www.wjpr.net Vol 3, Issue 8, 2014. 467 MICROWAVE SYNTHESIS- CHARACTERIZATION AND ANTIMICROBIAL STUDIES OF NOVEL LIGAND (2, 6- DIACETYLPYRIDINE AND 5-AMINO-1, 3,
4-THIADIAZOLE-2-THIOL) AND ITS METAL COMPLEX
1
Dr.Mohammed.Fakruddin Ali Ahamed*, 2Segni Asafa
Department of chemistry, College of Natural Science, Jimma University, Post-Box No:
378-Ethiopia.
ABSTRACT
Metal complexes of Cu (II) with a new Schiff base derived from
2,6-Diacetyl pyridine-5-amino -1,3,4-thiadiazole -2-thiol (DAPATT) in
methanol are reported. The complexes have been characterized using
chemical analysis, (IR, UV-VIS, H1-NMR), conductometric data.
According to these data, we propose an octahedral geometry for metal
(II) complexes. The invitro antibacterial activities of the investigated
complexes were evaluated against few microorganisms by well
diffusion technique. It was found that the metal complexes have higher
activity that the standard drugs. Antibacterial activity of the ligand and
its complexes were studied against to gram positive bacteria;
Staphylococcus aureus and bacteria Salmonella typhi and
Escherichia coli.
KEYWORDS: Hexa-dentate Schiff base, 2,6Diacetyl pyridine5amino 1,3,4thiadiazole -2-thiol (DAPATT), Biological activity.
INTRODUCTION
A microwave is a form of electromagnetic energy, which falls at the lower end of the
electromagnetic spectrum. Recently, Microwave heating has emerged as a powerful
technique to promote a variety of chemical reactions[1,2].Microwave synthesis is attractive in
offering reduced pollution, low cost, saving time and offer high yields together with
simplicity in processing and handling [3].The recent introduction of single-mode technology
assures safe and reproducible experimental procedures and microwave synthesis has gained
acceptance and popularity among the synthetic chemist community.
Volume 3, Issue 8, 467-480. Research Article ISSN 2277 – 7105
Article Received on 22 July 2014,
Revised on16 August 2014, Accepted on 10 Sept 2014
*Correspondence for Author
Dr. Mohammed. Fakruddin Ali Ahmed
Department of chemistry,
College of Natural Science,
Jimma University, Post-Box
www.wjpr.net Vol 3, Issue 8, 2014. 468 Microwave irradiation now a day is an accepted tool for accelerating the organic and
inorganic reactions. It leads to the higher reaction selectivity and utilization of the
inexpensive reagents. In addition to providing an eco-friendly “green chemistry” approach to
the reaction, it is free of environmental impacts [4-7].the application of microwave irradiation
towards the acceleration of wide range of organic and inorganic reactions has received
concealable attention [8-13] .It also allowed a greener approach [14]. Schiff base of an important
class of ligands in coordination chemistry and have many applications [15], in different fields.
The chemistry of Schiff base complexes continues to attract many researchers [16, 17]. because
of their wide application in food industry, dye industry ,analytical chemistry catalysis
,antimicrobial activity, agro-chemical activity[18] and pharmacological applications [19]. The
application of microwave irradiation to organic synthesis has been the focus of considerable
attention in recent years and is becoming an increasingly popular technology.
Schiff bases are generally bi-or tri- dentate ligands capable of forming very stable complexes
with transition metals. Schiff bases have number of applications. Some are used as liquid
crystals. In organic synthesis, Schiff base reactions are useful in making carbon-nitrogen
bonds. Schiff bases appear to be an important intermediate in a number of enzymatic
reactions involving interaction of an enzyme with an amino or a carbonyl group of the
substrate. One of the most important types of catalytic mechanism is the biochemical process
which involves the condensation of a primary amine in an enzyme usually that of a lysine
residue, with a carbonyl group of the substrate to form an imine, or Schiff base.
