Pharmacological Treatment of Urinary Incontinence
Lucio M.A. Cipullo, MD, Fulvio Zullo, MD, Cosimo Cosimato, MD, Attilio Di Spiezio Sardo, MD,
Jacopo Troisi, MD, and Maurizio Guida, MD
Abstract: We present an overview of the current pharmacological treatment of urinary incontinence (UI) in women, according to the latest evidence available. After a brief description of the lower urinary tract re-ceptors and mediators (detrusor, bladder neck, and urethra), the potential sites of pharmacological manipulation in the treatment of UI are discussed. Each class of drug used to treat UI has been evaluated, taking into account published rate of effectiveness, different doses, and way of administration. The prevalence of the most common adverse effects and overall compli-ance had also been pointed out, with cost evaluation after 1 month of treat-ment for each class of drug. Moreover, we describe those newer agents whose efficacy and safety need to be further investigated. We stress the importance of a better understanding of the causes and pathophysiology of UI to ensure newer and safer treatments for such a debilitating condition. Key Words:urinary incontinence, pharmacological treatment, anticholinergic, USA pharmacotherapy cost evaluation (Female Pelvic Med Reconstr Surg2014;20: 185Y202)
T
he termurinary incontinence(UI) refers to any type of in-voluntary urinary loss [International Continence Society (ICS)]. Urinary incontinence has an observed prevalence of 25%, and this tends to increase with age.1Normal functions of the female lowerurinary tract are the storage of urine within the bladder and the timely release during micturition at appropriate intervals. Bladder and urethra act together as a functional unit during filling and voiding phases. Effective micturition and bladder control both require a well-functioning nervous system altogether with local regulatory factors.2Functional impairment at various levels may
result in bladder control disorders, which can be roughly classi-fied as disturbances of storage and disturbances of emptying. The various types of UIs in women, according to the ICS, are reported in Table 1.3The annual incidence of UI in women ranges from 2%
to 11%, with the highest incidence occurring during pregnancy. Rates of complete remission of UI range from 0% to 13%, with the highest remission rates after pregnancy.4The annual incidence
of overactive bladder (OAB) ranges from 4% to 6%, with annual remission rates of OAB ranging from 2% to 3%.5Although
con-servative and behavioral therapy are important in the UI manage-ment in women, many patients may benefit from pharmacological therapy. The aim of this article was to provide an overview of the current therapeutical options in the pharmacological management of women with UI. New developments in the drug treatment of this condition are also pointed out. Drug therapy recommenda-tions are based on the Oxford Classification System for Levels of Evidence.6The prices of the single drugs reported in this article
and the weighted average cost for a monthly treatment are based on the US rates.
FUNCTIONAL ANATOMY AND NEUROPHYSIOLOGY OF THE LOWER
URINARY TRACT
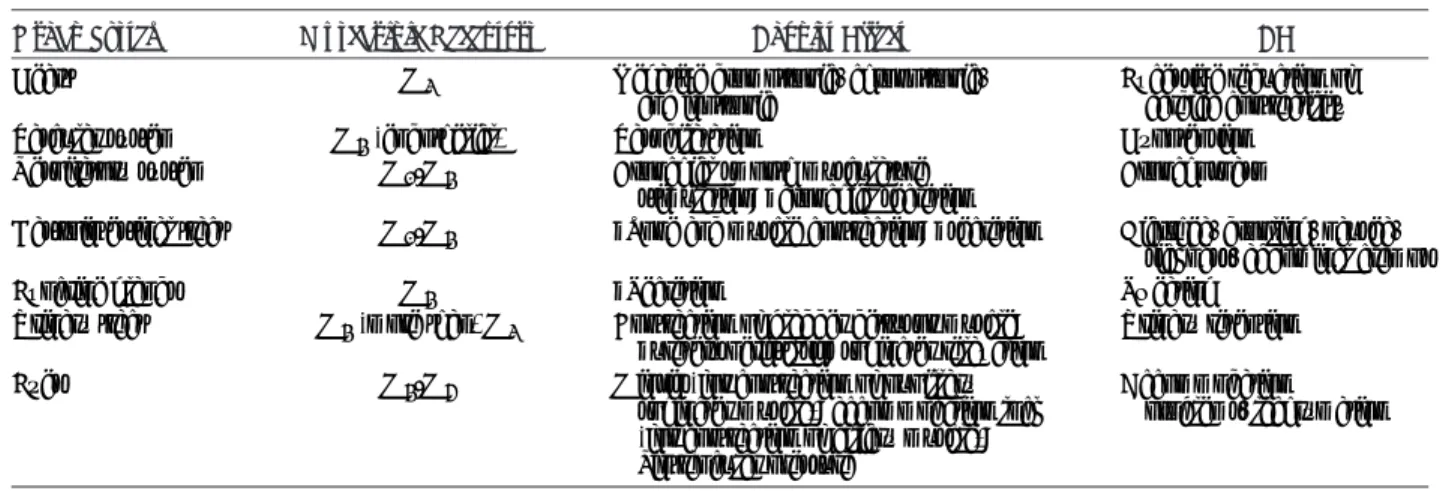
The female bladder and urethra have a somatic (pudendal) and an autonomic (sympathetic and parasympathetic) innervation (Fig. 1). The pudendal nerve arises from the sacral nerve roots S2YS4 and supplies the external urethral sphincter. The parasympathetic nerves, which govern bladder contraction, are derived from the second, third, and fourth sacral segments of the spinal cord to the detrusor muscle. Sympathetic nerve supply of the bladder origi-nates in the intermediolateral nuclei in the thoracolumbar region (T10-L2) of the spinal cord. Bladder storage and voidance involve a complex interplay of efferent and afferent signals in a way that parasympathetic, sympathetic, somatic, and sensory nerves can work synergically.7 Contraction of the detrusor smooth
mus-cle and relaxation of the outflow region result from the activation of parasympathetic neurons (S2YS4). The predominant effects of the sympathetic nerves in the lower urinary tract are inhibition of the parasympathetic pathways at spinal and ganglion levels and mediation of contraction of the bladder base and the urethra. During bladder filling, the outlet is closed, and the bladder smooth muscle is quiescent. When bladder volume increases to the mictu-rition threshold, the activation of a mictumictu-rition center in the dorso-lateral pons (the pontine micturition center) elicits bladder contraction and the reciprocal relaxation of the urethra, leading to bladder emptying. During voiding, sacral parasympathetic pelvic nerves provide an excitatory input (cholinergic and purinergic) to the bladder and an inhibitory input (nitrergic) to the urethra (Fig. 2). These peripheral networks are integrated by means of a feedback and feedforward regulation at spinal cord and brain levels.8There is
increasing evidence showing that the urothelium has got specialized sensory and signaling properties, including expression of nicotinic, muscarinic, tachykinin, adrenergic, bradykinin, and transient re-ceptor potential rere-ceptors, close physical association with the af-ferent nerves, and ability to release chemical molecules such as adenosine triphosphate (ATP), acetylcholine (ACh), and nitric oxide (NO).8Y10At present, the functional role of the muscarinic
receptors in the urothelium has largely been indirectly investi-gated, that is, by studying the effects after urothelium removal or administration of pharmacological inhibitors. Thus, it seems that the muscarinic receptors in the urothelium also contribute to the overall bladder function regulation, but their specific roles have not been fully established.11The parasympathetic pelvic nerves
stimulate the detrusor muscle via the muscarinic receptors M2and
M3. These receptors are activated by ACh, whereas purinergic
receptors (P2X1) are activated by ATP, inducing relaxation of the urethral smooth muscle, mediated by NO. Apparently, most musca-rinic receptors in the bladder are found on the detrusor smooth muscle cells. Although the detrusor expresses far more M2(80%)
than M3(20%) receptors, it seems that detrusor contraction under
physiological conditions is largely (if not exclusively) mediated by the M3receptor.12Y16The>1-adrenoceptors (ARs) and the
3A-AR subtypes (A1,A2, andA3) have been investigated in the
human detrusor. Human urothelium as well contains all 3 receptor subtypes.17,18Real-time PCR and immunostaining revealed high
concentrations ofA3-AR throughout the urothelium, the detrusor
From the Department of Gynecology and Obstetrics of San Giovanni di Dio and Ruggi d’Aragona Hospital, University of Salerno, Salerno, SA, Italy. Reprints: Lucio M.A. Cipullo, MD, University of Salerno, Largo Ippocrate
n-1, 84131 Salerno, SA, Italy. E-mail: luciocipullo@hotmail.com. The authors have declared they have no conflicts of interest. Copyright*2014 by Lippincott Williams & Wilkins DOI: 10.1097/SPV.0000000000000076
smooth muscle, and the peripheral nerves,19Y21and the functional
evidence of their important role in both normal and neurogenic bladders is convincing.22Y28The human detrusor also contains
A2-ARs: most probably, both receptors are involved in the
physi-ological effects (relaxation) of noradrenaline in this structure.21,29,30
Sympathetic postganglionic neurons release noradrenaline (NA), which activates A3 adrenergic receptors to relax the bladder
smooth muscle and activates>1-adrenergic receptors to contract
the urethral smooth muscle. Somatic axons in the pudendal
nerve also release ACh, which produces a contraction of the ex-ternal sphincter striated muscle by activating nicotinic cho-linergic receptors. Parasympathetic postganglionic nerves also release ATP, which excites the bladder smooth muscle, and NO, which relaxes the urethral smooth muscle. Targets of the stress urinary incontinence (SUI) pharmacological treatment are the> andA-ARs, whereas in the OAB/urgency urinary incontinence (UUI) treatment, the site of interaction is the muscarinic receptor (Figs. 3 and 4).
