S
ilver has been used for its antimicrobial properties for a long time; even ancient Greeks applied silver chips or granules to wounds of injured soldiers to prevent infection. Ingestible silver in different soluble prepa-rations has also been used, with the intention to cure many diseases. Silver nitrate and silver sulphadiazine have been used extensively in the care of burns for many years, with few questions asked. However, in 1977, Bridges and Lowbury1raised a question about possible microbial resistance to silver, something that has been brought to the fore today when drug resistance is discussed.With the extended use of different silver dressings, additional side effects such as staining of the skin, reduced wound healing, and increased wound pain of patients treated repeatedly with silver dressings have been reported and described.2,3These phenomena have rightfully caught the attention of researchers. For example, Trop4 presented a case in which raised liver
Accumulation of Silver and Delayed
Re-epithelialization in Normal
Human Skin: An
ex-vivo
Study of
Different Silver Dressings
Camilla Fredriksson, Med Lic;
1Gunnar Kratz, MD, PhD,
Professor;
1,2Fredrik Huss, MD, PhD
1,2WOUNDS 2009;21(5):116–123
From the 1Institution of Clinical and
Experimental Medicine, Department of Experimental Plastic Surgery, Faculty of Health Science, Linköping University, Linköping, Sweden; 2Department of Plastic,
Hand, and Burn Surgery, University Hospital of Linköping, Linköping, Sweden
Address correspondence to:
Camilla Fredriksson, Med, Lic
Department of Experimental Plastic Surgery, Faculty of Health Science Linköping University Sandbäcksgatan 7 S-581 83 Linköping, Sweden Phone: 46 13-22 73 37 E-mail: camilla.fredriksson@liu.se
Abstract: Silver is commonly used in wound dressings and topical for-mulations to assist in the management of wounds that are infected or at risk of becoming infected. They provide potent broad-spectrum antimicrobial activity, but should not cause sustained staining of the skin, dermal or systemic accumulation of silver, or discomfort to the patient. However, clinicians and healthcare personnel have been con-cerned about topical staining of the skin and complaints of addition-al pain from patients treated with certain silver dressings. Some delay in re-epithelialization has also been noticed and reported. The reasons for this are not clear, and the authors believed further study regarding the possible effects of silver accumulation and silver dressings’ effect on re-epithelialization was required. The authors studied possible sil-ver accumulation and re-epithelialization in normal human dermal skin. The results showed that most of the dressings or treatments dis-colored the wound surface and that there was a dermal accumulation of what were assumed to be silver particles. Varying grades of accu-mulation were found in deep dermal tissue, particularly around blood vessels, depending on the dressing used. The results also indicated that all of the tested products delayed re-epithelialization in this model.
DO
NOT
enzymes and argyria-like symptoms were noted when a patient with 30% total body surface area (TBSA) burns was treated with Acticoat™ (Smith & Nephew, Fort Lauderdale, Fla). Poon and Burd5 described silver to be highly cytotoxic to keratinocytes and suggestions were made that consideration of the cytotoxic effects of silver and silver-based products should be taken into account when deciding on what dressings to use, particularly when using cultured keratinocytes in situ. This is playing an increasing role in contemporary care of wounds and burns. Vlachou et al6performed studies on systemic silver absorption in patients using Acticoat and showed that only small quantities of silver were absorbed systemical-ly, which led to their recommendations for using Acticoat in the treatment of burns. Innes et al3 reported that donor sites treated with Acticoat needed more time to heal compared with the control wounds treated with Allevyn™ (Smith & Nephew, Fort Lauderdale, Fla).3The donor sites treated with Acticoat had worse scarring by 2 months and the authors stated that their results did not support its use on donor sites.3 A blackening of the wound and surrounding skin that was visible when treat-ing a wound with products containtreat-ing silver is often merely transient binding of silver to wound debris and epidermal cells, which will be shed as the wound heals. However, silver particles from topical applications can penetrate human tissues and be found systemically, though in small quantities.6Despite this, we could find few reports regarding the accumulation of silver in der-mal tissue, which theoretically would be the tissue sub-jected to the highest concentrations.
