; the remaining 8 patients were treated by drain placement or uroki-nase instillation.
Of note, only 1 patient of the 103 subjects analyzed in our study needed a thora-cotomy, as compared with 4 of 30 in the VATS group and an unspecified number in the urokinase group in the trial by Sonnappa et al.26 All 4 patients in that study were converted to thoracotomy during the initial VATS procedure, based on the surgeon’s perception that the pleural peel would impede proper expan-sion of the lung. Because the radiologic and functional course of our previously treated patients had demonstrated that residual pleural thickening resolves spontaneously over follow-up,25 our surgical protocol in the present study did not include debridement of pleural peel on the lung. Debridement is not
parenchymal injury is higher when attempts are made to remove thefibrin layer. In our series, findings on radio-graphic follow-up at 3 months after hospital discharge were considered normal in 66.7% of patients in the VATS group and 59.5% of those receiving urokinase. There were no later hospital referrals for“trapped lung.”Therefore, we believe that the surgeon’s subjective perception of lung distensibility during the acute phase of empyema should not guide the surgery performed.
Our study has some limitations that should be mentioned. First, it was con-ducted without previous calculation of the sample size because of the unfea-sibly large number of patients who would be needed to perform an equiv-alence study. Nonetheless, the number of patients ultimately randomized for this trial is the largest among the existing prospective studies having the same objective. Second, the diagnostic sono-graphic images did not undergo cen-tralized review, and there is a possibility of variability between the readers in the different centers.
The present study has some practical implications. Many second-level pediat-ric hospitals have the capability to per-form ultrasound diagnosis of severe empyema and image-guided chest tube placement, enabling treatment with fi -brinolytic drugs. Surgical treatment
transferred to third-level hospitals with surgeons trained to treat them. Second, VATS is a procedure lasting∼1 hour that requires general anesthesia and involves considerable aggressiveness in a severely ill patient, as was noted in the recent review by Islam et al.23Urokinase instillation is much less invasive. Lastly, although we did not perform a compar-ative cost study between the 2 treat-ments, previous trials have shown that the surgical option is more costly than fibrinolytic therapy.26,27
CONCLUSIONS
Initial treatment of septated para-pneumonic empyema withfibrinolytic agents is as effective as VATS in children. This multicenter study adds strength to current recommendations23,24 to use chemical debridement as first-line therapy in these patients.
ACKNOWLEDGMENTS
The authors are grateful to the members of the Departments of Pediatric Surgery, Pediatrics, and Radiology of the partici-pating hospitals, whose invaluable help contributed to make this study possible. We thank Xavier Vidal (Department of Clinical Pharmacology, Hospital Vall d’Hebron, Barcelona) for his help with the statistical analysis and Celine Cavallo for English language support.
REFERENCES
1. Durbin WJ, Stille C. Pneumonia.Pediatr Rev.
2008;29(5):147–158, quiz 159–160
2. Andrews NC, Parker EF, Shaw RR, et al
Management of nontuberculous empyema:
a statement of the Subcommittee on
Sur-gery.Am Rev Respir Dis. 1962;85:935–936
3. Martinón-Torres F, Bernaola Iturbe E,
Giménez Sánchez F, et al. Why are pediatric
empyemas on the increase in Spain? [in
Spanish]. An Pediatr (Barc). 2008;68(2):
158–164
4. Tan TQ, Mason EO Jr, Wald ER, et al. Clinical
characteristics of children with complicated
pneumonia caused by Streptococcus
pneu-moniae.Pediatrics. 2002;110(1 pt 1):1–6
5. Grijalva CG, Nuorti JP, Zhu Y, Griffin MR.
Increasing incidence of empyema
compli-cating childhood community-acquired
pneumonia in the United States. Clin
In-fect Dis. 2010;50(6):805–813
6. Byington CL, Spencer LY, Johnson TA, et al.
An epidemiological investigation of a
sus-tained high rate of pediatric parapneumonic
empyema: risk factors and microbiological
associations.Clin Infect Dis. 2002;34(4):434–
440
7. Obando I, Muñoz-Almagro C, Arroyo LA, et al.
Pediatric parapneumonic empyema, Spain.
Emerg Infect Dis. 2008;14(9):1390–1397
8. Calbo E, Díaz A, Cañadell E, et al; Spanish
Pneumococcal Infection Study Network.