TABLE 1. Types of UI in Women (Modified)* Type of Incontinence Definition/Symptoms SUI Involuntary urine loss during
physical exertion/exercise (coughing, sneezing, sports) without urgency
Urge incontinence (UI)
Involuntary urine loss combined with sudden sensation of urgency YWith detrusor instability (formerly
motor urge incontinence)
YWithout detrusor instability (formerly sensory urge incontinence)
Mixed incontinence (MI)
Involuntary urine loss associated not only with urinary urgency but also with physical exertion. It can be with predominant SUI or UUI symptoms Special forms Among others, neurogenic incontinence,
extraurethral incontinence (eg, in the presence of fistula), overflow incontinence, giggle incontinence
*From Bump et al.3 FIGURE 2. Micturition reflexes. [Representation from Yoshimura
et al.8
FIGURE 1. Innervation of the lower urinary tract (LUT) (A) with relative distribution of the different classes of afferent fibers in the bladder wall and urethra (B) and efferent pathways and neurotransmitter mechanisms that regulate the LUT (C). Adapted from Kanai et al9 and Fowler et al.10
A thorough knowledge of the body distribution of muscarinic receptors is mandatory to start an appropriate pharmacological treatment (Table 2). It is important to keep in mind that drugs acting on the muscarinic receptors are not specific for those present in the lower urinary tract.2,12,13This suggests that these drugs may cause
systemic adverse effects (AEs) (Tables 2b and 2c). Theoretically, drugs with selectivity for the bladder could be obtained if the receptor subtype(s) mediating bladder contraction and those
producing the main AEs of antimuscarinic drugs were different. Unfortunately, this does not seem to be the case.
PHARMACOTHERAPY TREATMENT OF OAB AND UUI
Overactive bladder is the term used to describe the symptom complex of urgency, with or without urge incontinence, usually with frequency and nocturia. Overactive bladder symptoms are FIGURE 3. Continence with drugs for UUI (pooled with random effects from RCTs). [Representation from Shamliyan et al.31
FIGURE 4. Treatment discontinuation due to AEs from drugs for UUI (pooled results from RCTs by using rate arcsine transformation). [Representation from Shamliyan et al.31
TABLE 2A. Distribution of Muscarinic Receptors
Type
G Protein and Transduction Mechanism
Location
(Decreasing Concentration) Agonists Antagonists
M1 Gq/11 Brain (cortex, hippocampus);
spinal cord; salivary glands
Ach Atropine
jIP3, DAG Oxotremorine Scopolamine
,K+conductance Muscarine Diphenhydramine
jCa2+conductance Carbachol Dimenhydrinate
Dicycloverine Thorazine Tolterodine Oxybutynin Ipratropium Pirenzepine Telenzepine Chlorpromazine Haloperidol
M2 Gi/o Heart; brainstem, cerebellum;
gastrointestinal (GI), bladder
ACh Atropine
,cAMP Methacholine Dicycloverine
Conductance: Carbachol Thorazine
,Ca2+jK+ Oxotremorine Diphenhydramine Muscarine Dimenhydrinate Tolterodine Oxybutynin Ipratropium Methoctramine Tripitramine Gallamine Chlorpromazine
M3 Gq/11 Exocrine glands, bladder; GI wall
smooth muscle and sphincters
ACh Atropine
jIP3, DAG Bethanechol Diphenhydramine
jCa2+conductance Carbachol Dimenhydrinate
Oxotremorine Dicycloverine Pilocarpine Tolterodine Oxybutynin Ipratropium Darifenacin Tiotropium
M4 Gi/o CNS ACh Atropine
,cAMP Carbachol Diphenhydramine
Conductance: Oxotremorine Dimenhydrinate
,Ca2+jK+ Dicycloverine
Tolterodine Oxybutynin Ipratropium
M5 Gq/11 CNS; ciliary muscle of eye ACh Same to M4
Carbachol Oxotremorine
due to involuntary contractions of the detrusor muscle during the filling phase of the micturition cycle. These involuntary contrac-tions are termeddetrusor overactivity(DO) and are mediated by ACh-induced stimulation of the bladder muscarinic receptors. Urgency urinary incontinence is an involuntary loss of urine as-sociated with urgency. Urodynamic testing shows an involuntary leakage from the urethra synchronous with the sensation of a sudden, compelling desire to void that is difficult to defer; during filling cystometry, involuntary detrusor muscle contractions can be detected.31Drug therapy continues to have an integral role in
the management of women with OAB, and there are now a number of different agents available (Table 3). Despite the clinical advantages of many of the pharmacological agents available, the occurrence of significant AEs and impaired compliance has lim-ited their use. The availability of bladder selective drugs in the near future, once daily dosing and differing the routes of admin-istration, may increase the compliance. However, in many cases, drugs may be considered as an adjunct to conservative therapy. An optimal treatment should be individualized, taking into con-sideration a number of factors, such as the patient’s comorbidities
and concomitant medications, together with the pharmacological profiles of different drugs.
DRUGS WITH MIXED ACTION Oxybutynin
Oxybutynin (Table 4a) is a tertiary amine that undergoes an extensive first-pass metabolism to its active metabolite, N-desmethyl oxybutynin, which occurs in high concentrations and is thought to be responsible for a significant part of the action of the parent drug. T1/2= 2 hours. It has a mixed action consisting of32,33:
- Nonselective antimuscarinic = M1 and M399M2 is its primary mechanism of action;
- Direct smooth muscle relaxation (antispasmodic) = may involve blockage of Ca2+channels;
- Local anesthetic properties = important only for intravescical infusion;
- Antihistamine properties.
TABLE 2B. Muscarinic Agonist Effect and Related AEs in Different Districts
Organ System Muscarinic Receptors Agonist Effect AE
Heart M2 Negative dromotropic, chronotropic,
and inotropic
Excessive reduction of cardiac contractility
Vascular system M3(endothelial) Vasodilatation Hypotension
Respiratory system M1-M3 Bronchial smooth musculature
stimulation;jbronchial secretion
Bronchospasm
Gastrointestinal tract M1-M3 jTone and muscle contraction;jsecretion Diarrhea, drooling, nausea,
sickness, abdominal cramps
Exocrine glands M3 jSecretion Sweating
Urinary tract M3(more than) M2 Contraction of bladder detrusor muscle;
jureters peristalsis; sphincter relaxation
Urinary retention
Eyes M3-M5 Miosis (for contraction of pupillary
sphincter muscle); accommodation lock (for contraction of ciliary muscle); ,intraocular pressure
Accommodation problems, lachrymation
TABLE 2C. Muscarinic Antagonist Effect and Related AE in Different Districts
Organ System Muscarinic Receptors Antagonist Effect AE
Heart M2 jHeart rate Tachycardia (for vagal block);
arrhythmias Vascular system M3(endothelial) Slightly vasodilation
Respiratory system M1-M3 Bronchodilation;jbronchial
secretion; prevention of laryngospasm in anesthesia
Gastrointestinal tract M1-M3 ,Motility;,secretion Dry mouth; constipation;
Exocrine glands M3 ,Secretion
Urinary tract M3(more than) M2 ,Tone and contraction of bladder
and ureters;,urination rate
Urinary retention; difficulty voiding Eyes M1-M3-M5 Mydriasis; accommodation lock Cycloplegia; blurry vision
CNS M1-M2-M4-M5 ,Cognition = sedation, amnesia Delirium (rare); hallucination (?);
drowsiness Ganglia and autonomic
nerves
M1 Inhibition of slow postsynaptic
potentials;jrelease ACh (for lock of presynaptic receptors)
Oxybutynin has showed improvement in quality of life (QoL) and leakage episodes or voids in the 24 hours, when com-pared to placebo, in several trials. When comcom-pared to tolterodine, there are no differences in terms of clinical efficacy, but fewer withdrawals due to adverse events with tolterodine [relative risk (RR), 0.52; 95% confidence interval (CI), 0.40Y0.66] and less risk of dry mouth (RR, 0.65; 95% CI, 0.60Y0.71).34Y36
Oxybutynin-based drugs include immediate release (IR) formulation, extended
release formulation (ER or XL), syrup, and a transdermal release. The OROS-based oxybutynin is an ER formulation (Ditropan XL). Oxybutynin ER ensures a smoother plasma concentration-time profile and a lower maximum plasma concentration than those seen with oxybutynin IR. The ER formulation improves drug tolerability, facilitating one-daily intake. Long-term and short-term studies have reported significant improvements in health-related QoL with oxybutynin ER therapy. In addition, pharmacoeconomic studies have suggested that oxybutynin ER is more cost effective than oxybutynin IR, and at least as cost effective as tolterodine IR.37The use of oxybutynin gel elicits more local AEs compared
to placebo (6.8% vs 2.8%).38However, the gel formulation may
offer a better combination of reduced local and systemic AEs and is an alternative to oral preparations in those women who expe-rienced intolerable antimuscarinic AEs (Tables 4b, 4c, and 4d). Propiverine
Propiverine hydrochloride is a benzilic acid derivative com-pound. It is rapidly absorbed and has a high first-pass metabolism. Its mixed action involves antimuscarinic and Ca2+channel an-tagonism.33Propiverine has more recently been introduced as a
long-acting once-daily preparation and may be useful in those women unable to tolerate other antimuscarinic drugs. Adverse effects are dry mouth (14%) and other AEs not well documented.