Any study of a physiological process demands a model that resembles the conditions seen in vivo, but in-vivo
wounds are difficult to standardize, as factors that affect wound healing vary among individuals—eg, age, nutri-tional state, and the presence of infections. In-vitro
wound models that consist of either a cell monolayer or cells cultured in 3-dimensional matrices give repeatable results, but do not completely resemble the complexity of the healing process that is seen in human skin. The in-vitro model, developed by Emanuelsson and Kratz,7 in which the re-epithelialization of the wounds was fol-lowed histologically throughout the time of incubation (14 days) by fixating and staining wounds every second day. After 14 days of incubation, the viability of the cells in the epidermis and dermis was confirmed by isolation and culture in vitro. The wounds incubated in 10% fetal calf serum were shown to heal after 7 days, whereas wounds incubated in 2% serum did not show any signs
of re-epithelization. However, both epidermal and dermal cells from wounds incubated in 2% serum were shown to be viable after 2 weeks of incubation.7Thus, wounds incubated in 2% fetal calf serum could be compared to a chronic nonhealing wound.
The present study concentrates on accumulation of sil-ver in the dermis. Additionally, re-epithelialization was studied and graded to estimate whether the different dressings affected the healing of the wounds in any way.
Materials and Methods
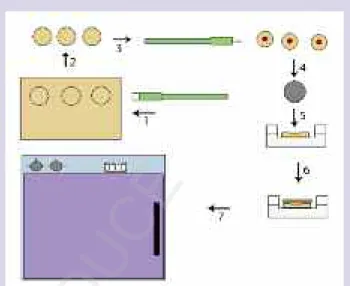
Wound model. Normal human skin from surgical waste (abdominoplasty) was transferred sterile to the laboratory in gauze soaked in physiological saline. The skin was rinsed twice in phosphate buffered saline sup-plemented with antibiotics and mycotics (penicillin 50 IU/mL, streptomycin 50 g/mL). Subcutaneous fat was removed with scissors and circular discs of skin were made from the remaining tissue (dermis and epidermis) using an 8-mm diameter skin biopsy punch (Kai Medical, Solingen, Germany). A dermal wound was made in the center of each disc roughly 1-mm deep using a 3-mm diameter skin biopsy punch. The discs (3 skin discs/group) were subsequently transferred to Transwell cell culture inserts (pore size 0.4 µm) in 6-well plates (BD Falcon, Stockholm, Sweden). Cell culture medium was added to the outer wells, leaving the discs in the air and the liquid interface in the inner cell culture insert, to facilitate the application of the viscous dressings—silver sulphadiazine (Flamazine®, Smith & Nephew, Fort Lauderdale, Fla) and silver nitrate—to prevent the dress-ings from floating off the discs (Illustration 1). Under sterile conditions, pieces of Acticoat, Aquacel® Ag (ConvaTec, Princeton, NJ), PolyMem®Silver™(Ferris Mfg Corp, Burr Ridge, Ill), SilvaSorb®(Medline, Mundelein, Ill), and Silverlon® (Argentum Medical, Chicago, Ill), roughly 8-mm in diameter, were cut and applied to the wounds. A thin layer of Flamazine was applied with a sterile cotton swab, and a drop of silver nitrate was dripped on to the wound. The dressings were moistened with sterile water (as recommended by the dressing manufacturers) and the wounds were incubated at 37˚C (5% carbon dioxide and 95% humidity) for 14 days before sampling. All silver dressings used were applied to the wounds and changed according to the manufacturers’ directions (Table 1). The culture media was changed every second day throughout the study. Skin discs were cultured in Dulbecco’s modified Eagle’s medium (DMEM), supple-mented with antibiotics and mycotics (penicillin 50
DO
NOT
IU/mL and streptomycin 50 µg/mL), and with 10% (posi-tive control and treatment groups), or 2% (nega(posi-tive con-trol) fetal calf serum (FCS), respectively. All media and supplements were purchased from Invitrogen AB (Lidingö, Sweden). Skin from a single donor was used for each experiment (n = 4).
Sampling. After 14 days of culture, the wounds were fixed in 4% neutral buffered formaldehyde, dehydrated through a xylene-ethanol series and embedded in paraf-fin for later histological examination.
Histology. Cross sections of the paraffin-embedded wounds (7- m thick) were stained using hematoxylin and eosin, and re-epithelialization was measured with an Olympus BX41 microscope. Images were captured with an Olympus DP70 CCD camera.