In-vasive pneumococcal disease among
impact of pneumococcal conjugate vaccine. Clin Microbiol Infect. 2006;12(9):867–872
9. Chan W, Keyser-Gauvin E, Davis GM, Nguyen LT, Laberge JM. Empyema thoracis in chil-dren: a 26-year review of the Montreal Children’s Hospital experience. J Pediatr Surg. 1997;32(6):870–872
10. Mayo P, Saha SP, McElvein RB. Acute em-pyema in children treated by open
thora-cotomy and decortication. Ann Thorac
Surg. 1982;34(4):401–407
11. Carey JA, Hamilton JR, Spencer DA, Gould K, Hasan A. Empyema thoracis: a role for open
thoracotomy and decortication. Arch Dis
Child. 1998;79(6):510–513
12. Khakoo GA, Goldstraw P, Hansell DM, Bush A. Surgical treatment of parapneumonic em-pyema.Pediatr Pulmonol. 1996;22(6):348–356
13. Balfour-Lynn IM, Abrahamson E, Cohen G, et al; Paediatric Pleural Diseases Sub-committee of the BTS Standards of Care Committee. BTS guidelines for the man-agement of pleural infection in children. Thorax. 2005;60(suppl 1):i1–i21
14. Thomson AH, Hull J, Kumar MR, Wallis C, Balfour Lynn IM. Randomised trial of intrapleural urokinase in the treatment of
childhood empyema. Thorax. 2002;57(4):
343–347
15. Singh M, Mathew JL, Chandra S, Katariya S, Kumar L. Randomized controlled trial of intrapleural streptokinase in empyema thoracis in children.Acta Paediatr. 2004;93 (11):1443–1445
16. Kern JA, Rodgers BM. Thoracoscopy in the
management of empyema in children. J
Pediatr Surg. 1993;28(9):1128–1132
17. Klena JW, Cameron BH, Langer JC, Winthrop AL, Perez CR. Timing of video-assisted
thor-acoscopic debridement for pediatric empy-ema.J Am Coll Surg. 1998;187(4):404–408
18. Stovroff M, Teague G, Heiss KF, Parker P, Ricketts RR. Thoracoscopy in the manage-ment of pediatric empyema.J Pediatr Surg.
1995;30(8):1211–1215
19. Grewal H, Jackson RJ, Wagner CW, Smith SD. Early video-assisted thoracic surgery in
the management of empyema.Pediatrics.
1999;103(5). Available at: www.pediatrics. org/cgi/content/full/103/5/e63
20. Kercher KW, Attorri RJ, Hoover JD, Morton D Jr. Thoracoscopic decortication asfi rst-line therapy for pediatric parapneumonic empyema. A case series.Chest. 2000;118 (1):24–27
21. Merry CM, Bufo AJ, Shah RS, Schropp KP, Lobe
TE. Early definitive intervention by thoracoscopy in pediatric empyema.J Pediatr Surg. 1999; 34(1):178–180, discussion 180–181
22. Schneider CR, Gauderer MW, Blackhurst D,
Chandler JC, Abrams RS. Video-assisted thoracoscopic surgery as a primary inter-vention in pediatric parapneumonic effusion
and empyema.Am Surg. 2010;76(9):957–961
23. Islam S, Calkins CM, Goldin AB, et al; APSA
Outcomes and Clinical Trials Committee, 2011-2012. The diagnosis and management
of empyema in children: a comprehensive review from the APSA Outcomes and Clini-cal Trials Committee.J Pediatr Surg. 2012;
47(11):2101–2110
24. Paraskakis E, Vergadi E, Chatzimichael A,
Bouros D. Current evidence for the
manage-ment of paediatric parapneumonic effusions.
Curr Med Res Opin. 2012;28(7):1179–1192
25. Marhuenda C, Barceló C, Molino JA, Guillén G,
Moreno A, Martínez X. Treatment of loculated
parapneumonic empyema. Video assisted
thoracoscopy or fibrinolytics? [in Spanish]. An Pediatr (Barc). 2011;75(5):307–313
26. Sonnappa S, Cohen G, Owens CM, et al.
Com-parison of urokinase and video-assisted
thoracoscopic surgery for treatment of
childhood empyema.Am J Respir Crit Care
Med. 2006;174(2):221–227
27. St Peter SD, Tsao K, Spilde TL, et al.
Thoracoscopic decortication vs tube
thoracostomy withfibrinolysis for empyema in children: a prospective, randomized trial
[published correction in J Pediatr Surg.