ANTIMUSCARINIC DRUGS
Recent large meta-analyses of the most widely used anti-muscarinic drugs have clearly shown that these drugs provide a significant clinical benefit (Table 5).31,39,40However, none of the
commonly used antimuscarinic drugs are an ideal first-line treat-ment for OAB/DO patients. There is no consistent evidence that 1 antimuscarinic drug is superior to an alternative antimuscarinic drug for the cure or the improvement of UUI. An optimal treatment should be individualized, taking into consideration any associate conditions and concomitant medications and the pharmacological profiles of different drugs. Recommendations on the use of these drugs41are shown in Table 6.
Contraindications are uncontrolled narrow-angle glaucoma, significant cardiac arrhythmias, urinary retention, gastric retention, myasthenia gravis, severe renal and hepatic diseases, acquired cog-nitive impairment and dementia (Alzheimer disease), and con-comitant treatment with acetylcholinesterase (AChE) inhibitors.
The treatment of OAB/DO in the elderly deserves specific considerations. Overactive bladder is more common in the elderly, where it causes a detrimental effect on the QoL. It has been estab-lished that 30.9% of women older than 65 years have this condi-tion. Studies have shown that 40% of patients with Alzheimer dementia experience OAB.42 Many of these patients are taking
AChE inhibitors like donepezil, galantamine, and rivastigmine. AChE inhibitors fall under the parasympathomimetic group of medications, whose effects are antagonized by antimuscarinic drugs.43Overactive bladder treatment in patients taking AChE
inhibitors remains difficult. A pilot study44found improvement
in OAB symptoms with the use of propiverine hydrochloride in patients taking Donepezil for cognitive impairment, without changes in cognition. More research is required in this field. Solifenacin
This compound is a bladder-selective antimuscarinic33agent
that has greater specificity for the M3receptors over the M2
re-ceptors and has much higher potency against M3 receptors in
smooth muscle than it does against M3 receptors in salivary
glands (Table 7a). It shows a long half-life. Its long-term efficacy has been assessed in a 12-month follow-up study of 1637 patients.45 TABLE 3. Drugs Used in the Treatment of OAB/UUI*
(Modified)
Drug LoE GoR
Antimuscarinic drugs Darifenacin 1 A Fesoterodine 1 A Solifenacin 1 A Tolterodine 1 A Trospium 1 A Propantheline 2 B Atropine 3 C
Drugs acting on membrane channels
Calcium antagonist 2
K+-channel openers 2
Drugs with mixed actions
Oxybutynin 1 A Propiverine 1 A Antidepressants Duloxetine 2 C Imipramine 3 C >-Adrenoreceptor antagonists Alfuzosin 3 C Doxazosin 3 C Prazosin 3 C Terazosin 3 C Tamsulosin 3 C A-Adrenoreceptor agonists Mirabegron (A3) 2 B Albuterol (A2) 3 C Terbutaline (A2) 3 C COX inhibitors Indomethacin 2 C Flurbiprofen 2 C Toxins
Botulinum toxin (neurogenic), injected into bladder wall
2 A
Botulinum toxin (idiopathic), injected into bladder wall
3 B
Capsaicin (neurogenic), intravescical 2 C RTX (neurogenic), intravescical 2 C Other drugs
Baclofen, intrathecal 3 C
Hormones
Estrogen 2 C
Desmopressin, for nocturia. 1 A
*Assessments have been done according to the Oxford modified system and ICS.7
COX inhibitor indicates cyclooxygenase inhibitor; GoR, grade of recommendation; K+, potassium; LoE, level of evidence.
There was a significantly greater reduction in urgency episodes in the solifenacin arm compared to the placebo arm (PG0.001) and the difference in the median length of warning time was 31.5 seconds
in the solifenacin group compared to the 12.0 seconds of the placebo group (P= 0.032).38When compared with oxybutynin,
solifenacin showed a better efficacy with lower AEs.42There were
statistically significant differences in QoL, patient reported cure, all favoring solifenacin. The recommended starting dose of 5 mg once daily has been compared to 10 mg, showing lower rate of frequency and urgency. The dry mouth was significantly lower with solifenacin when compared to tolterodine. Solifenacin 5 mg once daily is the usual starting dose, which could be increased to
TABLE 4A. Oxybutynin Costs Overview
Drug Name Active Ingredient Dose Prices for 30 d of Treatment
Oxytrol patch (3.9 mg) Oxybutynin 36-mg patch applied twice weekly (every 3Y4 d); delivers 3.9 mg/24 h; rotate administration sites
(abdomen, hip, buttock)
$42.71
Gelnique (gel) Oxybutynin chloride 10% , 1 g applied daily to dry, intact skin; rotate application (abdomen, thigh, shoulder, upper arm)
$39.09
Anturol (Gel) Oxybutynin chloride 3%, 3 pumps (84 mg); applied as above; may rotate site if necessary
,$150 Ditropan XL (ER)
(5 mg) (10 mg)
Oxybutynin chloride 5Y10 mg q.d.; may be increased to a maximum of 30 mg/d; swallowed whole; should not be chewed, divided, or crushed
$88.76 (5 mg) $94.46 (10 mg)
Oxybutynin Cl ER (5 mg) (10 mg)
Oxybutynin chloride 2.5Y5 mg b.i.d; max 5 mg q.i.d. $24.3 (5 mg) $20.52 (10 mg) Oxybutynin Cl
(IR) (5 mg)
Oxybutynin chloride 2.5Y5 mg b.i.d. or t.i.d.; max 5 mg q.i.d. $4.03 Oxybutynin UD syrup
(200 mL) (480 mL)
Oxybutynin chloride Usual dose is 1 teaspoonful (5 mg/5 mL) b.i.d or t.i.d. Max q.i.d.
$37.04 (200 mL) $6.45 (480 mL) b.i.d. indicates twice daily; q.d., once daily; q.i.d., 4 times daily; t.i.d., 3 times daily.
TABLE 4B. Efficacy and Safety of Oxybutynin*
Conclusion SoE
&Increased continence rates and improved UI High &Increased treatment discontinuation due to AEs; dry mouth was the most common AE High &IR oxybutynin resulted in greater rates of AEs and dry mouth compared with controlled-release oral or transdermal oxybutynin Low &Higher vs lower doses resulted in greater improvement in UI, the same rates of dry mouth, and greater rates of treatment withdrawal Low
*Adapted from Shamliyan et al.31
SoE indicates strength of evidence.
TABLE 4C. Prevalence of AEs of Oxybutynin Compared With Placebo (Modified)*
AE RA, % RC, %
&Treatment failure 12.2 22.9
&Dry mouth 34 15
&Dry skin 10 10.4
&Blurred vision 10.4 9.1 &Constipation 7.3 5.5 &Discontinuation: AEs 10 5
&Headache 4.1 4.5
&Serious AEs 3.7 2.0 &Urine retention 3.2 0.5
&Dysuria 0.8 0.2
*Adapted from Shamliyan et al.31
RA indicates rate in active treatment group; RC, rate in control group.
TABLE 4D. Comparative Effectiveness*
Head to Head SoE
Oxybutynin vs tolterodine:
&Greater rate of treatment discontinuation due to AEs High &No difference in improvement in UI rates Moderate &Low adherence to drug treatment;950% of women
stopped treatments within 1 y
Moderate *Adapted from Shamliyan et al.31
10 mg once daily for better efficacy but with increased risk of dry mouth (Tables 7b and 7c).