Immunostaining for von Willebrand factor. Immunoassays that targeted von Willebrand factor were used to verify the structures in the tissue where the deposits were found. Non-specific protein binding was blocked with 2% normal horse serum in phosphate buffered saline (PBS) for 20 minutes. Immunohistochemical analysis for endothelial cells was performed using a mouse monoclonal antibody raised against the glycoprotein von Willebrand factor (Dako M0616, DakoCytomation, Glostrup, Denmark). The sec-tions were incubated with primary antiserum diluted in the ratio of 1:25 in PBS for 30 minutes at room tempera-ture, rinsed in PBS, and incubated with a biotinylated
sec-ondary antibody (2 g/mL) for 30 minutes. After washing, the bound antibody was localized with an avidin-peroxi-dase Vectastain® VIP-kit (Vector Laboratories Inc., Burlingame, Calif) with hydrogen peroxide as peroxidase substrate. Native skin served as positive control for the immunohistochemistry; negative control was omission of the primary antibody. Sections were examined using an Olympus BX41 microscope, and images were cap-tured with an Olympus DP70 CCD camera.
Results
Re-epithelialization.All groups were stained using hematoxylin and eosin. Wound re-epithelialization was studied. Wounds in the positive control groups (incubat-ed in 10% FCS) show(incubat-ed complete re-epithelialization after 14 days of incubation (Figure 1A). Wounds in the negative control groups (incubated in 2% FCS) showed no signs of re-epithelialization (Figure 1B). Wounds treat-ed with Acticoat, Silverlon, SilvaSorb, or silver nitrate did not show any signs of re-epithelialization (Figure 1C–G). Wounds treated with Flamazine and PolyMem Silver showed signs of re-epithelialization, such as buds of epithelial cells at the wound margins, and a small tongue of epithelial cells extending toward the center of the wounds (Figure 2A). Wounds treated with Aquacel Ag showed a tongue of epithelial cells extending over the wound bed, representing active (but not yet complete) re-epithelialization (Figure 2B).
Illustration 1. A schematic of how the wounds were created in human skin from surgical waste (1–4); placed in a cell culture insert in a 6-well plate (5); and dressed with silver dressing (6) before incubation (7).
Group Treatment interval
(dressing changes)
Positive control No treatment
Negative control No treatment
Acticoat Every second day
Aquacel Ag Every second day
Silver sulphadiazine (Flamazine) Every day
PolyMem Silver Every seventh day
SilvaSorb Every second day
Silverlon Every second day
Silver nitrate Every day
Table 1. Treatment regimens used in the study. All groups were cultured for 14 days. Skin discs/wounds were cultured in Dulbecco’s modified Eagle’s medium supplemented with antibiotics/-mycotics (penicillin 50 U/mL and streptomycin 50 µg/mL) and with 10% (positive control and treatment groups) or 2% (nega-tive controls) fetal calf serum, respec(nega-tively. All culture mediums were changed every second day throughout the study.
DO
NOT
Silver deposition. No particles, discoloration, or deposits were seen in any of the control groups at any level or structure (Figure 3A, B).
Acticoat. Acticoat is a dressing consisting of an absorbent rayon/polyester core, covered on both sides by silver plated, high-density polyethylene. The silver in the dressing has a nanocrystalline structure with about 30 to 50 silver atoms in each crystal.
Findings. A black discoloration was seen in the epi-dermal layer. Black or gray deposits were also seen in cells at the wound margins and around blood vessels in the
dermal tissue. Wounds treated with Acticoat showed more deposits than all other treatment groups (Figure 4A, B).
Aquacel Ag. Aquacel Ag is a dressing consisting of sodium carboxymethylcellulose fibres in the form of a fleece, containing 1.2% silver in ionic form. When in con-tact with (wound) fluids the Aquacel Ag dressing forms a
Figure 1A, B.A) Wound incubated in 10% FCS for 14 days th a t i s c o mp l e te l y h e a l e d w i th a th i n l a y e r o f keratinocytes bridging the wound surface. B) Wound incubated in 2% FCS for the same period shows no signs of re-epithelialization.
Figure 2A, B. A) Wound treated with Flamazine for 14 days. At the wound margin, the keratinocytes have formed an epithelial bud, a sign of re-epithelialization. B) A wound treated with Aquacel Ag with a keratinocyte bud and a thin layer of keratinocytes traveling against the wound surface.
Figure 3A, B. A) Wounds incubated in 10% FCS and B) 2% FCS. No discoloration or aggregation is found throughout the tissue in any of these wounds.
Figure 4A, B. A) Wound treated with Acticoat. B) Cells containing small (black) aggregates are visible in great numbers at the wound margins and throughout the whole dermis. Some of these aggregate-containing cells are seen around blood vessels, and particles deposited around blood vessels are seen throughout the whole skin disc.
