Radiotherapy Combined with Transarterial Infusion
Chemotherapy and Concurrent Infusion of a
Vasoconstrictor Agent for Nonresectable Advanced
Hepatic Hilar Duct Carcinoma
Shunro Matsumoto,M.D. Hiro Kiyosue,M.D. Eiji Komatsu,M.D. Masaki Wakisaka,M.D. Kenichiro Tomonari,M.D. Yuzo Hori,M.D. Akira Matsumoto,M.D. Hiromu Mori,M.D.
Department of Radiology, Oita Medical University, Oita, Japan.
Address for reprints: Shunro Matsumoto, M.D., Department of Radiology, Oita Medical University, Hasama-machi, Oita, 879-5593, Japan; Fax: (011) 81-97-586-0025; E-mail: matsushu@oita-med. ac.jp
Received September 19, 2003; revision received March 4, 2004; accepted March 12, 2004.
BACKGROUND.The treatment of patients with advanced hepatic hilar duct carci-noma is a challenging problem. The current study was performed to evaluate the outcome of patients with advanced hepatic hilar duct carcinoma who received external beam radiotherapy (EBRT) combined with transarterial chemotherapy and infusion of a vasoconstrictor.
METHODS.Between April 1993 and December 2002, 23 patients with histopatho-logically confirmed hilar duct carcinoma entered the study. The median total dose of EBRT was 41.4 grays (Gy). Transarterial chemotherapy was performed twice during EBRT. It was comprised of an infusion of a cocktail of 20 mg of epirubicin, 10 mg of mitomycin C, and 500 mg of 5-fluorouracil and was administered 1 minute after injection of epinephrine via a catheter introduced in the hepatic arteries. After the combined treatment, the patients underwent biliary endopros-thesis after evaluation of the initial response to treatment by percutaneous trans-hepatic cholangiography (PTC). The initial responses based on PTC were classified into four categories: CR, no stenosis; PR, relief of stenosis/obstruction; NC, no change; and PD, progressive stenosis/obstruction. The outcome parameters were survival rates and time, as well as frequency and type of complications.
RESULTS. Excluding 1 patient who discontinued the treatment, the initial re-sponses of 22 patients were 1 CR (5%), 8 PR (36%), 11 NC (50%), and 2 PD (9%). The response rate was 41%. The overall survival rates at 1 year, 2 years, and 3 years after treatment were 59%, 36%, and 18%, respectively.
CONCLUSIONS.The combination of radiotherapy, transarterial infusion chemother-apy, and concurrent infusion of a vasoconstrictor can be delivered safely with good efficacy for patients withadvanced hilar duct carcinoma.Cancer2004;100:2422–9.
© 2004 American Cancer Society.
KEYWORDS: bile duct carcinoma, hilar cholangiocarcinoma, radiotherapy, intraar-terial chemotherapy, vasoconstrictor.
C
arcinoma of the hepatic hilar duct is a rare malignant neoplasm associated with poor prognosis.1–7 Despite recent advances indiagnostic and therapeutic technologies, patients with carcinoma of the hepatic hilar duct are often diagnosed in the advanced stage and the majority (approximately 58 – 83%) of patients can only be treated palliatively.1–7 The reported median survival time for patients with
unresectable hilar duct carcinoma ranges from 3 to 11 months.4 –9
To our knowlege, there currently is no standard therapy to treat patients with advanced hilar duct carcinoma. A few investigators have reported a good initial response of hilar duct carcinoma using
exter-© 2004 American Cancer Society DOI 10.1002/cncr.20265
nal beam radiotherapy (EBRT) alone or in combina-tion with brachytherapy,10 –12but others reported that
EBRT had no apparent effect on either survival or late toxicity.6,7
Bile duct carcinoma is basically less chemosensi-tive compared with other tumors in the gastrointesti-nal tract. This is believed to be due to inherent cellular resistance or poor drug delivery.13 5-fluorouracil
(5-FU) is most commonly used as a single agent to treat patients with bile duct carcinoma and was reported to have response rates ranging from 10% to 20%.14
Har-vey et al.15reported that a combination of 5-FU,
mit-omycin C, and doxorubicin administered to 17 pa-tients with advanced or recurrent bile duct carcinoma was an effective (a 31% response rate) and safe treat-ment. Several types of malignant neoplasms, such as metastatic liver tumors and head and neck carcinoma, are treated with transarterial infusion chemothera-py.16,17 5-FU injected via the hepatic artery and
con-formal radiotherapy reportedly produced long-term freedom from hepatic carcinoma progression and sur-vival for patients with bile duct carcinoma in whom intraarterial hepatic 5-FU was used as a radiosensiti-zation.13 However, to our knowledge, no studies of
independent transarterial chemotherapy for hilar duct carcinoma have been reported.