Tolterodine
Tolterodine (Table 8a) is a tertiary amine which is rapidly absorbed. Its low lipid solubility implies a poor capacity to cross the blood-brain barrier.33T
1/2= 2Y3 hours. This drug is
metabo-lized into the liver to its 5-hydroxymethyl derivative, which is an active metabolite having a similar pharmacokinetic profile and is thought to significantly contribute to the therapeutic effect.46It is
a competitive muscarinic receptor antagonist with relative func-tional selectivity for bladder receptors,47and, although it shows no
specificity for receptor subtypes, it does seem to target the bladder over the salivary glands.48No differences in QoL and improvement
of leakage episodes are reported when comparing tolterodine with oxybutynin in 8 trials. Where the prescribing choice is between oral IR oxybutynin and tolterodine, tolterodine might be preferred
for reduced risk of dry mouth. The starting dose is 2 mg twice daily. The effects of 1-, 2-, and 4-mg doses were similar for leakage episodes and micturitions in the 24 hours, with a greater risk of dry mouth with the 2- and 4-mg doses at 2 to 12 weeks (Tables 8b and 8c).36
Trospium
Trospium chloride is a quaternary ammonium compound (this means that it crosses the blood-brain barrier to a limited extent and hence would seem to have few cognitive effects) that shows low biological availability (Table 9a).40T
1/2= 20 hours. Its
mechanism of action is nonselective for muscarinic receptor subtypes: it blocks detrusor smooth muscle receptors as well as receptors in the ganglia.33The efficacy and tolerability of once
daily trospium chloride has also been confirmed in a further large study of 564 patients with OAB49; this drug seems to have a
meaningful impact on QoL.50However, in 4 comparative studies, TABLE 5. Patient-Centered Clinically Important Outcomes With Pharmacologic Interventions for UUI Compared With Placebo (Modified)*
Outcome and Drug RCTs RA, % RC, % RR (95% CI) Absolute Risk Difference (95% CI) SoE Continence
&Fesoterodine 2 61 48.5 1.3 (1.1 to 1.5) 0.13 (0.06 to 0.20) Low &Oxybutynin 4 27 16 1.7 (1.3 to 2.1) 0.11 (0.06 to 0.16) High &Solifenacin 5 39.2 28.1 1.5 (1.4 to 1.6) 0.11 (0.06 to 0.16) High &Tolterodine 4 53.2 43.7 1.2 (1.1 to 1.4) 0.09 (0.04 to 0.13) High &Trospium 4 28.3 16.6 1.7 (1.5 to 2.0) 0.11 (0.08 to 0.14) High Clinically important
improvement in incontinence
&Darifenacin 3 48.4 33 1.3 (1 to 1.5) 0.12 (0.06 to 0.17) High &Fesoterodine 2 42 32 1.3 (1.2 to 1.5) 0.10 (0.06 to 0.15) High &Oxybutynin 9 53 32 1.5 (1.2 to 1.9) 0.17 (0.10 to 0.24) Moderate &Solifenacin 2 60.2 43 1.5 (1.0 to 2.1) 0.18 (0.10 to 0.26) Low &Tolterodine 7 45 37 1.3 (1.1 to 1.4) 0.10 (0.04 to 0.15) High &Trospium 2 32.4 25.4 1.1 (0.6 to 2.0) 0.08 (j0.10 to 0.25) Low
*Adapted from Shamliyan et al.31
RCT indicates randomized controlled trial.
TABLE 6. Recommendations for Antimuscarinic Drugs*
GR Offer IR or ER formulations of antimuscarinic drugs as initial drug therapy for adults with UUI. A If IR formulations of antimuscarinic drugs are unsuccessful for adults with urge urinary incontinence, offer ER formulations
or longer-acting antimuscarinic agents.
A Consider using transdermal oxybutynin if oral antimuscarinic agents cannot be tolerated due to dry mouth. B Offer and encourage early review (of efficacy and AEs) of patients on antimuscarinic medication for urge urinary incontinence (G30 d) A When prescribing antimuscarinic drugs to elderly patients, be aware of the risk of cognitive AEs, especially in those receiving
cholinesterase inhibitors.
C Avoid using oxybutynin IR in patients who are at risk of cognitive dysfunction. A Consider use of trospium chloride in patients known to have cognitive dysfunction. B Use antimuscarinic drugs with caution in patients with cognitive dysfunction. B Do an objective assessment of mental function before treating patients whose cognitive function may be at risk. C Check mental function in patients on antimuscarinic medication if they are at risk of cognitive dysfunction. C *From EAU Guidelines on Urinary Incontinence, edition presented at the 27th EAU Annual Congress, Milan 2013. ISBN 978-90-79754-71-7.
trospium seems not to be better than oxybutynin in terms of efficacy.51Y55It is available in oral IR and XR formulations. A
comparison that might benefit from further research is the eval-uation of trospium versus oxybutynin or tolterodine in terms of safety. Because trospium is a quaternary amine and oxybutynin and tolterodine are tertiary amines, we could expect less neuro-logical AEs in patients with cognitive impairment. Patients al-ready on multiple medications could benefit from this compound because of its low metabolism by liver enzymes (Tables 9b and 9c). Darifenacin
Darifenacin is a tertiary amine with a long half-life. It has moderate lipophilicity and is a highly selective M3 receptor
antagonist which has been found to have a 5-fold higher affin-ity for the human M3 receptor, compared to the M1 receptor
(Table 10a).56Its efficacy has been investigated in a multicenter,
double-blind, placebo-controlled, parallel-group study which en-rolled 561 patients with symptoms of OAB.57It is available for
oral intake. Significant decreases in frequency and severity of urgency, micturition frequency, and number of incontinence epi-sodes were also observed, along with an increase in bladder capacity. Darifenacin was well tolerated. The incidence of CNS
TABLE 7A. Solifenacin Costs Overview Drug Name Active Ingredient Dose Prices for 30 d of Treatment VESIcare Solifenacin succinate 5Y10 mg q.d.; swallowed whole with water $70.23 (5 mg) $70.62 (10 mg)
TABLE 7B. Efficacy and Safety of Solifenacin*
Conclusion SoE
&Increased continence rates and greater benefits with the higher dose in women with urgency and mixed UI
High
&Increased risk for dry mouth, constipation, and blurred vision; 10 mg increased
the risk for severe dry mouth and constipation
High
&Resulted in treatment discontinuation due to AEs more often than did placebo
High *Adapted from Shamliyan et al.31
TABLE 7C. Prevalence of AEs of Solifenacin Compared With Placebo (Modified)*
AE RA, % RC, %
&Treatment failure 27.7 30.1
&Dry mouth 21 5
&Dizziness 3 2
&Blurred vision 4 4
&Constipation 11 3
&Discontinuation: AEs 5 4
&Headache 3 4
&Urine retention 2.4 0.8 &Discontinuation: treatment failure 1.5 1.3
*Adapted from Shamliyan et al.31
TABLE 8A. Tolterodine Costs Overview Drug Name Active Ingredient Dose Price for 30 d of Treatment Detrol LA 2 mg (IR) Tolterodine tartrate 2Y4 mg q.d.; swallowed whole with liquid ,$77 (for 2 mg) Detrol LA 4 mg (IR) Tolterodine tartrate 4 mg q.d.; swallowed whole with liquid ,$80.89 Detrusitol XL 4 mg Tolterodine tartrate 4 mg q.d.; swallowed whole with liquid ,$87 Tolterodine tartrate 2 mg Tolterodine tartrate 2Y4 mg q.d.; swallowed whole with liquid ,$29 (for 2 mg) Tolterodine tartrate 4 mg Tolterodine tartrate 4 mg q.d.; swallowed whole with liquid ,$32.33
TABLE 8B. Efficacy and Safety of Tolterodine*
Conclusion SoE
&Increased continence rates and improved UI High
&Improved QoL Low
&AEs, including autonomic nervous system disorders, abdominal pain, dry mouth, dyspepsia, and fatigue, were significantly more common in women taking tolterodine
High
&Discontinuation of the treatment and stopping the treatment because of AEs did not differ compared with placebo
High
*Adapted from Shamliyan et al.31
TABLE 8C. Prevalence of AEs of Tolterodine Compared With Placebo (Modified)*
AE RA, % RC ,%
&General body disorders 22.3 18 &Treatment failure 9 16
&Dry mouth 18.4 6.7
&Autonomic nervous system disorder 27.2 15.5 &Blurred vision 1.3 3
&Constipation 4 3
&Discontinuation: AEs 4 3
&Headache 4 4
&Urine tract infection 2 3 &Discontinuation: treatment failure 0.7 1.6 &Nasopharyngitis 3 3
&Diarrhea 2 2
&Serious AEs 1.8 3.1 *Adapted from Shamliyan et al.31
and cardiovascular adverse events were comparable to placebo (Tables 10b and 10c).58
Fesoterodine
Fesoterodine is a new and novel derivative of 3,3-diphenylpropyl-amine. Fesoterodine is a competitive muscarinic receptor antagonist (Table 11a).59 After oral administration,
the compound is rapidly hydrolyzed in its active metabolite, 5-hydroxymethyl tolterodine (5-HMT), which is responsible for the antimuscarinic activity of the drug and has the same activity as tolterodine. T1/2= 7Y8 hours [approved by the Food and Drug
Administration (FDA) for the treatment of OAB]. The lipophilicity and permeability across biological membranes has been shown to be considerably lower for 5-HMT as compared to tolterodine, and 5-HMT formation from fesoterodine by the ubiquitous nonspecific esterases is more consistent.60The efficacy of fesoterodine versus
ER tolterodine has been evaluated in 3 trials, later included in a recent Cochrane review36: fesoterodine was superior to
tolter-odine in terms of reduction of urgency episodes, frequency, and
leakage episodes. Patients taking fesoterodine had higher risk of withdrawal due to adverse events. Fesoterodine, 8 mg daily, is more effective than tolterodine ER, 4 mg daily, for the cure and the improvement of UUI (Tables 11b, 11c, and 11d).41
Atropine Sulfate Drug Name: Levsin
