ODONTOGENIC TUMOR DISTRIBUTION IN SOUTH INDIAN
SAMPLE POPULATION: A RETROSPECTIVE ANALYSIS OF 111
CASES ACCORDING TO WHO 2005 CLASSIFICATION
Dr. Praveen S. Basandi., M.D.S1, Dr. Adarsh H., M.D.S*2,Dr. Vaidehi Nayak, M.D.S3
1
Professor, Department of Oral and Maxillofacial Pathology and Microbiology, College of Dental Sciences and Hospital, Davangere, Karnataka.
2
Associate Professor, Dept. of Dentistry, BGS Global institute of Medical Sceinces, Bengaluru, Karnataka.
3
Reader, Department of Oral and Maxillofacial Pathology and Microbiology, Rajarajeshwari Dental College and Hospital, Bengaluru, Karnataka.
ABSTRACT
Objective: To study the frequency and distribution of odontogenic tumors (OTs) and their clinico-pathological features among South Indian sample population by analyzing biopsy specimens obtained from the archives of the Department of Oral and Maxillofacial Pathology, College of Dental Sciences, Davangere, Karnataka, India, from January 2000 to June 2014. Study design: Data for the study were retrieved from the case records of patients and the analyzed clinical variables included age, gender and anatomical location. Histopathological features of the lesions were reevaluated and those fitting the World Health Organization (2005) histological classification for odontogenic tumors, including Kerato Cystic Odontogenic tumor (KCOT) were analyzed in the study. Results: Of the 3148 biopsy reports analyzed, 111 cases (3.52%) were odontogenic tumors, including ameloblastoma (52.2%), KCOT (27.9%), odontogenic myxoma (6.3%), adenomatoid odontogenic tumor (5.4%), odontoma (2.7%),
Volume 6, Issue 8, 2323-2340. Research Article ISSN 2277– 7105
*Corresponding Author
Dr. Adarsh H.
Associate Professor, Dept.
of Dentistry, BGS Global
institute of Medical
Sceinces, Bengaluru,
Karnataka. Article Received on 19 June 2017,
Revised on 10 July 2017, Accepted on 31 July 2017
Ameloblastoma is the most frequent odontogenic tumor followed by KCOT in this study. Including KCOT under odontogenic tumors, has numerically raised the overall percentage of OTs and brought down the percentage of individual lesions, in particular ameloblastoma. The concept of presence of geographic and regional variations in the occurrence of odontogenic tumors is favored by the study results.
KEYWORDS: Ameloblastoma, Kerato Cystic Odontogenic Tumor, Odontogenic Tumor.
INTRODUCTION
Odontogenic tumors (OTs) encompass a group of heterogenous lesions arising from the epithelium and/or mesenchymal remnants of tooth forming apparatus.[1] The odontogenic cell rests such as epithelial remains of Malassez, cell rests of Serres, or the enamel organ may get entrapped within the bone tissue or gingival tissue of the jaws, and on active proliferation, give rise to different forms of odontogenic tumors. These tumors have a broad range of clinical presentations from hamartomatous proliferations to benign and malignant neoplasms with varying degree of aggressiveness and metastatic possibility.[2]
The World Health Organization (WHO) first classified odontogenic tumors in the year 1971, but by 1992, a revised edition of WHO classification was proposed by Philipsen and Reichart. In 2005, WHO published recent revision on classification including keratocystic odontogenic tumor (KCOT), as new entity under benign odontogenic tumor.[3]
Despite the large number of studies on odontogenic tumors in the literature, information regarding the demographic profile of these lesions in different populations shows varying prevalence. Thus, the objective of the present study is to determine the distribution of odontogenic tumors in South Indian sample population, in and around Davangere, Karnataka, India over a period of January 2001 to June 2014.
METHODOLOGY
and eosin were selected and re-evaluated according to the current concepts outlined by the WHO 2005.[3]
Inclusion criteria involved the histological confirmation of odontogenic tumors. Lesions with histological findings that were not compatible with odontogenic tumor were excluded from the study. Cases with incomplete clinical data were also excluded.
The following clinical variables were analyzed: prevalence rate, age, gender, and anatomical site and the recurrent lesions, if reported were considered as single. The following anatomical sites were defined: anterior, posterior, and anterio-posterior segments in both the maxilla and mandible.
The anterior segment was defined as the region between distal aspect of right canine to distal aspect of left canine. The posterior segment began from the mesial aspect of first premolar and extended towards molar region. In case of mandible, angle-ramus-condylar region were also included under posterior segment. The anterior-posterior segment was defined as involving both these anterior and posterior segments. Utility of imaging aids like intraoral periapical radiographs, orthopantamographs, computed tomography, lateral oblique, occlusal view was done to determine the extent of the lesion in the available cases.
Data were subjected to descriptive statistical analyses with the SPSS version 16.0 statistical software package (SPSS Inc., Chicago, USA).