Figure 1C–F. Wounds treated with C) Acticoat, D) SilvaSorb, E) Silverlon, and F) silver nitrate incubated for 1 4 d a y s . N o n e o f t h e w o u n d s s h o w s i g n s o f re
-epithelialization.
DO
NOT
hydrophilic gel that traps microbes and releases silver ions through the interaction with sodium ions.
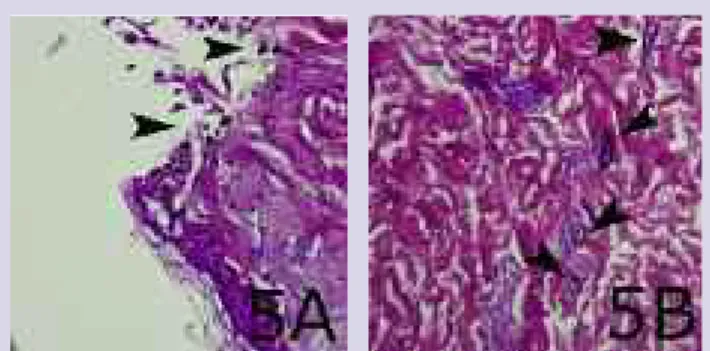
Findings. There was no discoloration in the ker-atinocyte layer in the wounds treated with this product. Particles and some aggregate-containing cells were seen at the wound margins together with particles aggregat-ed adjacent to blood vessels throughout the dermis. Wounds treated with Aquacel Ag had moderate deposits (Figure 5A, B).
Flamazine. Flamazine consists of 1.0% micronized silver sulphadiazine in a hydrophilic cream base. Sulphadiazine, as well as silver, provides an antimicrobial effect.
Findings.Slight discoloration of the keratinocyte layer was seen in these wounds. Cells with deposits were seen at the wound margins, throughout the wound, and also assembled around some blood vessels. Wounds treated with Flamazine had moderate deposits (Figure 6A, B).
PolyMem Silver. PolyMem Silver is composed of a polyurethane matrix on a semi-permeable thin film back-ing that promotes oxygen and vapor exchange. The dressing contains silver in a nanocrystalline form.
Findings. There was no discoloration in the ker-atinocyte layer in the PolyMem Silver group. There were no deposits in these wounds at any structure or level (Figure 7A, B).
SilvaSorb.SilvaSorb is composed of a synthetic poly-acrylate, with a highly absorbent MicroLattice® matrix containing stabilized silver. There was no discoloration in the keratinocyte layer in this group.
Findings. There were no particles deposited around blood vessels and no deposits in these wounds at any structure or level (Figure 8A, B).
Silverlon. Silverlon is a 3-dimensional mesh of nylon fibers, plated with silver, through the use of an autocat-alytic reduction-oxidation plating technology, resulting in silver released into the wound fluid in ionic form (Ag+) and is not colloidal (Ag0).
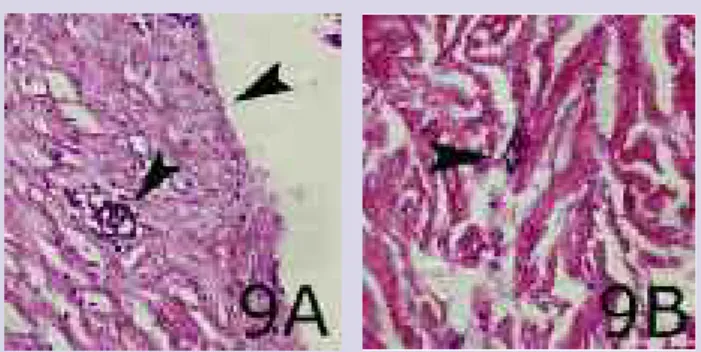
Findings. There was no discoloration in the ker-atinocyte layer. However, cells with black or gray deposits were seen at the wound margins and throughout the der-mis. Particles had also aggregated around blood vessels.
Figure 5A, B. A) Wound treated with Aquacel Ag. The keratinocyte layer has no discoloration, but the surface of the wound contains particles. B) Deeper in the tissue, particles and aggregate-containing cells are visible around blood vessels.
Figure 6A, B. A) Wound treated with silver sulphadiazine (Flamazine) where the keratinocyte layer shows a slight discoloration, but no particles. However, black particles are visible at the wound surface, at the wound margins, and extend down to dermal tissue. B) Cells containing aggregates assembled around blood vessels in deep dermal tissue.