It is well known that vasoconstriction of tumor blood vessels, which are immature, is defective due to the lack of both an adrenergic nerve supply and smooth muscles in the vessel wall.18 Several studies
have demonstrated that infusion of vasoconstrictors (including epinephrine, angiotensin II, vasopressin, and endothelin I) increases the tumor-to-liver blood flow ratio in liver metastasis and potentially enhances the tumor response rates to chemotherapy.19 –23
How-ever, to our knowledge there are no previous reports describing the effects of vasoconstrictors on extrahe-patic bile duct tumors.
Based on the poor prognosis of patients with non-resectable hilar duct carcinoma, a prospective treat-ment approach was developed at Oita Medical Uni-versity (Oita, Japan) in an attempt to improve the clinical outcome of these patients. Our approach com-bined radiotherapy and transarterial chemotherapy. In this treatment design, a vasoconstrictor was admin-istered to enhance the effect of the transarterial che-motherapy. The current study analyzes the effects of this combination treatment in patients with nonre-sectable hilar duct carcinoma.
MATERIALS AND METHODS
Patient Selection CriteriaThe patients included in the study satisfied the follow-ing criteria: histopathologically or cytologically
con-firmed cholangiocarcinoma; a performance status of 0 –2 as determined by the World Health Organization (WHO) criteria; and no active synchronous carcinoma. In addition, patients were required to have adequate bone marrow function (leukocyte countⱖ4000/L), a platelet count ⱖ 50,000/L, and adequate renal (se-rum creatinine concentration⬍1.5 mg/dL) and liver function after biliary drainage (total bilirubin [T-Bil] level ⱕ 4.0 mg/dL, serum transaminase level ⱕ 80 IU/L). Patients with distant metastases, active syn-chronous malignancy, or uncontrolled concurrent medical illness were excluded. The patients fully un-derstood the risks and benefits of the combination treatment and signed an informed consent form ac-cording to institutional regulations.
Patient Background
Sixty-four patients with suspected hilar duct carci-noma underwent diagnostic percutaneous transhe-patic cholangiography (PTC) between April 1993 and December 2002 (Table 1). Forty-one of these patients were excluded from the current study because 25 pa-tients were surgical candidates and 16 had unresect-able lesions but did not satisfy the above criteria. Therefore, 23 patients with unresectable hilar duct carcinoma entered the study. The study sample in-cluded 15 men and 8 women with a median age of 67 years (range, 48 – 83 years).
TABLE 1
Patient Characteristics
Characteristics No. of patients
Median age (yrs) (range) 67 (48–83) WHO performance status 0 7 1 14 2 2 Gender Male 15 Female 8 Anatomic locationa Type III 4 Type IV 19 Morphologic type Mass-forming 10 Infiltrating 13 LN enlargement LN0 11 LN1 9 LN2 3
WHO: World Health Organization. LN: lymph nodes; LN0: no enlargement of lymph nodes; LN1: enlargement of regional lymph nodes; LN2: enlargement of paraaortic lymph nodes.