Schiff bases are important class of compounds due to their flexibility, structural similarities
with natural biological substances and also due to presence of imine (N=CH-) which imports
in elucidating the mechanism of transformation and racemization reaction in biological
system which have capable of forming coordinate bonds with many of metal ions through
either/both azomethine nitrogen and phenolic oxygen. A large number of Schiff bases and
their complexes are of significant interest and attention because of their biological activity
including anti-tumor, antibacterial, fungicidal, anti-carcinogenic and catalytic activity [20,21].
Heterocyclic amineshave been widely used for the synthesis of new Schiff’s bases. Azoles,
thiadiazole and their derivatives continue to draw the attention of synthetic organic and
inorganic chemists due to the large group of compounds possessing a wide spectrum of uses.
Heterocyclic compounds possessing the 1, 3, 4-thiadiazole ring system shows antifungal,
www.wjpr.net Vol 3, Issue 8, 2014. 469 number of compounds which display biological activity. The biological activity of the
compounds is mainly dependent on their molecular structures.1, 3, 4-thiadiazoles are very
interesting compounds due to their important applications in many pharmaceutical, biological
and analytical field.
N
C H
3C H
3N
O
N
N
S
S H
Proposed structure of ligand-fig-1 MATERIALS AND METHODS
The entire chemical used was of analytical grade. The solvents were dried and distilled before
use according to standard procedures. Melting points were determined in a Electro thermal
9200.H1NMR spectra in CDCl3 and DMSO were recorded on NMR spectrophotometer. The
IR spectra (methanol/KBr) were recorded in the range400-4000 cm-1 by KBr pellet using
Perkin-Elmer 457 spectrophotometer. Conductance was measured in DMF at room
temperature using a Digital conductivity bridge. The UV-Visible spectra in CH3OH were
recorded on a shimadzu UV 1800 spectrophotometer. The metal contents were determined
gravimetrically.
Preparation of DAPATT
The reaction mixture containing 2,6-Diacetyl pyridine , (2g,0.077mol in 20ml of methanol )
5-amino -1,3,4-thiadiazole -2-thiol (0.848g ,0.077 mol in 20ml of methanol dissolved in hot
condition) was taken in 250‐ml round bottom flask and refluxed for 8h. On cooling the reaction mixture, deep-red coloured product was formed. It was collected by filtration and
washed with hot water and 50 % cold methanol. This compound was recrystallised from
www.wjpr.net Vol 3, Issue 8, 2014. 470
N
CH
3CH
3O
O
+
N
N
S
SH
NH
2Con HCl
Reflux
N
CH
3CH
3N
O
N
N
S
[image:4.595.93.507.79.242.2]SH
Fig. 2: preparation of Ligand – DAPATT
RESULT AND DISCUSSION Physical characteristics
The details of physical characterization of the ligand and metal complexes are tabulated in
table 1.
Table 1.Analytical and physical data for the ligand and its complex
Compound Colour M.pt (oC) Tield% Physical appearance Ligand
(DAPATT) brown 195-197 74.82 Powder Cu Complex of
(DAPATT) green 276-277 71.24 Powder
Molar Conductance Measurements
The molar conductance of Cu complex was measured in 10-3 M solution in DMSO as
solvents at room temperature. It showed a molar conductivity values 139.4 µs/cm. So the
molar conductance of this Cu complex 139.4 Ω-1 cm2 mol-1.From this concluded that Cu
complex is electrolyte.