Figure 8A, B. Wo u n d tre a te d w i th S i l v a S o rb . T h e keratinocyte layer is free from particles and no aggregates are found at any levels or structures in the tissue.
Figure 7A, B. Wound treated with PolyMem Silver with no discoloration, particles, or aggregates in the tissue.
DO
NOT
Wounds treated with Silverlon had moderate deposits com-pared to groups treated with other products (Figure 9A, B). Silver nitrate. The effects of silver nitrate probably result from silver ions readily combining with sul-phydryl-, carboxyl-, phosphate-, amino-, and other biolog-ically important chemical groups.
Findings. There was no discoloration in the ker-atinocyte layer of wounds treated with silver nitrate, but a thick layer of black particles covered the surfaces of the wounds. Cells containing black or gray aggregates were seen at the wound margins, throughout the wound bed, and also around a few blood vessels in the dermis. These wounds also showed moderate deposits (Figure 10A, C). Immunohistochemistry. Immunoassays that target-ed von Willebrand factor were ustarget-ed to verify that the cir-cular structures surrounded by black or gray shadows were in fact blood vessels. Cross-sections from the
con-trol groups showed staining for von Willebrand factor in the endothelial cells in native skin incubated with pri-mary antibody (Figure 11A) and no staining of the con-trols with the omission of the primary antibody (Figure 11B). Wounds from all groups treated with silver dress-ings, except for PolyMem Silver and SilvaSorb, had similar circular deposits around structures in the dermal layers (Figure 12A, B).
Discussion
The Western world has used the silver ion as a topical antimicrobial agent in burn care for many years. Klasen8,9 dated the use of silver back several hundred years. The toxicity of silver ions has not attracted much attention over the years, even though there are studies about it. Bridges and Lowbury1 suggest that silver sulphadiazine induced severe chronic inflammation in burns, but Vlachou et al6 state Acticoat is safe to use and should remain a standard part of the treatment at their clinic.
In the present study, black discoloration or particle deposits (or both) were seen throughout the tissue in all
Figure 9A, B. A) Wound treated with Silverlon that is free of particles and discoloration. B) Particles aggregated around blood vessels in deep dermal tissue.
Figure 10A–C. Wo u n d treated with silver nitrate. A) The keratinocyte layer is discolored and B) a thick layer of black particles is visible across the wound s u rfa c e . C ) C e l l s w i th gray/black aggregates are a s s e mb l e d n e a r b l o o d vessels.
Figure 11A, B.A) Negative controls (omission of antibody) show no staining. B) Positive staining for von Willebrand factor in endothelial cells from a control wound incubated with the primary antibody.
Figure 12A, B. A) Wound treated with Aquacel Ag. Immunoassay for von Willebrand factor reveals particles near blood vessels. B) Wound treated with Acticoat and immunoassayed for von Willebrand factor shows particles and aggregates near a blood vessel in deep dermal tissue.
DO
NOT
wounds treated with products containing silver, except for PolyMem Silver and SilvaSorb. At the wound margin and around blood vessels, there were clusters of cells that contained black particles and dark deposits around structures that were confirmed to be blood vessels through immunostaining for von Willebrand factor. However, not all blood vessels had deposits, and the num-ber of particles around individual blood vessels varied. Sets of controls, incubated without any products con-taining silver, showed no signs of deposits or discol-oration, which eliminates the medium and the wounds themselves as the source of discoloration. This leads to the conclusion that it is the treatment with products con-taining silver that results in black deposits throughout the tissue, not any surrounding factor. It seems unlikely to be anything other than silver, as all products have been manufactured in different ways and contain different materials and substances; silver is the only common denominator. Therefore, there is a strong likelihood that the discoloration and deposits in the wounds and sur-rounding tissue consist of silver that was released during treatment. However, not all products tested in this study left deposits in the tissues. Groups treated with PolyMem Silver and SilvaSorb showed no signs of discoloration or deposits, whereas all other products caused varying degrees of deposits near blood vessels and wound mar-gins. Acticoat caused the most deposits, and was the only dressing to cause black discoloration of the keratinocyte layer. Aquacel Ag caused the second most deposits, fol-lowed by Silverlon, Flamazine, and SilvaSorb. The results also indicate that all products tested delayed wound heal-ing. The wounds that were treated with Acticoat, Silverlon, and SilvaSorb showed no signs of re-epithelial-ization, and of all the products tested, seemed to decrease re-epithelialization the most. Contrary to our results, Demling and DeSanti10compared Acticoat with a standard Xeroform and 8-ply gauze dressing that was continually moistened with a 0.01% neomycin and polymyxin solution, and found that silver increased re-epithelialization by more than 40%, which was signifi-cant. They concluded that silver released into a moist environment significantly increased the rate of re-epithe-lialization compared with a standard antibiotic solution. Some degree of re-epithelialization was seen in the groups treated with Flamazine and PolyMem Silver. Aquacel Ag had the least effect on re-epithelialization, showing epithelial buds at the wound margins as well as an epithelial tongue that almost covered the entire wound bed. The controls incubated in 10% FCS showed
complete re-epithelialization and healing; whereas, the groups incubated in 2% FCS showed no re-epithelializa-tion after a 2-week incubare-epithelializa-tion, as expected, as this corre-sponds to a chronic wound. None of the groups treated with products containing silver showed the same grade of re-epithelialization or healing as the controls incubat-ed in 10% FCS. All 7 products delayincubat-ed re-epithelialization in this model.