RESULTS
Prevalence rate
Gender and Age
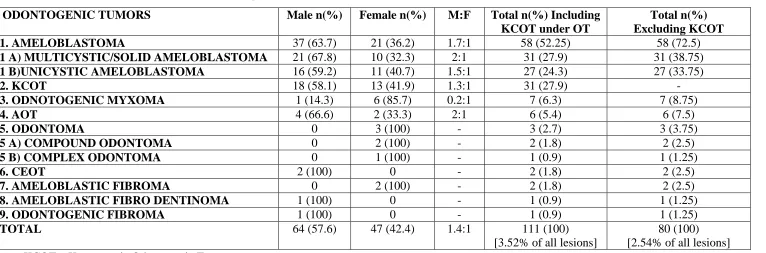
Of the 111 odontogenic tumors, 64 cases (57.6%) were observed in men and 47 cases (42.4%) were seen in women, with the male: female ratio of 1.4:1. On individual attention, all lesions except odontoma and odontogenic myxoma showed slight male predilection. The two lesions, CEOT and odontogenic fibroma occurred exclusively in male, whereas odontoma occurred exclusively in female (Table 1).
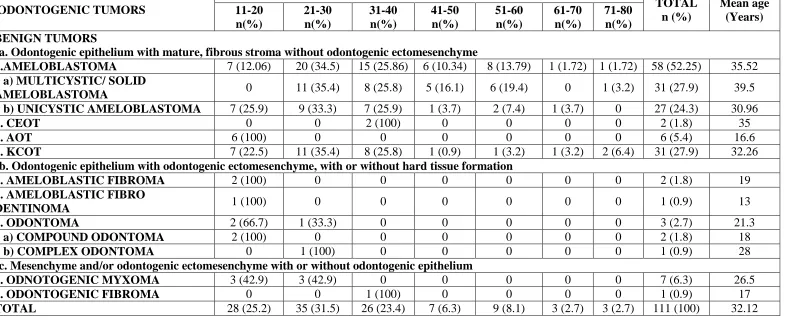
The mean age for diagnosis of odontogenic tumors in our study series was 32.12 years (range: 11–80 years). 28 cases (25.2%), 35 cases (31.5%) and 26 cases (23.4%) were diagnosed in the second, third and fourth decades of life, respectively, thereby 80.1% of lesions had occurred between the second and fourth decades of life. Ameloblastoma and KCOT, the first and second most common odontogenic tumors in this study respectively, showed peak occurrence in third decade. 70.68 % of ameloblastoma lesions (96.7% of solid ameloblastoma, 62.9% of unicystic ameloblastoma) occurred in third to fifth decade, while 85.18% of unicystic ameloblastoma and 83.8% of KCOT occurred between second and fourth decade. AOT, compound odontoma, ameloblastic fibroma, ameloblastic fibro– dentinoma occurred exclusively in younger age group, at second decade. A single case of complex odontoma occurring at third decade, 2 cases of CEOT and 1 case of odontogenic fibroma occurring at fourth decade had been reported so far. Odontogenic myxoma showed equal occurrence in both second and third decades (Table 2).
Anatomical Site
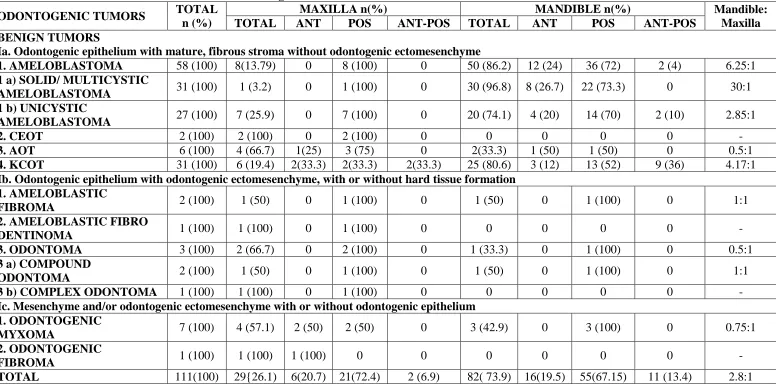
Mandible [82 cases (73.9%)] was the predominant site of occurrence for these odontogenic tumors and 29 cases (26.1%) were on the maxilla, with the mandible: maxilla ratio of 2.8:1. In the mandible, the most commonly affected segment was the posterior region [55 cases (67.15 %)], followed by anterior [16 cases (19.5%)] and anterio-posterior segment [11 cases (13.4%)]. Similarly, in maxilla, posterior segment was mostly affected [21 cases (72.4 %)], followed by anterior [6 cases (20.7%)] and anterio-posterior segment [2 cases (6.9%)]. In maxilla, KCOT alone showed anterio-posterior extension and in mandible, KCOT and unicystic ameloblastoma involved anterio-posterior segments (Table 3).
ameloblastoma was common in the mandible (20 cases, 74.1%), that too at the posterior segment of mandible [14 cases (70%)]. In maxilla, 7 cases (25. 9%) of unicystic ameloblastoma were seen and all at the posterior segment. 80.64% of KCOT involved mandible and remarkably, 35.5% of KCOT lesions were involving both anterior and posterior segments [2 cases in maxilla (33.3%) and 9 cases in mandible (36%)].