Chemistry of Ligand and Its Copper Complex
The purity of the synthesized Schiff bases and its copper complex was checked on TLC
plates and the spot was visualized under ultraviolet light. Also their purity was checked on
melting point. The functional group on synthesized ligands was established through
spectroscopic data IR. The below spectra presents the characterization of the compounds,
www.wjpr.net Vol 3, Issue 8, 2014. 471
[image:5.595.179.419.93.238.2]
Fig 1.IR spectra of ligand (DAPATT)
fig 2. IR spectra of Cu complex
The Data Stated From The Above IR Spectra f Ligand (DAPATT) And Its Cu Complex Compound (C-N)
cm-1
(C-S) cm-1
(C=N) cm-1
(C=O
) cm-1
(-CH3)
cm-1
(C-H) cm-1
(Cu-N) cm-1
ν(Cu-O) cm-1 L ~1500 ~1000 ~1625 1700–
1721
2856
2931 - -
[CuLH2O]SO4-2 1458 1150 1595 1617 2856 2931 490 554
Infra-Red Spectroscopy
Spectroscopy is the study of the interaction of energy and matter. Absorption of energy of
different magnitudes causes different changes in matter. The magnitude of energy absorbed is
determined by the structure of the matter under study. If we understand how the structure of
matter influences the energy absorbed, we can work backwards to elucidate certain features
www.wjpr.net Vol 3, Issue 8, 2014. 472 A spectroscopic method that determines the functional groups is infrared (IR) spectroscopy.
In this technique, we expose the molecule in question to infrared photons. Functional groups
absorb infrared photons of characteristic energies. We then make a plot of photon energy
versus intensity of absorption, called the infrared spectrum. Therefore IR spectroscopy allows
us to deduce the functional groups that arepresent and absent in a molecule.
Bond Polarity and Absorption Intensity
Like any other type of spectrum, an IR spectrum is a plot of energy (expressed as frequency
or wavelength of photons) versus intensity of absorption or transmittance. Bond polarity and
absorption intensity show correlation IR spectra; less polar bonds cause weaker absorptions
(smaller peaks) than more polar bonds. For example, the C-H bonds are slightly polar
whereas the C≡C bond is non polar (because it is symmetrical). In the IR spectrum, the C–H
stretches appear at 2963-2669 cm-1 whereas the C≡C stretch which usually occurs ~2200 Cm
-1
so weak that it cannot be seen.
The 4000-1450 cm-1 portion of the IR spectrum, sometimes called the functional group
region. The segment of the IR spectrum below 1450 cm-1 is called the fingerprint region. The
fingerprint region contains much absorption. Features that influence stretching frequency
(ring strain, conjugation, hydrogen bonding, electron-withdrawing or electron-donating
effects, etc.)
In case of this study FTIR spectrum of the ligand (L) showed some characteristic stretching
bands at: 2931, 2856, 1700, assigned to–CH3, C-H and C=O bond respectively which could
be found in complexes and 2760, 1625,673 assigned to S-H, C = N of thiadiazole ring, and
the last one is for stretching of C-S bond, respectively which could be found in Cu complex
[22- 24] .
The band of S-H in the ligand was disappeared when complexation occur. Characteristic
absorption new bands for (Cu-N) and (Cu-O) of the complexes appear respectively at
stretching frequency 490 cm-1and 554 cm-1[25].And the band of C = N and C=O is shifted to
the lower frequency due to complexation, but the other bands such as - CH3, C-H aromatic
were didn’t show any shifting because they aren’t participate in the complexation[26].
NMR Spectroscopic Analysis of the Schiff Base Ligand (DAPATT)
Over the past fifty years nuclear magnetic resonance spectroscopy, commonly referred to as
www.wjpr.net Vol 3, Issue 8, 2014. 473 compounds. Of all the spectroscopic methods, it is the only one for which a complete analysis
and interpretation of the entire spectrum is normally expected. Nuclear Magnetic Resonance
(NMR) spectroscopy is an analytical chemistry technique used in quality control and research
for determining the content and purity of a sample as well as its molecular structure. For
example, NMR can quantitatively analyze mixtures containing known compounds. For
unknown compounds, NMR can either be used to match against spectral libraries or to infer
the basic structure directly. Once the basic structure is known, NMR can be used to determine
molecular conformation in solution as well as studying physical properties at the molecular
level such as conformational exchange, phase changes, solubility, and diffusion.