Conclusion
A blackening of the wound bed is often transient bind-ing of silver to wound debris that will be shed as the wound heals. However, as suggested in this study, silver might also be deposited deeper into the tissue. It can only be speculated as to what extent these deposits might be harmful to patients and their wounds. Silver deposited near blood vessels could cause damage, and sil-ver might escape into the systemic circulation leading to silver protein complexes in different parts of the body (eg, skin, liver, kidney, bone marrow, or eyes).
When considering the effect of silver deposits in patients, the long-term effect of silver deposits in the der-mal layer of the skin must be taken into account. The only variable between the positive control groups and the treatment groups was the application of products containing silver, which makes it possible to estimate the effect of silver on re-epithelialization in this model.
The question remains as to whether the amount of sil-ver deposits in the tissue correlates with the antimicro-bial properties of the silver. One can surmise that nano-particles can penetrate further down and reach bacteria deeper into the tissue, thus exerting additional antibac-terial activity. One can also surmise that the same parti-cles are accumulated in the tissue, since they are too deep beneath the surface to be shed together with wound debris.
Before these results can be transferred to an in-vivo
situation, one must consider systemic factors and other elements that are different in vivo. For example, the con-centrations of applied silver on the wounds in a petri dish could be magnitudes greater than what would be applied to a metabolic unit, such as a chronic wound in vivo. Further study is needed to answer these questions.
Acknowledgements
The authors thank lab technicians Anita Lönn and Kristina Briheim for their help and support. We would also like to thank students Kristoffer Svensson and Malcolm Anderson for their help during the study.
DO
NOT
References
1. Bridges K, Lowbury EJ. Drug resistance in relation to use of silver sulphadiazine cream in a burns unit. J Clin Pathol. 1977;30(2):160–164.
2. Walker M, Cochrane CA, Bowler PG, Parsons D, Bradshaw P. Silver deposition and tissue staining associated with wound dressings containing silver. Ostomy Wound Manage. 2006;52(1):42–50.
3. Innes ME, Umraw N, Fish JS, Gomez M, Cartotto RC. The use of silver coated dressings on donor site wounds: a prospective, controlled matched pair study. Burns. 2001;27(6):621–627.
4. Trop M. Silver coated dressing Acticoat caused raised liver enzymes and argyria-like symptoms in burn patient.
J Trauma. 2006;61(4):1024.
5. Poon VK, Burd A. In vitro cytotoxity of silver: implication for clinical wound care. Burns. 2004;30(2):140–147. 6. Vlachou E, Chipp E, Shale E, Wilson YT, Papini R, Moiemen
NS. The safety of nanocrystalline silver dressings on burns: a study of systemic silver absorption. Burns. 2007;33(8):979–985.
7. Emanuelsson P, Kratz G. Characterization of a new in vitro burn wound model. Burns. 1997;23(1):32–36. 8. Klasen HJ. Historical review of the use of silver in the
treatment of burns. I. Early uses. Burns. 2000;26(2):117–130.
9. Klasen HJ. A historical review of the use of silver in the treatment of burns. II. Renewed interest for silver. Burns. 2000;26(2):131–138.
10. Demling RH, DeSanti L. The rate of re-epithelialization across meshed skin grafts is increased with exposure to silver. Burns. 2002;28(3):264–266.