Odontogenic myxoma [4 cases (57.1%)] and AOT [4 cases (66.7%)] showed maxillary predilection. Uncommonly, 75% of maxillary AOT (3 cases) were at posterior segment and mandible showed 2 cases (33.3%). Complex odontoma, CEOT, odontogenic fibroma, and ameloblastic fibro-dentinoma occurred only in maxilla. Compound odontoma and ameloblastic fibroma were distributed almost equally between maxilla and mandible (Table 3).
Table. 1: Gender wise distribution of odontogenic tumors.
ODONTOGENIC TUMORS Male n(%) Female n(%) M:F Total n(%) Including
KCOT under OT
Total n(%) Excluding KCOT
1. AMELOBLASTOMA 37 (63.7) 21 (36.2) 1.7:1 58 (52.25) 58 (72.5)
1 A) MULTICYSTIC/SOLID AMELOBLASTOMA 21 (67.8) 10 (32.3) 2:1 31 (27.9) 31 (38.75)
1 B)UNICYSTIC AMELOBLASTOMA 16 (59.2) 11 (40.7) 1.5:1 27 (24.3) 27 (33.75)
2. KCOT 18 (58.1) 13 (41.9) 1.3:1 31 (27.9) -
3. ODNOTOGENIC MYXOMA 1 (14.3) 6 (85.7) 0.2:1 7 (6.3) 7 (8.75)
4. AOT 4 (66.6) 2 (33.3) 2:1 6 (5.4) 6 (7.5)
5. ODONTOMA 0 3 (100) - 3 (2.7) 3 (3.75)
5 A) COMPOUND ODONTOMA 0 2 (100) - 2 (1.8) 2 (2.5)
5 B) COMPLEX ODONTOMA 0 1 (100) - 1 (0.9) 1 (1.25)
6. CEOT 2 (100) 0 - 2 (1.8) 2 (2.5)
7. AMELOBLASTIC FIBROMA 0 2 (100) - 2 (1.8) 2 (2.5)
8. AMELOBLASTIC FIBRO DENTINOMA 1 (100) 0 - 1 (0.9) 1 (1.25)
9. ODONTOGENIC FIBROMA 1 (100) 0 - 1 (0.9) 1 (1.25)
TOTAL 64 (57.6) 47 (42.4) 1.4:1 111 (100)
[3.52% of all lesions]
80 (100) [2.54% of all lesions]
KCOT – Keratocystic Odontogenic Tumor AOT – Adenomatoid Odontogenic Tumor
Table. 2: Age wise distribution of odontogenic tumors.
ODONTOGENIC TUMORS
Years
TOTAL n (%)
Mean age (Years) 11-20
n(%)
21-30 n(%)
31-40 n(%)
41-50 n(%)
51-60 n(%)
61-70 n(%)
71-80 n(%) BENIGN TUMORS
Ia. Odontogenic epithelium with mature, fibrous stroma without odontogenic ectomesenchyme
1.AMELOBLASTOMA 7 (12.06) 20 (34.5) 15 (25.86) 6 (10.34) 8 (13.79) 1 (1.72) 1 (1.72) 58 (52.25) 35.52
1 a) MULTICYSTIC/ SOLID
AMELOBLASTOMA 0 11 (35.4) 8 (25.8) 5 (16.1) 6 (19.4) 0 1 (3.2) 31 (27.9) 39.5
1 b) UNICYSTIC AMELOBLASTOMA 7 (25.9) 9 (33.3) 7 (25.9) 1 (3.7) 2 (7.4) 1 (3.7) 0 27 (24.3) 30.96
2. CEOT 0 0 2 (100) 0 0 0 0 2 (1.8) 35
3. AOT 6 (100) 0 0 0 0 0 0 6 (5.4) 16.6
4. KCOT 7 (22.5) 11 (35.4) 8 (25.8) 1 (0.9) 1 (3.2) 1 (3.2) 2 (6.4) 31 (27.9) 32.26
Ib. Odontogenic epithelium with odontogenic ectomesenchyme, with or without hard tissue formation
1. AMELOBLASTIC FIBROMA 2 (100) 0 0 0 0 0 0 2 (1.8) 19
2. AMELOBLASTIC FIBRO
DENTINOMA 1 (100) 0 0 0 0 0 0 1 (0.9) 13
3. ODONTOMA 2 (66.7) 1 (33.3) 0 0 0 0 0 3 (2.7) 21.3
3 a) COMPOUND ODONTOMA 2 (100) 0 0 0 0 0 0 2 (1.8) 18
3 b) COMPLEX ODONTOMA 0 1 (100) 0 0 0 0 0 1 (0.9) 28
Ic. Mesenchyme and/or odontogenic ectomesenchyme with or without odontogenic epithelium
1. ODNOTOGENIC MYXOMA 3 (42.9) 3 (42.9) 0 0 0 0 0 7 (6.3) 26.5
2. ODONTOGENIC FIBROMA 0 0 1 (100) 0 0 0 0 1 (0.9) 17
Table. 3: Anatomical site distribution of odontogenic tumors.