Derived from the plantAtropa belladonna, this is the proto-type of all antimuscarinic agents.33It is rarely used for the
treat-ment of OAB/DO because of its systemic AEs, which preclude its use as an oral treatment. However, in patients with neurogenic DO (NDO), intravesical atropine may be effective for increasing blad-der capacity without causing any systemic AEs, as shown in open pilot trials.60Y64Sublingual form may have fewer AEs, whereas
intravescical form may be effective without AEs.33
Propantheline Bromide Drug Name: Pro-Banthine
It is a quaternary ammonium compound with a nonselective antimuscarinic action. It has a low (5%Y10%) and individually varying biological availability. After metabolization has occurred,
TABLE 9A. Trospium Costs Overview
Drug Name Active Ingredient Dose Price for 30 d of Treatment
Sanctura (IR) (20 mg) Trospium chloride 20 mg b.i.d., at least 1 h before meals or on empty stomach
,$88 Sanctura XR (60 mg) Trospium chloride 60 mg q.d. in morning, at least 1 h before
breakfast, with water or on empty stomach
,$96.42 Trospium chloride (20 mg) Trospium chloride 20 mg b.i.d., at least 1 h before meals or on
empty stomach
,$69.45
Trospium chloride (60 mg) Trospium chloride 60 mg q.d. in the morning, at least 1 h before breakfast, with water or on empty stomach
,$39
TABLE 9B. Efficacy and Safety of Trospium*
Conclusion SoE
&Increased continence rates High &Dry mouth, dry eye, dry skin, and constipation
occurred more often than with placebo
Moderate &AEs resulted in treatment discontinuation more
often than did placebo
High *Adapted from Shamliyan et al.31
TABLE 9C. Prevalence of AEs of Trospium Compared With Placebo (Modified)*
AE RA, % RC, %
&Dry mouth 15.1 4.5
&Diarrhea 2.5 4
&Central nervous system disorders 3.9 3.8 &Constipation 9.3 2.6 &Discontinuation: AEs 5.8 3.9
&Headache 3.3 3.5
&Urinary tract infections 2.6 1.3 *Adapted from Shamliyan et al.31
TABLE 10A.Darifenacin Costs Overview Drug Name Active Ingredient Dose Price for 30 d of Treatment Enablex ER (7.5 mg) (15 mg) Darifenacin hydrobromide 7.5Y15 mg q.d.; swallowed whole with liquid; should not be chewed, divided, or crushed $84.62 (7.5 mg) $84.64 (15 mg)
TABLE 10B. Efficacy and Safety of Darifenacin*
Conclusion SoE
&At 7.5 and 15 mg, improved urgency UI and several domains of QoL when compared with placebo
High &Caused AEs more often than did placebo; among
examined AEs, darifenacin increased rates of constipation, dry mouth, dyspepsia, and headache
Moderate
&Higher dosage (30 mg/d) did not result in better benefits but caused greater rates of AEs
High &Treatment discontinuation rates due to AEs were
the same with darifenacin and placebo
High *Adapted from Shamliyan et al.31
its metabolites remain inactive.65The effect of propantheline on
OAB/DO has not been well documented in controlled trials sat-isfying current standards; it can be considered effective, and may, in individually titrated doses, be clinically useful.7
>-ADRENERGIC ANTAGONISTS
Their mechanism of action is urethral sphincter tone in-crease. So far, there are no controlled clinical trials showing that
>-adrenergic antagonist is an effective alternative in the treatment of OAB/DO.7A randomized controlled trial (RCT), comprising
364 women with OAB, revealed no effect of tamsulosin versus placebo.66On the other hand, voiding symptoms in women with
functional outflow obstruction, or lower urinary tract symptoms, were successfully treated with an>1-AR antagonist.67,68In women,
these drugs may produce stress incontinence due to their>-lytic mechanism.69Tamsulosin demonstrated effectiveness in female
patients with voiding dysfunction, independently of the obstruc-tion grade. A recent study on>1-AR antagonists offers an initial
treatment option for women affected by nonneurogenic voiding dysfunction.70
A-AR AGONISTS
A-Adrenoceptors have been previously treated. The exact mechanism of signaling pathway involving this class of drugs at the urothelium level has to be clarified. Several studies conducted in vitro and in vivo have shown a myorelaxant effect ofA-AR agonists; however, role of theA3-AR agonists remains to be
elu-cidated.7 A number of A
3-AR selective agonists, including
solabegron (phase II), are currently being evaluated as potential treatments for OAB in humans.
Mirabegron
In June 28, 2012, the FDA approved Myrbetriq (mirabegron, hitherto known as YM-178) to treat adults with OAB/UUI. Myrbetriq relaxes the detrusor smooth muscle during filling by activation ofA3-ARs, increasing bladder capacity (Table 12a).71,72
Its safety and efficacy were demonstrated in 3 double-blind, placebo-controlled, multicenter clinical trials (Table 12b).70
Recommended starting dose is 25 mg once daily; 25 mg is effective within 8 weeks.72 In a study by Nitti et al,73 1329
patients were randomized to receive placebo, mirabegron 50 mg, or 100 mg once daily for 12 weeks. At the final visit, mira-begron 50 and 100 mg showed statistically significant improve-ments in efficacy and mean volume voided/micturition compared with placebo.
Chapple et al74 compared the long-term administration
safety and efficacy of mirabegron 50 and 100 mg and tolterodine
TABLE 10C. Prevalence of AEs of Darifenacin Compared With Placebo (Modified)*
AE RA, % Pl, %
&Dry mouth 22 5.6
&Constipation 14.6 5.7 &Discontinuation: AEs 4.6 3.3
&Dyspepsia 4.4 1.3
&Urinary tract infection 2.9 2.3 &Serious AEs 1.2 2.1 &Discontinuation: treatment failure 1 1.7
*Adapted from Shamliyan et al.31
Pl indicates placebo.
TABLE 11A. Fesoterodine Costs Overview Drug Name Active Ingredient Dose Price for 30 d of Treatment Toviaz ER (4 mg) (8 mg) Fesoterodine fumarate 4Y8 mg q.d.; swallowed whole with liquid; should not be chewed, divided, or crushed
$90.57 (4 and 8 mg)
TABLE 11B. Efficacy and Safety of Fesoterodine*
Conclusion SoE
&Increased continence rate when compared with placebo Low &Improved urgency UI and better response with 8 vs 4 mg High
&Improved QoL Low
&Resulted in higher rates of AEs and discontinuation of the treatments due to AEs; AEs were more common with 8 than 4 mg
High
*Adapted from Shamliyan et al.31
TABLE 11C. Prevalence of AEs of Fesoterodine Compared With Placebo (Modified)*
AE RA, % RC, %
&Treatment failure 4 8
&Dry mouth 27 7
&Influenza-like symptoms 5.7 8
&Headache 7 6
&Constipation 11 3
&Discontinuation: AEs 6 3 &Discontinuation: treatment failure 2 3
&Back pain 2.1 3
&Upper respiratory tract infection 2 3.5 &Nasopharyngitis 2.5 3.3
&Nausea 2 3.1
&Abdominal pain 3.7 2.7 &Urinary tract infection 2 2
*Adapted from Shamliyan et al.31
TABLE 11D. Comparative Effectiveness and Safety*
Head to Head SoE
Fesoterodine vs tolterodine
Greater rates of continence Low
Greater rates of reduced UI High
Greater rate of treatment discontinuation due to AEs Moderate *Adapted from Shamliyan et al.31
in a 12-month, 3-arm, parallel-group study (with no placebo arm). Both mirabegron and tolterodine improved key OAB symptoms from the first measurement after 4 weeks, and effectiveness was maintained throughout the 12-month treatment period. Van Kerrebroeck et al75have demonstrated a significant reduction of
incontinence episodes and micturition frequency.
In patients with severe renal impairment or with moderate hepatic dysfunction, maximum dose is 25 mg once daily. In patients with end-stage renal disease or with severe hepatic impairment, the use of this drug is not recommended. In preg-nancy, it can be used only if the benefit to the mother outweighs the potential risk of the fetus. Myrbetriq is not recommended in breast-feeding mothers because it is excreted in human milk.
In a proof-of-concept study of mirabegron 100 and 150 mg twice daily,76AEs were reported by 45.2% of patients, and the
incidence was similar among those treated with placebo (43.2%) and mirabegron (43.8%Y47.9%). The most commonly reported AEs were treatment-related gastrointestinal disorders, including constipation, dry mouth, dyspepsia, and nausea. There was no patient-reported acute retention.
In a study reported by Khullar et al,77the incidence of AEs
was similar across the placebo, mirabegron 50 and 100 mg groups (50.1%, 51.6%, and 46.9%, respectively; Table 12c). Comparative studies with tolterodine,78,79shown in Table 12d,
were also carried out.
NEUROTOXINS Botulinum
Botulinum toxin (BONT onabotulinumtoxinA) is a neu-rotoxin produced byClostridium botulinum (Table 13). Unin-hibited urinary bladder contractions in people with some neurological conditions can lead to the inability to store urine. The treatment consists of Botox injection into the bladder that induces detrusor relaxation, an increase in its storage capacity, and a decrease in UI.80In 2011 the FDA approved Botox
in-jections to treat UI in people with neurologic conditions, such as spinal cord injury and multiple sclerosis, experiencing OAB. Observational studies are currently in progress with the aim to assess Botox efficacy and safety in neurogenic treatment in patients affected by Parkinson disease, and to assess risks and
benefits of its long-term use for OAB/UUI treatment.81Y83The
recommended dose is 200 U of BOTOX per treatment, and should not be exceeded.