DEPT spectra
DEPT stands for Distortion less Enhancement by Polarization Transfer. It is a very useful
method for determining the presence of primary, secondary and tertiary carbon atoms.
The DEPT experiment differentiates between CH, CH2 and CH3 groups by variation of the
selection angle parameter (the tip angle of the final 1H pulse):
1. 135° angle gives all CH and CH3 in a phase opposite to CH2
2. 90° angle gives only CH groups, the others being suppressed
3. 45° angle gives all carbons with attached protons (regardless of number) in phase
Signals from quaternary carbons and other carbons with no attached protons are always
absent (due to the lack of attached protons).
1
[image:7.595.134.457.515.717.2]H - NMR Spectroscopic Analysis of the Schiff Base (DAPATT)
www.wjpr.net Vol 3, Issue 8, 2014. 474 The 1H - NMR spectra of the Schiff bases were recorded in DMSO (Fig 3). For CH aromatic
proton,the ligand shows singlet in the region 8.135 ppm. The1H -NMR signal at =2.694
ppm sharp and singlet peak is due to –CH3 proton [27, 28] .
13
[image:8.595.143.453.163.370.2]C - NMR spectroscopic analysis of the Schiff base (DAPATT)
Fig. 4 13C NMR Spectra of Ligand (DAPATT) 13
C NMR showed that carbon (RCH3) (a) of this compound appeared at 25.807 ppm, (R3CH)
(e) at 39.943 ppm, (N=C) azomethine (imines) group at 152.644 ppm, (N=C-R) at 139.401
ppm and carbon(C=C- RH) (d) at 125.003 ppm in pyridine ring. As well as 13C NMR showed
that carbon (N=C-S2) (h) at 181.421 ppm and Carbon (N=C-SN) (g) at 161.940 ppm in
thiadiazole ring. The carbonyl carbon (b) also deshielded and appeared at 199.225ppm due to
inductive effect (Oxygen attracting electron from carbonyl carbon)[29].
[image:8.595.142.455.524.723.2]www.wjpr.net Vol 3, Issue 8, 2014. 475
The above DEPT-135 Spectra show that the compound contains CH and CH3 carbon (there is
no CH2 carbon).The quaternary carbon was absent.
Electronic spectroscopy
Electronic spectroscopy is an analytical technique to study the electronic structure and its
dynamics in atoms and molecules. Electronic absorption spectra are often very helpful in the
evaluation of results furnished by other methods of structure investigation. The electronic
spectral measurements were used for assigning the stereochemistry of metal ions in the
complexes based on the positions and numbers of d-d transition bands.In general an
excitation source such as X- rays, electrons, or synchrotron radiation will eject an electron
from an inner-shell orbital of an atom. The Beer-Lambert law states that the absorbance of a
solution is due to the solution's concentration. Thus UV/vis spectroscopy can be used to
determine the concentration of a solution. It is necessary to know how quickly the absorbance
changes with concentration.
The method is most often used in a quantitative way to determine concentrations of an
absorbing species in solution, using the Beer-Lambert law:
A= -log (I /I0) =. C. l
Where A is the measured absorbance, I0 is the intensity of the incident light at a given
wavelength, I is the transmitted intensity, / is the path length through the sample, and c is the
concentration of the absorbing species. Samples for UV /vis spectrophotometer are most
often liquids, although the absorbance of gases and even of solids can also be measured.
Samples are typically placed in a transparent cell, known as a cuvette.Cuvettes are typically
rectangular in shape, commonly with an internal width of 1 cm. (This width becomes the path
length, /, in the Beer-Lambert law).