ODONTOGENIC TUMORS TOTAL
n (%)
MAXILLA n(%) MANDIBLE n(%) Mandible:
Maxilla
TOTAL ANT POS ANT-POS TOTAL ANT POS ANT-POS
BENIGN TUMORS
Ia. Odontogenic epithelium with mature, fibrous stroma without odontogenic ectomesenchyme
1. AMELOBLASTOMA 58 (100) 8(13.79) 0 8 (100) 0 50 (86.2) 12 (24) 36 (72) 2 (4) 6.25:1
1 a) SOLID/ MULTICYSTIC
AMELOBLASTOMA 31 (100) 1 (3.2) 0 1 (100) 0 30 (96.8) 8 (26.7) 22 (73.3) 0 30:1
1 b) UNICYSTIC
AMELOBLASTOMA 27 (100) 7 (25.9) 0 7 (100) 0 20 (74.1) 4 (20) 14 (70) 2 (10) 2.85:1
2. CEOT 2 (100) 2 (100) 0 2 (100) 0 0 0 0 0 -
3. AOT 6 (100) 4 (66.7) 1(25) 3 (75) 0 2(33.3) 1 (50) 1 (50) 0 0.5:1
4. KCOT 31 (100) 6 (19.4) 2(33.3) 2(33.3) 2(33.3) 25 (80.6) 3 (12) 13 (52) 9 (36) 4.17:1
Ib. Odontogenic epithelium with odontogenic ectomesenchyme, with or without hard tissue formation 1. AMELOBLASTIC
FIBROMA 2 (100) 1 (50) 0 1 (100) 0 1 (50) 0 1 (100) 0 1:1
2. AMELOBLASTIC FIBRO
DENTINOMA 1 (100) 1 (100) 0 1 (100) 0 0 0 0 0 -
3. ODONTOMA 3 (100) 2 (66.7) 0 2 (100) 0 1 (33.3) 0 1 (100) 0 0.5:1
3 a) COMPOUND
ODONTOMA 2 (100) 1 (50) 0 1 (100) 0 1 (50) 0 1 (100) 0 1:1
3 b) COMPLEX ODONTOMA 1 (100) 1 (100) 0 1 (100) 0 0 0 0 0 -
Ic. Mesenchyme and/or odontogenic ectomesenchyme with or without odontogenic epithelium 1. ODONTOGENIC
MYXOMA 7 (100) 4 (57.1) 2 (50) 2 (50) 0 3 (42.9) 0 3 (100) 0 0.75:1
2. ODONTOGENIC
FIBROMA 1 (100) 1 (100) 1 (100) 0 0 0 0 0 0 -
Table 4: Worldwide epidemiological studies on odontogenic tumors according to WHO 2005 (including KCOT).
Region/ Country of study
Number of OTs studied
Ameloblastoma (%)
KCOT (%)
Odontoma (%)
AOT (%)
Odontogenic
myxoma (%) M:F Man:Max
Malignant OTs (%)
Peak age of occurrence (Decades) ASIAN COUNTRIES
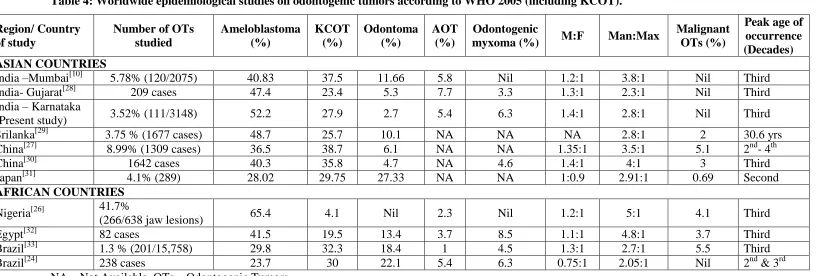
India –Mumbai[10] 5.78% (120/2075) 40.83 37.5 11.66 5.8 Nil 1.2:1 3.8:1 Nil Third India- Gujarat[28] 209 cases 47.4 23.4 5.3 7.7 3.3 1.3:1 2.3:1 Nil Third India – Karnataka
(Present study) 3.52% (111/3148) 52.2 27.9 2.7 5.4 6.3 1.4:1 2.8:1 Nil Third Srilanka[29] 3.75 % (1677 cases) 48.7 25.7 10.1 NA NA NA 2.8:1 2 30.6 yrs China[27] 8.99% (1309 cases) 36.5 38.7 6.1 NA NA 1.35:1 3.5:1 5.1 2nd- 4th China[30] 1642 cases 40.3 35.8 4.7 NA 4.6 1.4:1 4:1 3 Third Japan[31] 4.1% (289) 28.02 29.75 27.33 NA NA 1:0.9 2.91:1 0.69 Second
AFRICAN COUNTRIES
Nigeria[26] 41.7%
(266/638 jaw lesions) 65.4 4.1 Nil 2.3 Nil 1.2:1 5:1 4.1 Third Egypt[32] 82 cases 41.5 19.5 13.4 3.7 8.5 1.1:1 4.8:1 3.7 Third Brazil[33] 1.3 % (201/15,758) 29.8 32.3 18.4 1 4.5 1.3:1 2.7:1 5.5 Third Brazil[24] 238 cases 23.7 30 22.1 5.4 6.3 0.75:1 2.05:1 Nil 2nd & 3rd
Table. 5: Distribution of odontogenic tumours in India and Srilanka.