Adverse effects are urinary tract infections and retention. Vanilloid Receptors Agonist
These receptors are present on the afferent sensory neurons innervating detrusor and urethra. The rationale for intravesical vanilloid agonist application in patients with DO was offered by the demonstration that capsaicin, after bladder C-fiber desen-sitization, suppresses involuntary detrusor contractions depen-dent upon a sacral micturition reflex.84The C-fiber micturition
reflex is usually inactive but it was shown that it is enhanced in patients with chronic spinal-cord lesions above sacral segments and in those with chronic bladder outlet obstruction.85
Capsaicin
Capsaicin has been used for intravescical instillation in patients affected by NDO.7,33Capsaicin suppresses involuntary
detrusor contractions after chronic spinal cord lesions above
TABLE 12B. Efficacy and Safety of Mirabegron Conclusion
Mirabegron caused a statistically significant improvement from baseline compared with placebo in the numbers of urgency in-continence episodes and micturitions per 24 h.73
Mirabegron 25 and 50 mg, both doses were associated with sig-nificant improvements in efficacy measures of incontinence ep-isodes and micturition frequency.75
TABLE 12C. Prevalence of AEs of Mirabegron 50 mg, 100 mg Compared With Placebo (Modified)*
AE RA 50 mg, % RA 100 mg, % RC, %
Hypertension 6.1 4.9 6.6
Urinary tract infection 2.7 3.7 1.8
Headache 3.2 3 2
Nasopharyngitis 3.4 2.5 2.9
Dry mouth 2.8 2.8 2.6
Constipation 1.6 1.6 1.4
*Adapted from Khullar et al.77
TABLE 12A. Mirabegron Costs Overview Drug Name Active Ingredient Dose Price for 30 d of Treatment Myrbetriq 25 mg Mirabegron 25 mg q.d.; swallow whole with water, with or without food
,$240.99
Myrbetriq 50 mg
Mirabegron 50 mg q.d.; swallow whole with water, with or without food
,$240.99
TABLE 12D. Comparative Effectiveness Head to Head
Mirabegron vs tolterodine:
The incidence and severity of treatment-emergent serious AEs (pri-mary outcome parameters) were similar across the mirabegron 50 mg (59.7%), mirabegron 100 mg (61.3%), and tolterodine sustained-release 4 mg (62.6%) groups.76
During 12 mo of treatment, 2.8% of mirabegron 50 mg once daily recipients reported dry mouth compared with 8.6% with tolter-odine ER 4 mg once daily recipients.77
On the basis of descriptive analyses from a 12-mo trial, once-daily mirabegron 50 mg and tolterodine ER 4 mg were both efficacious in reducing urinary symptoms and improving health-related QoL.77
TABLE 13. Botulinum Costs Overview Drug Name Active Ingredient Dose Price for a Single Dose Botox 200UNT
OnabotulinumtoxinA Total dose 200 U, as 1 mL (~6.7 U) injections across 30 sites into the detrusor
sacral segments. Intravesical capsaicin for NDO was studied in 6 noncontrolled and 1 controlled clinical trials. After capsaicin has been dissolved in 30% alcohol and 100 to 125 mL of sterile water, 1 to 2 mM are instilled into the bladder and left in contact with the mucosa for 30 minutes. The pungency of alcoholic capsaicin solutions has prevented the widespread use of this compound.7 In the single randomized study comparing the
capsaicin solution against the use of 30% ethanol, a significant reduction of urge incontinence was found. The pungency of alcoholic capsaicin solutions has prevented the widespread use of this compound.
Resiniferatoxin
This compound, derived from the cactus-like plantEuphorbia, is 1000 times more potent, but much less pungent, than capsaicin.7,33
Different resiniferatoxin (RTX) concentrations, 10 nM, 50 nM, 100 nM, and 10 KM, were tested. Resiniferatoxin brought a rapid improvement or disappearance of UI in up to 80% of the selected patients and a 30% decrease in their daily urinary frequency.86
Furthermore, RTX also increased the volume to first detrusor contraction and the maximal cystometric capacity. In general, in patients receiving 50- to 100-nM RTX, the effect was long-lasting, with a duration of more than 6 months being reported. In patients treated with 10-KM doses, transient urinary retention may occur.87
Currently, new experimental tests are in progress to test its safety and efficacy.
PHARMACOLOGICAL TREATMENT OF SUI Stress urinary incontinence is defined as an involuntary loss of urine on effort or physical exertion (or on sneezing or coughing)7
associated with increased intra-abdominal pressure (stress test), in the absence of a detrusor contraction.31Although various agents
such as >1-AR agonists, estrogens, and tricyclic antidepressants
have all been used anecdotally in the past for the treatment of stress incontinence, duloxetine is the first drug to be specifically devel-oped and licensed for this indication.38Drugs used for SUI are
shown in Table 14.
ANTIDEPRESSANT Selective Norepinephrine and Serotonin Reuptake Inhibitors
Duloxetine
Duloxetine (Cymbalta) is a potent and balanced serotonin (5-hydroxytryptamine) and noradrenaline reuptake inhibitor (Table 15a).88T
1/2= 12 hours. Its elimination is mainly through
hepatic metabolism. Its ability to stimulate the pudendal moto-neurons (target: Onuf ’s nucleus, in the sacral spinal cord) and to increase striated urethral sphincter contractility is thought to be the basis for its efficacy in women with SUI. Dmochowski et al89randomized 683 women, 22 to 84 years old, to duloxetine
or placebo. There was a significant decrease in incontinence episode frequency with duloxetine compared with placebo (50% vs 27%,P, 0.001), with comparably significant improve-ments in QoL (11.0 vs 6.8,P, 0.001). Duloxetine has been ap-proved for the treatment of SUI in Europe, although the FDA
TABLE 14. Drugs Used for SUI Treatment (Modified)*
Drug LoE GoR
Duloxetine 1 B Midodrine 2 C Clenbuterol 3 C Estrogen 2 D Methoxantine 2 D Imipramine 3 D Ephedrine 3 D Norephedrine (phenylpropanolamine) 3 D *From Thu¨roff et al5and Andersson et al.7
GoR indicates grade of recommendation; LoE, level of evidence.
TABLE 15A. Duloxetine Costs Overview
Drug Name Active Ingredient Dose
Price for 30 d of Treatment Cymbalta Cap Oral 20 mg Duloxetine 40 mg b.i.d. ,$218.55 Cymbalta Cap Oral 40 mg Duloxetine 40 mg b.i.d. ,$132.84 Duloxetine 20 mg Duloxetine 40 mg b.i.d. ,$67.18 Duloxetine 40 mg Duloxetine 40 mg b.i.d. ,$50.99
TABLE 15B. Efficacy and Safety of Duloxetine* Conclusion
The mean IEF at baseline was 12.50 (2.2), and 9.02 (1.3), 7.50 (0.9), 6.02 (0.86) at the end of 1, 2, and 3 mo of treatment with duloxetine, respectively. This shows that there was a statistically significant reduction in the incontinence episode frequency (IEF) at the end of each month when compared to the baseline. Statistical reduction in the IEF at the end of 1 mo. In patients not
responding to treatment, the mean IEF at baseline was 13.10 (2.96) and 12.90 (2.88) at the end of 1 mo of treatment with duloxetine.
There was a decrease of 30% in first month, around 40% to 45% at the end of second month and over 50% decrease in IEF in third month.