Radiologic Evaluation
Extent of ductal involvement and portal/hepatic arterial involvement
These findings were assessed by PTC, computed to-mography (CT) scans, and angiography performed by three radiologists (S.M., H.K., and E.K.) and the final diagnosis was reached by consensus. Tumors were classified into one of four anatomic types using a modification of the original Bismuth–Corlette classifi-cation.3None of the lesions were classified as type I or
type II because these types represent resectable le-sions. Four lesions were classified as type III (lesions involving the biliary confluence and extending into one of the main hepatic ducts) and 19 lesions were classified as type IV (extension of the lesion to involve the secondary bile duct or the main trunk of the he-patic artery or portal vein).
Morphologic type
The hilar duct carcinomas were classified into two types after CT scans: infiltrative type and mass-form-ing type. The infiltrative type (n⫽13 carcinomas) was defined as a lesion with ductal wall thickness⬍5 mm without mass formation as observed on after contrast-enhanced CT scan. All other lesions were classified as the mass-forming type (n⫽10 carcinomas).
Lymph node enlargement
A positive finding of lymph node enlargement was determined as a maximum dimension of⬎10 mm as observed on a CT scan. Regional lymph nodes were classified as LN1 and paraaortic lymph nodes were classified as LN2. Twelve lesions (9 LN1 lesions and 3 LN2 lesions) were classified as a CT scan-positive lymph node enlargement.
Treatment Methods
Biliary drainage and endoprosthesis
All patients underwent percutaneous-transhepatic bil-iary drainage (PTBD) with two or three 7-French or 10-French drainage tubes. After the combined treat-ment, all patients underwent biliary endoprosthesis with self-expandable metallic stents after the evalua-tion of the initial response to treatment by PTC.
EBRT
The two opposing fields technique using 10-megavolt X-rays was employed. The clinical target volume was designed to adequately cover the tumor volume, with a margin ofⱖ2 cm, as well as the primary lymphatic drainage. The radiation fields were shaped on the basis of the findings of CT scan and PTC (Fig. 1). The
prescribed beam dose was 1.8 or 2.0 Gy per fraction, 5 times a week, for a total dose of 41.4 Gy.
Transarterial chemotherapy with concurrent infusion of a vasoconstrictor
Transarterial chemotherapy with concurrent infusion of a vasoconstrictor (TACCIV) was performed twice in the patients, at the beginning and during exposure to 24 –30 Gy of EBRT. There was a mean interval of 26 days (range, 20 –34 days) between the first and second TACCIV. The first TACCIV was performed 2–9 days before the initiation of ERBT. TACCIV was performed with a 4-French catheter introduced through the right femoral artery into the proper hepatic artery, common hepatic artery, or the origin of the right and left he-patic arteries.
To determine the interval between infusions of
FIGURE 1.Radiotherapy plan for patients with advanced hilar duct cancer. (A) The radiation field is determined by the findings of computed tomography scans and percutaneous transhepatic cholangiography and encompasses the tumor bed and regional lymph nodes. (B) Good dose distribution of the hilar duct carcinoma is obtained.
epinephrine and anticancer drugs, as well as the du-ration of the infusion of anticancer drugs, the first 4 patients underwent serial hepatic angiography and CT scans during hepatic angiography before and at 0 sec-onds, 30 secsec-onds, 60 –90 secsec-onds, 180 –240 secsec-onds, and 300 – 420 seconds after the infusion of epineph-rine. The concentration of contrast materials in the tumor was observed mainly from 1 minute to 3–5 minutes after the infusion of epinephrine (Fig. 2), in-fused at a dosage of 2– 4 g per injection via the catheter over a period of 1 minute. Based on the data described earlier, 1 minute after the infusion of epi-nephrine, administration of epirubicin (3–10 mg per injection), mitomycin C (1–5 mg per injection), and 5-FU (70 –250 mg per injection) commenced via the same catheter and continued for 3–10 minutes with confirmation of vasoconstriction status using periodic test injections of small volumes of contrast medium. This infusion was repeated two to five times,
depend-ing on the length of time for vasoconstriction. The total dosages of drugs used in the procedures for each treatment were 8 –10 g of epinephrine, 20 mg of epirubicin, 10 mg of mitomycin C, and 500 mg of 5-FU. The interval between the first infusion and the next infusion was approximately 3 minutes.