Test tubes can also be used as cuvettes in some instruments. The best cuvettes are made of
high quality quartz, although glass or plastic cuvettes are common. (Glass and most plastics
absorb in the UV, which limits their usefulness to visible wavelengths.) An ultraviolet-visible
spectrum is essentially a graph of light absorbance versus wavelength in a range of ultraviolet
or visible regions. Such a spectrum can often be produced by a more sophisticated
spectrophotometer. Wavelength is often represented by the symbol . For the given
substance, the wavelength at which maximum absorption in the spectrum occurs is called
www.wjpr.net Vol 3, Issue 8, 2014. 476 Electronic spectra of Schiff base (DAPATT) and its complexes
Figure 6.Electronic Spectra of Ligand (DAPATT)
Figure 7.Electronic Spectra of Copper Complex
The UV-Vis spectral data of the ligands (DAPATT) and the Cu complex were recorded in
DMSO in the wavelength range from 800 – 200 nm at room temperature. The UV-Vis spectra
of the ligand(DAPATT) showed bands at 220, nm 260 nm,295 nm,345 nm and 377 nm,
assigned to π → π* and n →π* transition with the molecular, these bands were slightly
shifted to lower wavelength region for the complexes due to coordination with metal ions.
The electronic spectra of copper complex displays bands, which are assigned as an
intraligand charge transfer band (382 nm) and d-d band (700 nm) which is due to 2B1g →2Eg
transition. This d-d band strongly favors square-planar geometry for the copper complex[30].
The various wavelengths, band assignments and the proposed geometry of the complex are
[image:10.595.144.453.325.520.2]www.wjpr.net Vol 3, Issue 8, 2014. 477 Table 2. Electronic Spectral Data of Schiff Base DAPATT and Its Copper Complexes
Compound (nm) Band assignments Geometry
Ligand (L)
220, 260 295 345 377
π→ π*, n →π*( C=O) (INCT) n →π*( C=O)
n →π*(C=N) n →π*(C=N)
-
[CuLH2O]SO4-2 382 700
INCT 2
B1g →2Eg transition Octahedral INCT-intraligand charge transfer
O H 2 N
N S
S H
N
C H 3 C H 3
N O
M
Cu-complex with ligand Antibacterial Activity of Ligand and Its Metal Complex
Disc Diffusion Assay
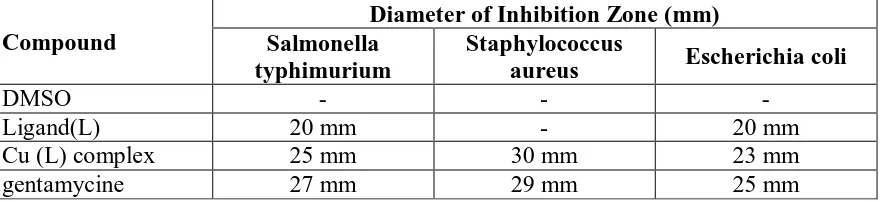
The in vitro antibacterial activity of the DMSO, ligand and its metal complexes were tested
against three different bacteria at a concentration of 10-2 M table 2.At this concentration the
Cu complex shows significant antimicrobial activity against the tested pathogens. The degree
of inhibition varied with the nature of the compound. These concentrations of the ligand and
complexes were used to get visible results. The highest zone of inhibition i.e. 2.5 and 3 cm
were measured in salmonella and staphelo coccus aurus when treated with Cu (II) complex.
The zone of inhibition is greatly affected by the thickness of the test agar layer. As the
thickness increases, the zone of inhibition decreases. This can be attributed to the decrease of
concentration of the ligand and its complexes per unit volume of the culture media. Another
factor, which influences the inhibition zone, is inoculum size (concentration of the organism
per unit volume). The diameter of the inhibition zone decreases with increase in the inoculum
www.wjpr.net Vol 3, Issue 8, 2014. 478 Table 3: Antibacterial screening data of investigated ligand (DAPATT) and its Cu complex
Compound
Diameter of Inhibition Zone (mm) Salmonella
typhimurium
Staphylococcus
aureus Escherichia coli
DMSO - - -
Ligand(L) 20 mm - 20 mm
Cu (L) complex 25 mm 30 mm 23 mm
gentamycine 27 mm 29 mm 25 mm
There is a significant reduction in the growth rate of microorganisms due to unfavorable
culture media, low temperature and acidic pH. The activity test was conducted at an optimum
temperature of 37 0C antibacterial activities. Synthesized compounds were investigated for
their antimicrobial activity by agar diffusion method [31].