Region of study Number of
OTs studied Most common OTs in the study (%) M:F Man:Max Malignant OTs (%) Peak age of occurrence
India – Mumbai[9]
(Without KCOT) 250
Ameloblastoma -61.5 AOT -12.4
O myx & odontoma –6% each
1.2:1 3.8:1 1.2
20-29 yrs [74 cases (29.6)] 10-19 yrs [55 cases (22)] 30-39 yrs [44 cases (17.6)] India – Tamil Nadu[22]
(Without KCOT) 489 (4.13%)
Ameloblastoma -67.69 AOT -9
Odontoma – 7.77
1.08:1 4.02:1 3
11-20 yrs[131 cases (26.8)] 21-30 yrs[129 cases (26.4)] 31-40 yrs [66 cases (13.5)] India – Mumbai[34]
(Without KCOT) 60
Ameloblastoma -66.67 Odontoma -20
AOT -10
1:1 2.3:1 Nil
20-29 yrs [18 cases (30)] 10-19 yrs [14 cases (23.3)] 30-39 yrs [13 cases (21.7)]
India – Gujarat[28] 209
Ameloblastoma – 47.4 KCOT – 23.4
AOT – 7.7
1.3:1 2.3:1 Nil
20-29 Yrs [82 cases (39.2)] 10-19 yrs [53 cases (25.3)] 30-39 yrs [41 cases (19.6)]
India – Mumbai[10] 120
Ameloblastoma - 40.83 KCOT – 37.5
Odontoma – 11.66
1.4: 1 2.75:1 Nil
20-29 yrs [32 cases(26.7)] 10-19yrs [29 cases (24.16)] 30-39yrs [22 cases (18.33)]
India – Karnataka
(Present study) 111 (3.52%)
Ameloblastoma – 52.2 KCOT – 27.9
Odontogenic myxoma – 6.3 AOT -5.4
1.4:1 2.8:1 Nil
21-30 yrs [35 cases (31.5)] 11-20 yrs [28 cases (25.2)] 31-40 yrs [26 cases ( 23.4)]
Srilanka[29] 1677 (3.75%)
Ameloblastoma – 48.7 KCOT – 25.7
Odontoma – 10.1
NA 2.8:1 2 Mean age – 30.6 yrs
Srilanka[23]
(Without KCOT) 226
Ameloblastoma -69.8 AOT – 9.29
Odontogenic myxoma – 4.86
DISCUSSION
Odontogenic tumors are lesions derived from epithelial and / or mesenchymal tissues of the tooth forming apparatus,[1] comprising about 1% of all jaw tumors.[4] Literature sources on the distribution of OTs are more in America, Europe, Africa and few Asian countries like China and Japan. South Asian countries, in particular India and Srilanka are coming up with valid contribution towards odontogenic tumors in this decade.
The parakeratinised variant of odontogenic keratocyst (OKC) is renamed as KCOT and WHO shifted this cystic lesion to benign OTs category in 2005. The worldwide studies on odontogenic tumors are showing varying results on the prevalence rate and clinical data[5-37], depicting the geographical and regional variation in the distribution of OTs. Also, underreporting of odontomas in developing countries and type of institution (medical college or dental college) reporting the study might also determine the prevalence rate.[25] A Nigerian study[26] has calculated the percentage of OTs among the jaw lesions, instead of among total biopsies (as done in our study and many other studies), resulting in differing values. Above these factors, including KCOT under OTs have brought remarkable changes in the numerical count of OTs[10, 26,27,28,29,30,31,32,33] and added to the diversity of study results.
After WHO 2005 classification, we are almost a decade ahead and still the acceptance of KCOT as tumor is being debated around the scientific world. Only countable number of epidemiological studies[10,24,26-33] on OTs has included KCOT in their work (Table 4) and we have focused more on those results for our comparison.