For the last 7 patients recruited to the study, fur-ther maintenance treatment with TACCIV was per-formed 2 or 3 times with an interval of 4 months, limited to within 1 year after the initial treatment. This was simply a protocol change adopted from June 2000 and was applied to the patients regardless of the initial treatment results provided informed consent was ob-tained from them.
Assessment Adverse side effects
Adverse side effects were evaluated using WHO stan-dard criteria.24Hematologic parameters were assessed
weekly and all other adverse reactions were evaluated during and after treatment.
Treatment effects
Two radiologists (S.M. and H.K.) independently as-sessed the initial treatment effects by PTC obtained within 1 week after treatment completion. Differences between the assessments of the two observers were resolved by consensus. The objective response based on PTC was classified into four categories as follows: complete response (CR), no stenosis; partial response (PR), relief of stenosis/obstruction; no change (NC), no significant change of stenosis/obstruction; pro-gressive deterioration (PD), propro-gressive stenosis/ob-struction. For patients who survived⬎6 months after treatment, the duration of the jaundice-free period was also assessed.
Statistical Analysis
The tumor responses for the various groups of pa-tients were evaluated by the chi-square test. If indi-vidual groups of patients comprised fewer than five patients in a 2⫻2 comparison, the Fisher exact test was utilized. The overall survival was measured from the time of study entry until death according to the Kaplan–Meier method. Univariate analysis of the prognostic factors was performed with log-rank tests.
P⬍0.05 denoted the presence of a statistically signif-icant difference.
RESULTS
Treatment Completion Rate
One patient (type IV; mass-forming type; LN2) discon-tinued the combined treatment at a dosage of 26 Gy of EBRT and 1 cycle of TACCIV because of hematologic
FIGURE 2.Common hepatic arteriograms were obtained (A) before and (B) after the infusion of epinephrine. Although the normal hepatic arteries are constricted by the epinephrine, the tumor vessels (arrow) are not.
toxicity (Grade 4 leukocytopenia and Grade 4 throm-bocytopenia) and severe nausea/emesis. She im-proved approximately 1 month later, after medical treatment. Therefore, this combined therapy was completed according to schedule in 22 patients, a treatment completion rate of 96% (22 of 23 patients). The side effects and treatment effects mentioned are based on the 22 patients who completed the treat-ment.
Adverse Side Effects
Hematologic toxicity was assessed based on the WHO classification. Acute and subacute adverse side effects less than Grade 3 were observed in 4 of 22 patients (18%). All improved within 2 weeks without any treat-ment. Grade 3 leukocytopenia and Grade 4 thrombo-cytopenia were observed in one patient each just be-fore the completion of the combination treatment. The patient with Grade 4 thrombocytopenia was treated with a blood platelet transfusion. With regard to other forms of Grade 3 toxicity, one patient devel-oped acute cholangitis after completion of the combi-nation treatment. This was believed to be due to an infection in the PTBD tube and the patient recovered after tube replacement. Grade 1/2 complications were observed in six patients. Of these, gastric ulceration and gastritis were observed in two patients each after the second session of TACCIV and improved with medical treatment such as H2-blockers. In addition, acute cholecystitis and cholangitis were observed in one patient each after the completion of the treat-ment. The acute cholecystitis was considered to be the result of a transient occlusion of the cholecystic duct because it developed just after the biliary endopros-thesis. These complications improved after intrave-nous administration of antibiotics.