In case of solvent control disc no zone of inhibition was observed as far as our study is
concerned DMSO, as a solvent is having no effect on the tested bacterial species. Hence we
can effectively conclude here that whole of the antimicrobial effect is due to the nature of the
metal complexes and the ligand used (synthesized) in this study. The antimicrobial behavior
of the Cu complex when compared with standard antibacterial drugs showed momentous and
identical biological properties [32]; even inStaphylococcus aurous showed more activity.
Due to the high antimicrobial activities and chelation of this metal ion in its complex, this
enhances the lipophylic character favoring its permeation through the lipid layer of cell
membrane.
CONCLUSION
A DAPATT ligand was synthesized by microwave method from precursor 5-mercapto-1, 3,
4-thiadiazole-2-thiol with 2, 6-diactylpyridine in purified ethanol. The copper complex was
synthesized by direct method. In direct method, DAPATT and Cu (II) ions were condensed.
Based on conductivity, UV-Vis, IR and NMR spectroscopy studies it is concluded that the
ligand bonds to the metal ion through azomethine nitrogen, carbonyl oxygen (-C=O),
pyridine nitrogen, water molecule oxygen (H2O) and 1, 3, 4-thiadiazole sulfur atoms. The
newly synthesized [CuLH2O] SO4-2 complex is concluded to have an octahedral geometry
with the sixth coordination site benig fulfilled by H2Oion. In addition, the Schiff base ligand
(DAPATT) and its copper complex were evaluated for their in vitro antibacterial activity
[image:12.595.71.512.114.215.2]www.wjpr.net Vol 3, Issue 8, 2014. 479 REFERENCE
1. P. Lidstrom, J.T., B. Wathey, J. Westman, Tetrahedron, , The rapid synthesis of
schiff-bases without solvent under microwave irradiation and their antimicrobial activity. 2001;
57: 9225.
2. Jnyanaranjan Panda, V.J.P., 1 BiswaMohan Sahoo,1 and JitendriyaMishra2, Green
Chemistry Approach for Efficient Synthesis of Schiff Bases of Isatin Derivatives and
Evaluation of Their Antibacterial Activities. 2013.
3. D. Adam, O.o.t.k., Nature, The rapid synthesis of schiff-bases without solvent under
microwave irradiation and their antimicrobial activity. 2003; 421: 571.
4. Ashry,E S H E1,Ramadan E,Kassem E, Kassem A A& Hager M,AdvHeterocycl Chem,
2005; 68.
5. Kappe C O & Loupy A, Microwave in Organic Synthesis (Wiley-VCH, Weinheim),
2002; 405.
6. Kappe C O,Curr Opinion Chem Bio, 2002; 6: 314.
7. Danida A, Arya K, Sati M &Gautam S, Tetrahedron,2004; 60: 5253.
8. Gedye R, Smith F,Westaway K, Ali H, Baldisera L, Laberge L &Rousell J, Tetrahedron
Lett. 1986; 27: 279.
9. Gedye R J, Bray T L & Duncan S M, Tetrahedron Lett. 1986; 27: 2945.
10.CaddickS,TetrahedronLett, 1994; 50: 10403.
11.Mingos D M P &Baghurst D R, Chem Rev, 1991; 20.
12.Whittaker A G &Mingos D M P,J Microwave Power Electomag Energy, 1994; 29: 195.
13.Mingos D M P,ResChemIntermid, 1994; 20: 85.
14.Whittaker A G, EducChem, 2002; 134.