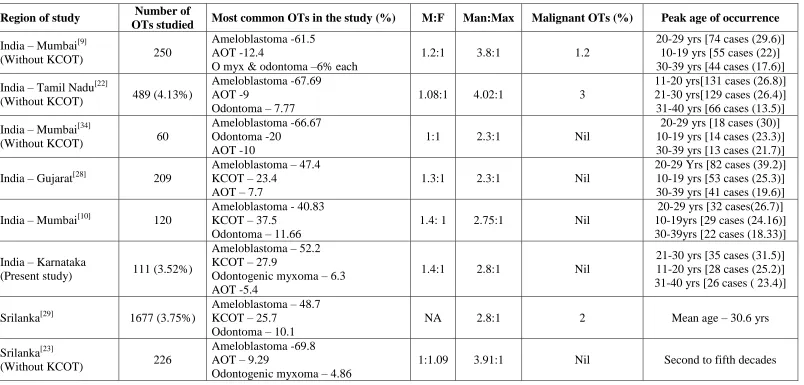
Studies on OTs from other parts of India[9,10,22,28,34] and Srilanka[23,29], where regional and geographical variation is minimal from ours, are also analyzed (Table 5). Ameloblastoma followed by odontoma or AOT are the most common lesions in those studies without KCOT[9,22,23,34], whereas in studies including KCOT[10,28,29], KCOT was the second most common, followed by odontoma or AOT.
Gupta et al[22] has given a broad review on odontogenic tumors (excluding KCOT), where South America and Europe are showing female predilection and North America, Africa, southern Asia and eastern Asia are showing male predilection in gender distribution. Few Asian studies done by Wu and Chan (China)[5], Saghravanian et al8 (Iran), also have shown female predominance. In the present study, there is a slight predilection in favor of men (57.6%) with male: female ratio of 1.4:1, similar to the other study values from India.[9,10,22,28] Study results from Nigeria[26], Egypt[32], Brazil[33] (South America), including KCOT under OTs also show slight male predilection (Table 4). Equal distribution among both gender is noticed in an Indian study (without KCOT)[34] and slight female predominance (1:1.09) in seen in the Srilankan study (without KCOT)[23] (Table 5). In our study, KCOT as an individual lesion has occurred slightly more common in males and the inclusion of KCOT has favored shift towards male gender in a negligible amount (0.01%). ―Will including KCOT under OTs will shift the gender predilection in OTs towards male and to what extend???‖ has to be answered by future studies.
In the present study, all OTs are seen between age group of 11-80 years. Nil tumor occurrence before ten years of age supports the hypothesis that OTs probably develops after crown formation,[11-13] arising from the quiescent remnants of the tooth germ.[13] In the present study, OTs are most frequent in the third decade, followed by second and fourth decades of life. All the Indian and Srilankan studies (Table 5) are reflecting the same, except the one by Gupta et al[22], where OTs are reported slightly more in second decade than third decade. Worldwide studies on OTs including KCOT, also says third decade to be more commonly affected (Table 4).
Ameloblastoma
Ameloblastoma (52.25%) is the most frequent OT in the present study similar to the previous studies from other Indian regions[9,10,22,28,34], Srilanka[23,29], other Asian countries[7,30] and Africa.[14,15,26,32] Western countries like Canada[17], Mexico[18], and Chile[19] reports odontoma to be the most common OT. Odontomas are clinically asymptomatic1 and are not brought into limelight for treatment without symptoms. The researchers substantiate that unlike in western countries, regular medical consultation in the developing countries is lacking, resulting in under reporting and lower incidence of odontomas.[25] Adding KCOT under OTs dilutes the percentage of individual OTs, in particular ameloblastoma [from 72.5% to 52.25% in our study (Table 1)] and KCOT emerges to be the most common OT in few studies[27,24,31,33] (Table 4).
Male are affected more than female (1.7:1) and in this study, 96.55% cases of ameloblastoma occurred in second to sixth decade, with peak occurrence in third decade. The average age of patients at the time of initial diagnosis was 35.52 years (Range: 11-73 years) similar to the findings of Reichart et al[21] (35.9 years).
Almost 86.2% of ameloblastoma are located in the mandible, with the mandible: maxilla ratio of 6.25:1 in our report and the mandibular molar–ramus region has been most frequently encountered (73.3%). Sriram et al[9] from India has reported the highest predilection of ameloblastomas in mandibular region (18.1:1), and viewed that OTs occurring in maxilla might be dealt by the medical fraternities like ENT surgeons, thereby extremely raising the ratio.