Treatment Effects
The objective responses based on PTC consisted of CR in 1 patient (5%), PR in 8 patients (36%), NC in 11 patients (50%), and PD in 2 patients (9%). There was a 41% initial response rate. With regard to the anatomic types, the objective response was better in patients with Bismuth type III (100%; 4 PR) than in patients with Bismuth type IV (28%; 1 CR, 4 PR, 11 NC, and 2 PD) (P⫽0.01). With regard to the morphologic type, the objective response was better in 12 patients clas-sified as having infiltrative-type tumors (58%; 1 CR, 6 PR, and 5 NC) than in the 10 patients classified as having mass-forming type tumors (20%; 2 PR, 6 NC, and 2 PD;P⫽0.09).
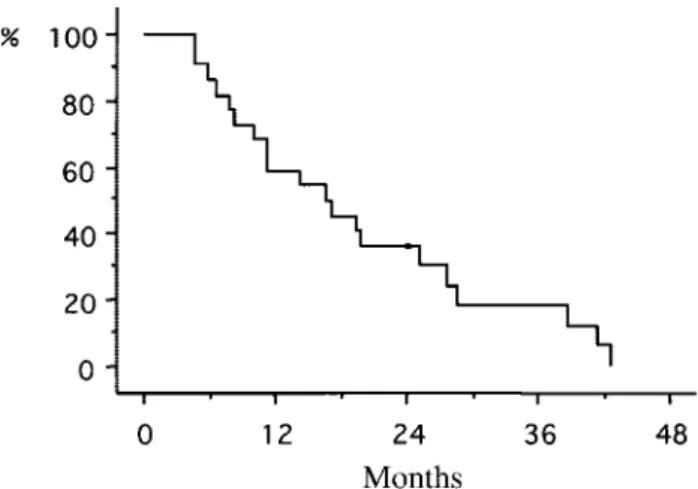
The overall survival rates (Kaplan–Meier) for all 22 patients are shown in Figure 3. The median follow-up was 17 months (range, 5– 43 months) and the 1-year,
2-year, and 3-year survival rates for the entire cohort were 59%, 36%, and 18%, respectively. The median survival time was 19.5 months. In patients with the infiltrative-type tumor, the survival rates were 75% at 1 year, 50% at 2 years, and 32% at 3 years, which were significantly better than the survival rates for patients with the mass-forming type tumor (40% at 1 year, 20% at 2 years, and 0% at 3 years;P⫽0.01). The median survival time also was found to be better in patients with the infiltrative-type tumor (25.7 months vs. 12.9 months; Fig. 4). For patients with Bismuth type III versus Bismuth type IV tumors, the 1-year survival rates were 75% versus 56% (Fig. 5). There were no significant differences with regard to the survival rates based on the Bismuth type (P ⫽ 0.15). The 1-year survival rates for patients with LN0, LN1, and LN2
FIGURE 3.Overall survival curve by the Kaplan–Meier method (median follow-up, 17 months; range, 5– 43 months).
FIGURE 4.Cumulative survival curves were constructed for patients with hilar ductal carcinoma by morphologic type. There is a significant difference in survival noted between the infiltrating type and the mass-forming type (P ⫽0.01). Solid line: infiltrative type; dashed line: mass-forming type.
disease were 64%, 67%, and 0%, respectively. The me-dian survival time was worse for patients with LN2 (7.1 months) compared with patients with LN0 (22.7 months) or LN1 (18.3 months; Fig. 6). The survival time was ⬎ 3 years in 3 patients (38.6 months, 41.4 months, and 42.5 months, respectively). Log-rank analysis did not identify a significant independent prognostic factor.
We also evaluated the duration of jaundice during the period from biliary endoprosthesis to recurrent jaundice among patients who survived ⬎ 6 months after treatment. At the time of last follow-up, 18 pa-tients were still alive at or 6 months after the
comple-tion of the combinacomple-tion treatment. Four of these 18 patients (22%) required re-PTBD because of recurrent obstructive jaundice. The median jaundice-free dura-tion was 14 months (range, 4 – 41 months).