*Adapted from Deepak et al.92
TABLE 15C. Prevalence of AEs of Duloxetine Compared With Placebo (Modified)* AE RA, % RC, % &Nausea 25.1 3.9 &Headache 14.5 8.7 &Insomnia 13.7 2.6 &Constipation 12.8 1.7
&Dry mouth 12.3 1.7
&Dizziness 11 2.6
&Fatigue 10.1 3.5
&Somnolence 8.4 0
&Anorexia 6.6 0
&Vomiting 6.2 1.7
&Increased sweating 5.7 0.9 &Discontinuation of AEs 91
has not approved it for this purpose. Liver toxicity and suicidal events represent a great concern. However, an observation study from Michel et al90 has shown that women with SUI treated
with duloxetine doses lower than recommended reported a low incidence of AEs and suicide attempts were not reported. Cymbalta was first used to treat major depressive disorder and generalized anxiety disorder. Subsequently, FDA approved Cymbalta to treat chronic musculoskeletal pain, including dis-comfort from osteoarthritis, chronic lower back pain, and fi-bromyalgia, but failed the US approval for SUI amid concerns over liver toxicity and suicidal events, whereas it was approved for this indication in Europe, where it is recommended as an add-on medication instead of surgery. The efficacy and safety of duloxetine (20, 40, and 80 mg; Table 15b) for treatment of SUI has been evaluated in a study by Norton et al,91that
in-volved 48 centers in the United States, including 553 women with SUI, and Deepak et al.92Duloxetine was associated with a
significant dose-dependent decrease in incontinence episode frequency; reductions were 41% for placebo and 54%, 59%, and 64% for the 20-, 40-, and 80-mg groups, respectively. Discontinuation rates were also dose dependent; 5% for placebo and 9%, 12%, and 15% of 20, 40, and 80 mg, respectively.93
Nausea is the most frequently reported AE. There was a sig-nificant decrease in incontinence episode frequency and im-provement in QoL in those women taking duloxetine 40 mg o.d. when compared to placebo.38Escalating the dose upon
initia-tion of the treatment has been shown to reduce the frequency of AEs.94From the whole group, 20% of patients cancelled surgery
after 2 months on duloxetine. The main adverse event was nau-sea, ranging from 23% to 25%. In these 6 trials, the percentage of withdrawals due to AEs resulted to be 17% in the drug group compared to 4% in the placebo arm.7The most frequent
AEs are reported in Table 15c. Dysphoric mood, irritability, agitation, paresthesias, anxiety, confusion, emotional lability, hypomania, and tinnitus are generally self-limiting; some have been reported to be severe. In a systematic review performed to assess duloxetine safety and tolerability for SUI, no case of suicide has been reported.95An observational study on the same
subject including 3233 women did not report any case of suicide during the treatment.90
Tricyclic Antidepressants Imipramine
Imipramine is a tertiary amine of the tricyclic antidepressant group; its mechanism of action is norepinephrine and serotonin reuptake inhibition and it is thought to improve contraction of the urethral smooth muscle (Table 16a).2This drug is derived
from chlorpromazine, so it has the same blocking effects on muscarinic (M1), adrenergic, and histaminergic receptors, that
cause the relative AEs. Several researchers have found a sig-nificant effect in the treatment of patients with DO96although
others report little effect.97 It may be used to treat SUI or
mixed incontinence. In the light of this evidence and the se-rious AEs associated with tricyclic antidepressants, their role in DO remains of uncertain benefit, although they are often useful in patients complaining of nocturia or bladder pain.38It
has been known for a long time that imipramine can have favorable effects in the treatment of nocturnal enuresis in children, with a success rate of 10% to 70% in controlled trials.98,99Adverse effects are shown in Table 16b.
>-Adrenergic Agonists
Several drugs with agonistic effects on>-ARs have been used in the treatment of SUI. However, ephedrine and nor-ephedrine (phenylpropanolamine) seem to have been the most widely used.100There was weak evidence to suggest that use
of an adrenergic agonist was better than placebo treatment.7
The limited evidence suggested that such drugs were better than placebo in reducing the number of pad changes and in-continence episodes, and in improving subjective symptoms. Phenylpropanolamine, clonidine (Catapres), ephedrine, and pseudoephedrine belong to this category.>-Adrenergic ago-nists are not selective for bladder receptors: they are not recommended in people with glaucoma, diabetes, hyperthy-roidism, heart disease, or high blood pressure. The selective
>1-AR agonist midodrine is approved for SUI in Portugal.101
Phenylpropanolamine
Phenylpropanolamine is a nonselective >-ARs agonist which is capable to bindA-adrenergic receptors and enhances release of NE by presynaptic neurons.33This drug can be
ef-fective in patients with mid-SUI, but it is no longer dispensed in the United States because of the AEs reported later. Phenyl-propanolamine is approved in Finland for the treatment of SUI.101 Adverse effects are as follows: cardiac arrhythmias,
hypertension, insomnia, headache, tremor, anxiety, and stroke (in women taking appetite suppressants).
A-AR Antagonists
A-Adrenoceptor antagonists inhibit urethral A-ARs and this may increase noradrenaline action on the urethral>-ARs.7
Propranolol has been reported to have beneficial effects in the
TABLE 16A. Imipramine Costs Overview
Drug Name Active Ingredient Dose Price for Package Tofranil CAP 25 mg Imipramine pamoate 25Y75 mg q.h.s. or b.i.d. ,$17 (for 25 mg q.h.s.) Tofranil CAP 50 mg Imipramine pamoate 25Y75 mg q.h.s. or b.i.d. $25.50 (for 50 mg q.h.s.) Tofranil CAP 75 mg Imipramine pamoate 25Y75 mg q.h.s. or b.i.d. $36.50 (for 75 mg q.h.s.) q.h.s. indicates every day at hours of sleep.
TABLE 16B. AEs of Imipramine* &Peripheral antimuscarinic effects &Orthostatic hypotension &Hypertension
&Blurred vision &Tinnitus &Rush &Headache &Palpitation &Dry mouth &Dizziness &Drowsiness &Tachycardia &Urinary retention
treatment of stress incontinence102,103but no RCTs to support
this action is present in literature.
A-AR Agonists
A-Adrenoceptor stimulation is generally conceded to de-crease urethral pressure,104 butA
2-AR agonists have been
re-ported to have a different action on fast- and slow-contracting skeletal muscle contractility.105Clenbuterol, a selectiveA
2-AR
agonist, is approved for the treatment of SUI in Japan.106
PHARMACOTHERAPY OF MIXED INCONTINENCE
The optimum treatment of mixed urinary incontinence (MUI) may often require multiple treatment modalities. One should treat most bothersome symptoms first.5Behavioral
ther-apy and lifestyle modification, such as moderate weight loss and caffeine reduction, should be considered first-line options for all women with MUI. Pharmacological treatment of the urge com-ponent with antimuscarinics is effective. The addition of pelvic floor muscle therapy may have an additional benefic effect. Often a surgical procedure for the incontinence stress component sig-nificantly improves both symptoms. Anti-incontinence surgery may have a positive impact on both the stress and the urge com-ponents of MUI; however, it seems that women with MUI may have lower cure rates compared to women with pure SUI.
If the initial pharmacological approach fails, consider the use of botulinum toxin or neuromodulation.
HORMONAL TREATMENT OF UI Desmopressin
Desmopressin (Table 17) is a synthetic analog of vasopressin (antidiuretic hormone). Desmopressin (DDVAP) was found to be well tolerated and resulted in a significant improvement com-pared to placebo in reducing nocturnal voids/UI and increasing the hours of undisturbed sleep.5Studies are in progress to test
if desmopressin improves nocturnal enuresis in patients after radical cystectomy with bladder reconstruction, and the impact on sleep and daytime functioning. The drug is available in a range of formulations: intranasal solution (spray), injectable solution, tablets and, most recently, an oral lyophilisate. Intra-nasal and oral formulations are generally well tolerated, and AEs are usually minor. Usual adult dose, in oral formulations, is 0.2 to 0.6 mg every day at hours of sleep. One of the most feared AEs (although rare) is hyponatremia. However, precau-tions in prescribing desmopressin for this condition and com-pliance monitoring will help prevent this complication. Adverse effects are as follows: dizziness, headache, mood change,
vomiting, weakness, loss of appetite, feeling restless or irri-table, confusion, and hallucinations.
Estrogens
Bladder, urethra, and pelvic floor estrogen sensibility plays an important role in the continence mechanism (Table 18).7
Many studies have shown that oral estrogen replacement, alone or combined with a progesterone, has poor results in terms of continence. In the women health initiative study, HRT was found to increase the incidence of all types of UI at 1 year in those women continent at baseline. The most recent meta-analysis on the effects of estrogen therapy on the lower uri-nary tract showed that overall systemic administration resulted in worse incontinence than that of placebo.107The use of local
estrogen therapy may improve incontinence (RR, 0.74; 95% CI, 0.64Y0.86), reducing frequency and urgency.107The subjective
improvement in symptoms may simply represent local estro-genic effects reversing urogenital atrophy rather than a direct effect on bladder function. Reliable data are too scant to suggest the dose, type of estrogens, and route of administration.
PRACTICAL SUGGESTIONS
Conclusively, considering the similar pharmacological profile of the different drugs used for the UUI treatment, we suggest starting with instant release form of antimuscarinic drugs. Dari-fenacin, solifenacin and tolterodine seem to offer a good bioavail-ability and efficacy with acceptable AEs. Assessment of efficacy and AEs of the treatment within 30 days from the beginning of the therapy is mandatory. Specific caution must be taken when using instant release oxybutynin in patient with cognitive dysfunction. Consider use of transdermal oxybutynin in those patients ex-periencing dry mouth. Regarding the use of duloxetine for SUI, the FDA has not given approval for this purpose. However, use of duloxetine in the daily practice with doses lower then recommended has shown a low incidence of AEs with a significant decrease in incontinence episode frequency and improvement in QoL.
SUGGESTIONS FOR FURTHER RESEARCH The following classes of drugs need to be investigated to find out if they could be helpful in the treatment of any form of incontinence:
- Solabegron (A3-adrenergic agonist): phase II; - Cizolirtine citrate (antimuscarinic agent): phase II; - Tramadol (K-receptor agonist): off-label;
- Gabapentin: off-label;
- Aprepitant (neurokinin-1 receptor antagonist): off-label
REFERENCES
1. Rortveit G, Hunskaar S. Urinary incontinence and age at the first and last delivery: the Norwegian HUNT/EPINCONT study.Am J Obstet Gynecol2006;195(2):433Y438.