2009;44(9):1865].J Pediatr Surg. 2009;44(1):
106–111, discussion 111
28. Cobanoglu U, Sayir F, Bilici S, Melek M.
Comparison of the methods offibrinolysis by tube thoracostomy and thoracoscopic
decortication in children with stage II and
III empyema: a prospective randomized
study.Pediatr Rep. 2011;3(4):e29
29. Avansino JR, Goldman B, Sawin RS, Flum
DR. Primary operative versus nonoperative
therapy for pediatric empyema: a
meta-analysis.Pediatrics. 2005;115(6):1652–1659
30. Gasior AC, Knott EM, Sharp SW, Ostlie DJ,
Holcomb GW III, St Peter SD. Experience
with an evidence-based protocol usingfi -brinolysis as first line treatment for em-pyema in children.J Pediatr Surg. 2013;48
(6):1312–1315
(Continued fromfirst page)
Address correspondence to Claudia Marhuenda, MD, Servicio de Cirugía Pediátrica, Hospital Universitari Son Espases, Carretera de Valldemossa, 79, 07010 Palma de Mallorca, Spain. E-mail: claudia.marhuenda@ssib.es
PEDIATRICS (ISSN Numbers: Print, 0031-4005; Online, 1098-4275).
Copyright © 2014 by the American Academy of Pediatrics
FINANCIAL DISCLOSURE:The authors have indicated they have nofinancial relationships relevant to this article to disclose.
FUNDING:This clinical trial was funded by a Health Research Fund grant (EC07/90385) from the Institute of Health Carlos III, under the Spanish Ministry of Science and Innovation. The funding within the program of support for research unrelated to commercial interests was essential for completion of this study.
POTENTIAL CONFLICT OF INTEREST:Dr Moreno-Galdó has been an advisory board member of and has received institutional research funding from AbbVie Inc; has received funding for travel to conferences by Actelion, Novartis, Gilead, Bayer Pharma AG, and Ferrer Laboratories; and received a fee for speaking from Merck Sharp & Dohme. Dr Pérez-Yarza is an advisory board member of AbbVie Inc, has received institutional research funding from AbbVie Inc, and has received fees for speaking from Merck Sharp & Dohme. The other authors have indicated they have no potential conflicts of interest to disclose.
DOI: 10.1542/peds.2013-3935 originally published online October 27, 2014;
2014;134;e1301
Pediatrics
Matute, María A. García-Casillas, Víctor Álvarez and Antonio Moreno-Galdó
A.
Indalecio Cano, María López, Francisco Hernández, Eduardo G. Pérez-Yarza, José
Claudia Marhuenda, Concepció Barceló, Inmaculada Fuentes, Gabriela Guillén,
Services
Updated Information &
http://pediatrics.aappublications.org/content/134/5/e1301
including high resolution figures, can be found at:
References
http://pediatrics.aappublications.org/content/134/5/e1301#BIBL
This article cites 30 articles, 7 of which you can access for free at:
Subspecialty Collections
http://www.aappublications.org/cgi/collection/pulmonology_sub
Pulmonology
nt_sub
http://www.aappublications.org/cgi/collection/med_tech_advanceme
Medical Technology and Advancement
following collection(s):
This article, along with others on similar topics, appears in the
Permissions & Licensing
http://www.aappublications.org/site/misc/Permissions.xhtml
in its entirety can be found online at:
Information about reproducing this article in parts (figures, tables) or
Reprints
http://www.aappublications.org/site/misc/reprints.xhtml
DOI: 10.1542/peds.2013-3935 originally published online October 27, 2014;
2014;134;e1301
Pediatrics
Matute, María A. García-Casillas, Víctor Álvarez and Antonio Moreno-Galdó
A.
Indalecio Cano, María López, Francisco Hernández, Eduardo G. Pérez-Yarza, José
Claudia Marhuenda, Concepció Barceló, Inmaculada Fuentes, Gabriela Guillén,
Multicenter Clinical Trial
Urokinase Versus VATS for Treatment of Empyema: A Randomized
http://pediatrics.aappublications.org/content/134/5/e1301
located on the World Wide Web at:
The online version of this article, along with updated information and services, is
by the American Academy of Pediatrics. All rights reserved. Print ISSN: 1073-0397.