DISCUSSION
Despite recent developments in diagnostic and ther-apeutic technology, carcinoma of the hepatic hilar duct remains among the most difficult malignancies to treat. The survival rates after surgical resection are better than those after palliative treatment and were reported to be 45– 87% at 1 year, 21–30% at 3 years, and 11–21% at 5 years.2–5 However, curative surgical
resection can be performed in only 30 – 40% of pa-tients with hilar duct carcinoma, even in recent re-ports.2,5Patients with unresectable hilar duct
carcino-mas often are treated by biliary drainage with or without radiotherapy.8 –11 The reported median
sur-vival for patients who received palliative biliary drain-age alone ranged from 3– 8 months.8,9
Several retrospective studies demonstrated that radiotherapy prolonged survival in these patients.10,11
However, to our knowledge, most of these studies included patients with common or distal bile duct carcinoma, who have a better prognosis than patients with hilar duct carcinoma. In 1985, Karani et al.12
reported excellent results after brachytherapy alone for hilar duct carcinoma. In their study, the median survival was 16.8 months. However, the clinical char-acteristics of their patients might be different from those enrolled in the current study because the resect-ability of hilar duct carcinoma has improved based on advances in surgical techniques in recent years.
The results of the current study showed favorable treatment results (median survival, 19.5 months; sur-vival rate at 1 year, 59%). Although there was no evi-dence of the major factor that affected the treatment results, the possibility exists that the concurrent infu-sion of a vasoconstrictor with the anticancer agents led to these good treatment effects.19 –23Several
vaso-constrictors (including epinephrine, angiotensin II, vasopressin, and endothelin I) have been used for this purpose in experimental studies.19,20 In the current
study, we used epinephrine as the vasoconstrictor agent because it is easily available and often used in angiography. The main disadvantage of this agent for use with chemotherapy is its short duration (1–3 min-utes) of effective vasoconstriction in the normal he-patic parenchyma. To our knowledge, the effects and toxicity of continuous injection of epinephrine cur-rently are unknown. In the current study, serial injec-tions of small amounts of the vasoconstrictor agent were applied with anticancer agents. Recent experi-mental studies of continuous injection of various
va-FIGURE 5.Cumulative survival curves constructed for patients with hilar ductal carcinoma by anatomic tumor type (according to the modification of the original Bismuth–Corlette classification). There is no significant difference noted between the survival rates of patients with Bismuth type III and type IV (P⫽0.15). Solid line: type III; dashed line: type IV.
FIGURE 6.Cumulative survival curves constructed for patients with hilar ductal carcinoma by lymph node (LN) factor. The LN2 group had a poor survival compared with the LN0 and LN1 groups. Solid line: LN0 group; dashed line: LN1 group; mixed solid and dashed line: LN2 group.
soconstrictor agents such as angiotensin II, vasopres-sin, and endothelin I showed that the tumor-to-normal liver ratio was increased transiently during the infusion of each agent, except for endothelin I, which increased the ratio throughout the infusion.19
Pro-longed infusion is favorable for influencing certain anticancer agents such as 5-FU. Therefore, prolonged infusion chemotherapy with concurrent infusion of long-acting vasoconstrictors such as endothelin I may enhance the effects of anticancer agents.
Several factors, such as radiation dose, local tu-mor extension, tutu-mor volume, and lymph node me-tastasis, influence the prognosis of patients with hilar duct carcinoma. Several groups demonstrated that the radiation dose was well correlated with the survival of patients with bile duct carcinoma.25,26Therefore, most
patients with bile duct carcinoma in recent studies were treated using combined EBRT and intraluminal brachytherapy. Some of these studies demonstrated the utility of a boost brachytherapy for prolongation of survival.26 The radiation dose escalation protocol
might increase the risk of treatment-related complica-tions such as the development of gastroduodenal ul-cers, bowel stricture, biliary-enteric fistulas, and cholangitis. Gonzalez et al.27warned against radiation
dose escalation. They investigated the effects and complications associated with brachytherapy com-bined with EBRT with a total dose of 50 – 68 Gy in 109 patients with proximal bile duct carcinoma (including 71 patients who received tumor resection). In their study, brachytherapy was not superior to EBRT alone and, therefore, they concluded that high radiation doses could be dangerous and have a detrimental effect on the prognosis.