Prevalence of KCOT (27.9%) is next to that of ameloblastoma in our study with the peak age of occurrence in third, fourth and second decades of life and the mandibular molar area being affected more commonly (80.64%) (Table 3). 36% of lesions in mandible and 33.3% of lesions in maxilla are involving both the anterior and posterior segments, supporting that KCOT has a tendency to expand anterio-posteriorly. Male predominance (58.1%) is seen in contrast to the study of Avelar et al[24] who has shown female predilection (56.6%).
The frequency of odontogenic myxoma is 6.3% of all OTs in the present study. It is about 6% in a study from Maharashtrian population[9], next to ameloblastoma and AOT (KCOT is not included), but only 2.66% in the Tamil linguistic Dravidian population.[22] Odontogenic myxoma showed equal occurrence in second and third decade with female predilection. The mean age of 26.5 years was significantly lower than that for ameloblastoma (35.52 years) in the present study, which is in agreement with Sriram et al[9] and Gupta et al.[22]
Adenomatoid odontogenic tumor make up 5.4% of all OTs in our sample population, similar to Varkhede A et al (5.8%),[10] but lower than that of Sriram et al9 (12.4%) and Gupta et al[22] (9%). In the present study, six cases are reported and all occurred exclusively in male patients in their second decade of life, in contrast to the female predilection observed in literature[1,5] whereas Gupta et al[22] has observed almost equal distribution between male and female. Sriram et al comments on the early occurrence of AOT, hypothesizing that ―AOT may cause expansion of the cortical plates in an early stage compared with ameloblastomas, which spread linearly within the cancellous bone before causing expansion/ resorption of the cortical plates‖. Higher maxillary predilection (66.7%), but predominantly in posterior region is noted in contrast to other Indian studies[9,22] and literature[1], where maxillary anterior region is observed more and increasing our sample size of AOT might resolve such controversies.
The two cases of CEOT (1.8%) are seen in male, at fourth decade. Both the lesions are in the maxillary posterior region, similar to the study results of Santos et al6 and Varkhede A et al.10 AF and AFD together have accounted for 2.7% of total OTs in present study, being noticed in second decade of life and at posterior jaws. One case of odontogenic fibroma (0.9%) in the anterior maxilla of male has been reported so far.
CONCLUSION
Science perpetuates itself at all times by incorporating changes with research based proofs and refreshes us with newer scenario over time. Thus, inclusion of KCOT has brought visible changes in the prevalence rate and numerical dependent clinical data of OTs. Based on this retrospective study, it was observed that the relative frequency of OTs was 3.52%, inclusive of KCOT. OTs showed male predilection and mandible as preferable location. Most OTs were diagnosed in the second, third and fourth decades of life. Worldwide comparison suggested many similarities and variations, whereas Indian and Srilankan studies, in particular those including KCOT are considerably in par with our study values, reflecting that geographical and regional variations do influence the distribution of OTs.
ACKNOWLEDGMENT
The authors are thankful to the patients, for their invaluable contribution to the study, and also the college management, faculty and post graduate students of various departments for their support and efforts in preserving the vital source of information. Our sincere thanks to our laboratory technicians, Mr. Mallikarjun KH, Mr. Manjunath and Mr. Kumar, for their excellent skill and contribution towards diagnosis.
REFERENCES
1. Regezi JA, Sciubba JJ, Jordan RC. Oral Pathology: Clinical Pathologic Correlation. 5th ed. St. Louis: Saunders: 2008; 261-75.
2. Mosqueda- Taylor A. New findings and controversies in odontogenic tumors. Med Oral Patol Oral Cir Bucal, 2008; 13: E555-8.
5. Wu PC, Chan KW. A survey of tumors of the jaw bones in Hong Kong Chinese: 1963‑1982. Br J Oral Maxillofac Surg, 1985; 23: 92-102.
6. Santos JN, Pinto LP, de Figueredo CR, de Souza LB. Odontogenic tumors: Analysis of 127 cases. Pesqui Odontol Bras, 2001; 15: 308-13.
7. Lu Y, Xuan M, Takata T, Wang C, He Z, Zhou Z, et al. Odontogenic tumors, a demographic study of 759 cases in Chinese population. Oral Surg Oral Med Oral Pathol Oral Radiol Endod, 1998; 86: 707-14.
8. Taghavi N, Rajabi M, Mehrdad L, Sajjadi S. A 10-year retrospective study on odontogenic tumors in Iran. Indian J Dent Res, 2013; 24: 220-4.
9. Sriram G, Shetty RP. Odontogenic tumors: A study of 250 cases in India teaching hospital. Oral Surg Oral Med Oral Pathol Oral Radiol Endod, 2008; 105: e14-21.
10.Varkhede A, Tupkari JV, Sardar M. Odontogenic tumors: A study of 120 cases in an Indian teaching hospital. Med Oral Patol Oral Cir Bucal, 2011; 16 (7): e895-9.
11.Tanaka N, Murata A, Yamaguchi A, Kohama G. Clinical features and management of oral and maxillofacial tumors in children. Oral Surg Oral Med Oral Pathol Oral Radiol Oral Radiol Endod, 1999; 88: 11-5.