15.Suma S, Kumar M R S,Nair C R &Prabhakaran C P, Indian J Chem,32A. 1993; 214.
16.Warad D U, Salish C D, Kulkarani V H &Bajgur C S,Indian J Chem,39A, 2000; 415.
17.Sen A K, Singh G, Singh K, Noren R K, Handa R N &Dubey S N,Indian J Chem,36A
1997: 891.
18.Ramarao N, Rao P Venkateduwar, Reddy G Venkat&Gasnorkar M C,Indian J Chem,26A
1987; 887.
19.Santoskar R S &Bhandarkar S D, Pharmacology and Pheumacothepeutic (Popular
Prakasan, Bombay), 1993; 648.
20.Srivastava, K.V., S. N. Rakesh, Singh. Der Chemica Sinica, Environmentally benign
studies of bivalent transition metal complexes of tridentate (NNO donor) Schiff base
www.wjpr.net Vol 3, Issue 8, 2014. 480 21.Arulmurugan, S.H., P.; Kavitha, B.; Venkatraman, R., Biological activities of Schiff base
and its complexes. a review. Rasayan J. Che, 2010; 3(3): 385-410.
22.Silvertein R, B.G.N.Y.J.W.S., Spectrometric identification of organic compounds. 1980.
23.pedro ortega-luoni *, claudio astudillo, miguel guzmán and pedro ortega-lópez2, Synthesis
of metallic azoderivatives of 2-amino-5-mercapto-1,3,4-thiadiazole. J. chil. chem. soc,
2007; 52.
24.Yousif E, H.A., Ameer A Synthesis and characterization of complexes of some
transition metals with 2-amino-5- (4-hexyloxyphenyl)-1,3,4-thiadiazole. J of Al-Nahrain
University, 2005; 8(1): 9-11.
24.S, Q., Synthesis and Characterization of some transition metal Complexes of Schiff base
derived from isonicotinic hydrazide and O-Vanillin. Diyala J Pure Sci, 2011; 7(2):
94-104.
25.Flifel I, K.S., Synthesis and Chracterization of 1,3,4- oxadiazole derivatives with some
new transition metal complexes. J of Kerbala Uni, 2012; 10(3): 197-209.
26.P. Venkatesh, Synthesis, charecterisation and anti microbial activity of various Schiff
base complex of zinc(II) and copper (II) ions. Asian Journal of Pharmaceutical and Health
Sciences, 2011; 1: 8-11.
27.Ahmed .T.AL- Jeboori ,R., schiff bases: facile synthesis, spectral characterization and
biocidal studies.Mar-2012; 3: 0976-4550.
28.Hashim Al-Noor, A.T.A.-J., Rasha. L. Sadawi Synthesis and Characterization of
Complexes of Schiff Base
[1,2-Diphenyl-2-2-{[1-(3-Amino-Phenyl)-Ethylidene]-Hydrazono Methyl}-Phenol] with Mn(II), Fe(II), Co(II), Cu(II), Zn(II), Cd(II), Ni(II), and
Hg(II) Ions Taghreed. 2013.
29.Al-Amery*, M.H.A., Synthesis, characterization and spectroscopic studies on Schiff base
complexes of 1-phenyl-2, 3-dimethyl-4-amino-5-oxo-pyrazole with salicylaldehyde with
some divalent transition metals Baghdad Science, 2011; 8(3): 187.
30.R. A. Sheikh, S.S., M. A. Malik, L. A. Khan, A. A. Hashmi, , Transition Metal
Complexes with Mixed Nitrogen-Sulphur (N-S) Donor Macrocyclic Schiff Base Ligand:
Synthesis, Spectral, Electrochemical and Antimicrobial Studies. J. Chem. Pharm. Res,
2010; 2: 133.
31.S. Shreaz, R.A.S., B. Rimple, A.A Hashmi, M. Nikhat, L.A. Khan,, Microbial