In the current study, we identified some factors that significantly influenced the prognosis of patients. The prognosis of patients with a mass-forming type tumor was poorer compared with patients with an infiltrative-type tumor (median survival: 25.7 vs. 12.9 months). Because the tumor volume in the mass-forming type most likely is larger than that in the infiltrative type, EBRT with a mean dose of 40 Gy in the current series might be less effective in patients with a mass-forming type tumor than in those with an infiltrative type tumor. In the mass-forming type, an escalation radiation dose with brachytherapy might be useful. However, the effective target dosage with brachytherapy cannot cover the entire tumor. Re-cently, the application of stereotactic radiotherapy to hepatic tumors has been reported.28,29These studies
described good local control of tumors and suggested that the method might become a feasible treatment for hepatic tumors in the future. The addition of ste-reotactic radiotherapy to the adequate radiation dose
in our protocol should enhance the local control of hilar duct carcinoma, especially of the mass-forming type.
The current study has some limitations. First, there were not enough patients to clarify the useful-ness of this treatment protocol. Second, this was not a prospective comparative study setting with a control group. Therefore, the major factor causing the favor-able treatment results was unclear. A prospective comparative trial is required to evaluate the relative efficacy of this treatment protocol. For example, a group of patients treated with radiation alone and a group treated with combination therapy of radiation and transarterial chemotherapy without the infusion of a vasoconstrictor should be prepared as controls.
Although further studies with larger population samples and a prospective comparative study are re-quired, the combination of radiotherapy and TACCIV is believed to be an effective and safe treatment for patients with advanced hepatic hilar duct carcinoma and appears to improve the clinical outcome of these patients.
REFERENCES
1. Chamberlain RS, Blumgart LH. Hilar cholangiocarcinoma: a review and commentary.Ann Surg Oncol. 2000;7:55– 66. 2. Lillemoe KD, Cameron JL. Surgery for hilar
cholangiocarci-noma: the Johns Hopkins approach.J Hepatobiliary Pan-creat Surg. 2000;7:115–121.
3. Bismuth H, Nakache R, Diamond T. Management strategies in resection for hilar cholangiocarcinoma.Ann Surg. 1992; 215:31–38.
4. Figueras J, Llado L, Valls C, et al. Changing strategies in diagnosis and management of hilar cholangiocarcinoma.
Liver Transplant. 2000;6:786 –794.
5. Bathe OF, Pacheco JT, Ossi PB, et al. Management of hilar bile duct carcinoma.Hepatogastroenterology. 2001;48:1289 – 1294.
6. Pitt HA, Nakeeb A, Abrams RA, et al. Perihilar cholangiocar-cinoma: postoperative radiotherapy does not improve sur-vival.Ann Surg. 1995;221:788 –798.
7. Jarnagin WR, Fong Y, DeMatteo RP, et al. Staging, resect-ability, and outcome in 225 patients with hilar cholangio-carcinoma.Ann Surg. 2001;234:507–517.
8. Nordback IH, Pitt HA, Coleman J, et al. Unresectable hilar cholangiocarcinoma: percutaneous versus operative pallia-tion.Surgery. 1994;115:597– 603.
9. Gibson RN, Yeung E, Hadjis N, et al. Percutaneous transhe-patic endoprostheses for hilar cholangiocarcinoma.Am J Surg. 1988;156:363–367.
10. Ohnishi H, Asada M, Shichijo Y, et al. External radiotherapy for biliary decompression of hilar cholangiocarcinoma.
Hepatogastroenterology. 1995;42:265–268.
11. Kuvshinoff BW, Armstrong JG, Fong Y, et al. Palliation of irresectable hilar cholangiocarcinoma with biliary drainage and radiotherapy.Br J Surg. 1995;82:1522–1525.