12.Sato M, Tanaka N, Sato T, Amagasa T. Oral and maxillofacial tumors in children: a review. Br J Oral Maxillofac Surg, 1997; 35: 92-95.
13.Ajayi OF, Ladeinde AL, Adeyemo WL, Ogunlewe MO. Odontogenic tumors in Nigerian children and adolescents—a retrospective study of 92 cases. World J Surg Oncol, 2004; 2: 39.
14.Odukoya O. Odontogenic tumors: analysis of 289 Nigerian cases. J Oral Pathol Med, 1995; 24: 454-7.
15.Arothiba JT, Ogunbiyi JO, Obiechina AE. Odontogenic tumors: a 15 year review from Ibadan, Nigeria. Br J Oral Maxillofac Surg, 1997; 35: 363-7.
16.Adebayo ET, Ajhile SO, Adeket EO. Odontogenic tumours in children and adolescent. A study of 78 Nigerian cases. J Craniomaxfac, 2002; 30: 267-72.
17.Daley TD, Wyoscki GP, Pringle GA. Relative incidence of odontogenic tumors and oral and jaw cysts in a Canadian population. Oral Surg Oral Med Oral Pathol Oral Radiol Endod, 1994; 77: 276-80.
collaborative retrospective study of 349 cases. Oral Surg Oral Med Oral Pathol Oral Radiol Endod, 1997; 84: 672-5.
19.Ochsenius G, Ortega A, Godoy L, Penafiel C, Escobar E. Odontogenic tumors in Chile: a study of 362 cases. J Oral Pathol Med, 2002; 31: 415-20.
20.Small IA, Waldron CA. Ameloblastomas of the jaws. Oral Surg Oral Med Oral Pathol, 1955; 8: 281-97.
21.Reichart PA, Philipsen HP, Sonner S. Ameloblastoma: Biological profile of 3677 cases. Eur J Cancer B Oral Oncol, 1995; 31B: 86-99.
22.Gupta B, Ponniah I. The pattern of odontogenic tumors in a government teaching hospital in the southern Indian state of Tamil Nadu. Oral Surg Oral Med Oral Pathol Oral Radiol Endod, 2010; 110: e32-9.
23.Okada H, Yamamoto H, Tilakaratne WM. Odontogenic tumors in Sri Lanka: analysis of 226 cases. J Oral Maxillofac Surg, 2007; 65: 875-82.
24.Avelar RL, Antunes AA, Santos T, Andrade E, Dourado E. Odontogenic tumors: clinical and pathological study of 238 cases. Rev Bras Otorrinolaringol, 2008; 74: 668-73.
25.Fregnani ER, Fillipi RZ, Oliveira CR, Vargas PA, Almeida OP. Odontomas and Ameloblastomas: variable prevalences around the world? Oral Oncol, 2002; 38: 807-8. 26.Lawal AO, Adisa AO, Olusanya AA. Odontogenic tumors: A review of 266 cases. J Clin
Exp Dent, 2013; 5(1): e13-7.
27.Luo HY, Li TJ. Odontogenic tumors: a study of 1309 cases in a Chinese population. Oral Oncol, 2009; 45: 706-11.
28.Gill S, Chawda J, Jani D. Odontogenic tumors in Western India (Gujarat): Analysis of 209 cases. J Clin Exp Dent, 2011; 3(2): e78-83.
29.Siriwardena BS, Tennakoon TM, Tilakratne WM. Relative frequency of odontogenic tumors in Srilanka: Analysis of 1677 cases. Pathol Res Pract, 2012; 208(4): 225-30. 30.Jing W, Xuan M, Lin Y, Wu L, Liu L, Zheng X, et al. Odontogenic tumors: a
retrospective study of 1642 cases in a Chinese population. Int J Oral Maxillofac Surg, 2007; 36: 20-5.
33.Da-Costa D, Mauricio A, de-Faria P, da-Silva L, Mosqueda-Taylor A, Lourenco S. Odontogenic tumors: A retrospective study of four Brazilian diagnostic pathology centers. Med Oral Patol Oral Cir Bucal, 2012; 17(3): e389-94.
34.Varkhede A, Tupkari JV, Mandale MS, Sardar M. Odontogenic tumors: A review of 60 cases. J Clin Exp Dent, 2010; 2(4): e183-6.
35.Buchner A, Merrell PW, Carpenter WM. Relative frequency of central odontogenic tumors: a study of 1088 cases from northern California and comparison to studies from other parts of the world. J Oral Maxillofac Surg, 2006; 64: 1343-52.
36.Tamme T, Soots M, Kulla A, Karu K, Hanstein SM, Sokk A. Odontogenic tumors, a collaborative retrospective study of 75 cases covering more than 25 years from Estonia. J Craniomaxillofac Surg, 2004; 32: 161-5.