TABLE 17. Desmopressin Costs Overview Drug Name Active Ingredient Dose Price for 30 d of Treatment DDAVP 60 mcg melt Desmopressin acetate 20Y60 mcg q.h.s. ,$72.98 (for 60 mcg) Minirin 0.2 mg Desmopressin acetate 0.2Y0.6 mg q.h.s. ,$149 (for 0.2 mg) Desmopressin 0.2 mg Desmopressin acetate 0.2Y0.6 mg q.h.s. ,$123.28 (for 0.6 mcg) Desmopressin 60 mcg Desmopressin acetate 20Y60 mcg q.h.s. ,$80 (for 60 mcg)
TABLE 18. Vagifem Costs Overview Drug Name Active Ingredient Dose Price for 30 d of Treatment Vagifem 10 mcg
17A-estradiol 1 tablet q.d. for 2 wk, followed by 1 tablet twice weekly
,$70.49
Vagifem 25 mcg
17A-estradiol 1 tablet q.d. for 2 wk, followed by 1 tablet twice weekly
2. Saks EK, Arya LA. Pharmacologic management of urinary incontinence, voiding dysfunction, and overactive bladder.Obstet Gynecol Clin North Am2009;36(3):493Y507.
3. Bump RC, Mattiasson A, Bø K, et al. The standardization of terminology of female pelvic organ prolapse and pelvic floor dysfunction.Am J Obstet Gynecol1996;175(1):10Y17. 4. Wennberg AL, Molander U, Fall M, et al. A longitudinal
population-based survey of urinary incontinence, overactive bladder, and other lower urinary tract symptoms in women.Eur Urol 2009;55(4):783Y791.
5. Thu¨roff JW, Abrams P, Andersson KE, et al. EAU guidelines on urinary incontinence.Eur Urol2011;59(3):387Y400.
6. CEBM (Centre for Evidence-based Medicine). Oxford Centre for Evidence-based MedicineVLevels of Evidence. 2009. Available at: http://www.cebm.net/index.aspx?o=1025. Accessed September 1, 2012. 7. Andersson KE, Chapple CR, Cardozo L, et al. Pharmacological
treatment of urinary incontinence. In: Abrams P, Cardozo L, Khoury S, et al, eds.Incontinence. 21st ed. Paris, France: Health Publication Ltd; 2009:631Y700.
8. Yoshimura N, Kaiho Y, Miyazato M, et al. Therapeutic receptor targets for lower urinary tract dysfunction.Naunyn Schmiedebergs Arch Pharmacol2008; 377(4Y6):437Y448.
9. Kanai A, Wyndaele JJ, Andersson KE, et al. Researching bladder afferentsVdetermining the effects ofA(3)-adrenergic receptor agonists and botulinum toxin type-A.Neurourol Urodyn2011;30(5):684Y691. 10. Fowler CJ, Griffiths D, de Groat WC. The neural control of micturition.
Nat Rev Neurosci2008;9(6):453Y466.
11. Andersson KE. How many drugs for LUTS due to BPH are too many? J Urol2008;180(3):811Y812.
12. Hegde SS, Choppin A, Bonhaus D, et al. Functional role of M2 and M3 muscarinic receptors in the urinary bladder of rats in vitro and in vivo. Br J Pharmacol1997;120(8):1409Y1418.
13. Chess-Williams R, Chapple CR, Yamanish T, et al. The minor population of M3-receptors mediate contraction of human detrusor muscle in vitro.J Auton Pharmacol2001;21(5Y6):243Y248. 14. Fetscher C, Fleichman M, Schmidt M, et al. M3 muscarinic receptors
mediate contraction of human urinary bladder.Br J Pharmacol 2002;136(5):641Y644.
15. Kories C, Czyborra C, Fetscher C, et al. Gender comparison of muscarinic receptor expression and function in rat and human urinary bladder: differential regulation of M2 and M3 receptors?Naunyn Schmiedebergs Arch Pharmacol2003;367(5):524Y531.
16. Schneider T, Fetscher C, Krege S, et al. Signal transduction underlying carbachol-induced contraction of human urinary bladder.J Pharmacol Exp Ther2004;309(3):1148Y1153.
17. Otsuka A, Shinbo H, Matsumoto R, et al. Expression and functional role of beta-adrenoceptors in the human urinary bladder urothelium. Naunyn Schmiedebergs Arch Pharmacol2008;377(4Y6):473Y481. 18. Kullmann FA, Downs TR, Artim DE, et al. Urothelial beta-3 adrenergic
receptors in the rat bladder.Neurourol Urodyn2011;30(1):144Y150. 19. Nomiya M, Yamaguchi O. A quantitative analysis of mRNA
expression of alpha 1 and beta-adrenoceptor subtypes and their functional roles in human normal and obstructed bladders.J Urol 2003;170:649Y653.
20. Tyagi P, Thomas CA, Yoshimura N, et al. Investigations into the presence of functional Beta1, Beta2 and Beta3-adrenoceptors in urothelium and detrusor of human bladder.Int Braz J Urol 2009;35(1):76Y83.
21. Michel MC.A-Adrenergic receptor subtypes in the urinary tract. Handb Exp Pharmacol2011;(202):307Y318.
doi: 10.1007/978-3-642-16499-6_15.
22. Igawa Y, Yamazaki Y, Takeda H, et al. Functional and molecular biological evidence for a possible beta3-adrenoceptor in the human detrusor muscle.Br J Pharmacol1999;126(3):819Y825. 23. Takeda M, Obara K, Mizusawa T, et al. Evidence for
beta3-adrenoceptor subtypes in relaxation of the human urinary bladder detrusor: analysis by molecular biological and pharmacological methods.J Pharmacol Exp Ther 1999;288(3):1367Y1373.
24. Yamaguchi O. Beta3-adrenoceptors in human detrusor muscle. Urology2002;59:25Y29.
25. Badawi JK, Langbein S. Selective beta-adrenoceptor agonists, calcium antagonists and potassium channel openers as a possible medical treatment of the overactive bladder and urge incontinence.Pharmazie 2006;61(3):175Y178.
26. Biers SM, Reynard JM, Brading AF . The effects of a new selective beta3-adrenoceptor agonist (GW427353) on spontaneous activity and detrusor relaxation in human bladder.BJU Int 2006;98(6):1310Y1314.
27. Badawi JK, Seja T, Uecelehan H, et al. Relaxation of human detrusor muscle by selective beta-2 and beta-3 agonists and endogenous catecholamines.Urology2007;69(4):785Y790.
28. Leon LA, Hoffman BE, Gardner SD, et al. Effects of the beta 3-adrenergic receptor agonist disodium 5-[(2R)-2-[[(2R)-2-(3- chlorophenyl)-2-hydroxyethyl]amino]propyl]-1,3-benzodioxole-2,2-dicarboxylate
(CL-316243) on bladder micturition reflex in spontaneously hypertensive rats.J Pharmacol Exp Ther2008;326(1):178Y185. 29. Andersson KE, Arner A. Urinary bladder contraction and relaxation:
physiology and pathophysiology.Physiol Rev2004;84(3):935Y986. 30. Yamada S, Ito Y.>(1)-Adrenoceptors in the urinary tract.Handb Exp
Pharmacol2011;(202):283Y306. doi: 10.1007/978-3-642-16499-6_14. 31. Shamliyan T, Wyman JF, Ramakrishnan R, et al. Benefits and harms of
pharmacologic treatment for urinary incontinence in women: a systematic review.Ann Intern Med2012;156(12):861Y874, W301-10. 32. Hughes KM, Lang JCT, Lazare R, et al. Measurement of oxybutynin
and its N-desethyl metabolite in plasma, and its application to pharmacokinetic studies in young, elderly and frail elderly volunteers. Xenobiotica1992;22:859Y869.
33. Siddighi S, Chuan S. Medicine used in urogynecology. In: Siddighi S, Hardesty JS, eds.Urogynecology and Female Pelvic Reconstructive Surgery: Just the Facts. New York, NY: McGraw-Hill-Medical Publishing Division; 2006.
34. Abrams P, Freeman R, Anderstro¨m C, et al. Tolterodine, a new antimuscarinic agent: as effective but better tolerated than oxybutynin in patients with an overactive bladder.Br J Urol1998;81(6):801Y810. 35. Homma Y, Kawabe K. Health-related quality of life of Japanese
patients with overactive bladder treated with extended-release tolterodine or immediate-release oxybutynin: a randomized, placebo-controlled trial.World J Urol2004;22(4):251Y256. 36. Madhuvrata P, Cody JD, Ellis G, et al. Which anticholinergic drug for
overactive bladder symptoms in adults.Cochrane Database Syst Rev 2012;1:CD005429.
37. Siddiqui MA, Perry CM, Scott LJ. Oxybutynin extended-release: a review of its use in the management of overactive bladder. Drugs2004;64(8):885Y912.
38. Robinson D, Cardozo L. New drug treatments for urinary incontinence. Maturitas2010;65(4):340Y347.
39. Chapple CR, Khullar V, Gabriel Z, et al. The effects of antimuscarinic treatments in overactive bladder: an update of a systematic review and meta-analysis.Eur Urol2008;54:543Y562.