12. Karani J, Fletcher M, Brinkley D, Dawson JL, Williams R, Nunnerley H. Internal biliary drainage and local radiother-apy with iridium-192 wire in treatment of hilar cholangio-carcinoma.Clin Radiol. 1985;36:603– 606.
13. Price P. Cholangiocarcinoma and the role of radiation and chemotherapy.Hepatogastroenterology. 2001;48:51–52. 14. Oberfield RA, Rossi RL. The role of chemotherapy in the
treatment of bile duct cancer.World J Surg. 1988;12:105– 108.
15. Harvey JH, Smith FP, Schein PS. 5-Fluorouracil, mitomycin (FAM) in carcinoma of the biliary tract.J Clin Oncol. 1984; 2:1245–1248.
16. Skitzki JJ, Chang AE. Hepatic artery chemotherapy for colo-rectal liver metastasis: technical considerations and review of clinical trials.Surg Oncol. 2002;11:123–135.
17. Fuwa N, Ito Y, Matsumoto A, et al. A combination therapy of continuous superselective intraarterial carboplatin infusion and radiation therapy for locally advanced head and neck carcinoma.Cancer. 2000;89:2099 –2105.
18. Ashraf S, Loizidou M, Crowe R, Trumaine M, Taylor I, Burn-stock G. Blood vessels in liver metastasis from both sarcoma and carcinoma lack perivascular innervation and smooth muscle cells.Clin Exp Metastasis. 1997;15:484 – 498. 19. Dworkin MJ, Carnochan P, Allen-Mersh TG. Effect of
con-tinuous regional vasoactive agent infusion on liver metas-tasis blood flow.Br J Cancer. 1997;76:1205–1210.
20. Hafstrom L, Nobin A, Persson B, Sundqvist K. Effect of catecholamines on cardiovascular response and blood flow distribution to normal tissue and liver tumors in rats.Cancer Res. 1980;40:481– 485.
21. Goldberg JA, Thomson JAK, Bradnam MS, et al. Angiotensin II as a potential method of targeting cytotoxic-loaded
mi-crospheres in patients with colorectal liver metastasis.Br J Cancer. 1991;64:114 –119.
22. Burke D, Davies MM, Zweit J, et al. Continuous angiotensin II infusion increases tumor:normal blood flow ratio in colo-rectal liver metastases.Br J Cancer. 2001;85:1640 –1645. 23. Hemingway DM, Angerson WJ, Anderson JH, Goldberg JA,
McArdle CS, Cooke TG. Monitoring blood flow to colorectal liver metastasis using laser Doppler flowmetry: the effect of angiotensin II.Br J Cancer. 1992;66:958 –960.
24. Miller AB, Hoogstranten B, Staquet M, Winkler A. Reporting results of cancer treatment.Cancer. 1981;47:207–214. 25. Hishikawa Y, Shimada T, Miura T, Imajyo Y. Radiation
ther-apy of carcinoma of the extrahepatic bile ducts.Radiology. 1983;146:787–789.
26. Foo ML, Gunderson LL, Bender CE, Buskirk SJ. External radiation therapy and transcatheter iridium in the treatment of extrahepatic bile duct carcinoma.Int J Radiat Oncol Biol Phys. 1997;39:929 –935.
27. Gonzalez GD, Gouma DJ, Raus EA, Van Gulik TM, Bosma A, Koedooder C. Role of radiotherapy, in particular intralumi-nal brachytherapy, in the treatment of proximal bile duct carcinoma.Ann Oncol. 1999;10:215–220.
28. Herfarth KK, Debus J, Lohr F, et al. Stereotactic single-dose radiation therapy of liver tumors: results of a phase I/II trial.
J Clin Oncol. 2001;19:164 –170.
29. Herfarth KK, Debus J, Lohr F, Bahner ML, Wannenmacher M. Stereotactic irradiation of liver metastasis. Radiologe. 2001;41:64 – 68.