Acta Cryst.(2002). E58, o1065±o1067 DOI: 10.1107/S1600536802015763 Agnieszka Mrozeket al. C20H14N2O2S
o1065
organic papers
Acta Crystallographica Section E Structure Reports
Online
ISSN 1600-5368
9-(Thio-2
000-methyl-4
000-nitrophenyl) acridine
Agnieszka Mrozek,a* Janina
Karolak-Wojciechowska,b
Pascale Amielcand Jacques
Barbec
aInstitute of General and Ecological Chemistry, Technical University of èoÂdzÂ, ul. Z.eromskiego 116, 90-924 èoÂdzÂ, Poland,bInstitute of General Chemistry, Technical University of èoÂdzÂ, Z.eromskiego 116, 90-924 èoÂdzÂ, Poland, and cGERCTOP / URA CNRS 1411, Faculte de Pharmacie, 27 blvd Jean Moulin, 13385 Marseille, Cedex 05, France
Correspondence e-mail: agmrozek@ck-sg.p.lodz.pl
Key indicators Single-crystal X-ray study
T= 293 K
Mean(C±C) = 0.006 AÊ
Rfactor = 0.058
wRfactor = 0.166
Data-to-parameter ratio = 10.8
For details of how these key indicators were automatically derived from the article, see http://journals.iucr.org/e.
#2002 International Union of Crystallography Printed in Great Britain ± all rights reserved
All interatomic distances in the title compound, C20H14N2O2S,
(I), are normal. The molecule consists of two cyclic moieties, an acridine and a substituted phenyl ring. The two rings are joinedviaa sulfur bridge [SÐC = 1.782 (3) and 1.764 (4) AÊ]. The substituted phenyl ring in (I) is nearly perpendicular to the acridine moiety, with a dihedral angle of 75.01 (7). The
acridine skeleton is slightly bent, with dihedral angles between the two terminal carbocyclic rings and the central heterocycle of 1.2 (1) and 0.5 (1). The molecules of the title compound
stack head-to-tail along theb axis, with a distance between molecules of about 4.381 AÊ. In order to investigate quantita-tively the aromaticity in acridines, we calculated the HOMA index [Krygowski (1993). J. Chem. Inf. Comput. Sci.33, 70.] for each of the acridine rings; values were 0.568 and 0.524 for the carbocyclic rings and 0.816 for the heterocycle.
Comment
Acridine derivatives are currently delivered to animals as antihelminthics agents (Durchheimeret al., 1980). In addition, acridine derivatives have shown in vitro activity against protozoan-like Trypanosoma cruzi (Ngadi et al., 1993) and
Leishmania donovani(Mesa-Valleet al., 1996). This work has been undertaken in the context of our studies on acridine derivatives with potential pharmacological properties (Karolak-Wojciechowskaet al., 1996; Karolak-Wojciechowska
et al., 1998). It was especially important to perform an X-ray analysis on single crystals of 9-(thio-2-methyl-4-nitrophenyl) acridine, (I), to obtain atomic coordinates which could be used as a starting point for further molecular modelling.
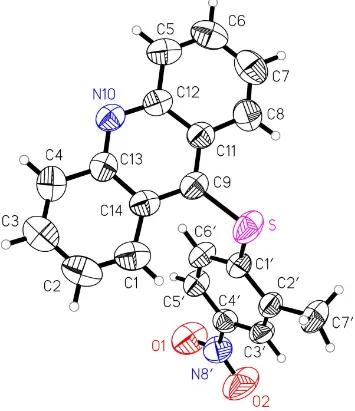
The molecular structure of (I) is presented in Fig. 1. The molecule consists of two cyclic moieties, an acridine and a substituted phenyl ring. The rings are joinedviaa sulfur bridge at C9 (S1ÐC9 = 1.782 (3) and S1ÐC10 = 1.764 (4) AÊ). The
substituted phenyl ring in (I) is nearly perpendicular to the acridine moiety, with a dihedral angle of 75.01 (7). The
molecules of (I) stack head-to-tail along the b axis, with a distance between molecules of about 4.381 AÊ. All bond distances and angles have normal values. The acridine
organic papers
o1066
Agnieszka Mrozeket al. C20H14N2O2S Acta Cryst.(2002). E58, o1065±o1067 skeleton is slightly bent, with dihedral angles between the twoterminal carbocyclic rings,A1 andA3, and the heterocycleA2 of 1.2 (1) and 0.5 (1), respectively. As has been shown for
various hydrocarbon systems, such small deviations from planarity of an aromatic species do not affect the aromaticity signi®cantly (Krygowskiet al., 2000; Krygowski & Cyranski, 2001). It should also be noted that such a slight deformation of the acridine skeleton is in accordance with data for 34 C9-substituted acridines retrieved from the Cambridge Structural Database (Version of November 2001; Allen & Kennard, 1993). Most of these molecules include an acridine moiety with a very slight boat conformation. Only two structures found in the CSD contain acridine in a chair conformation. The average value of the dihedral angles between the terminal and central rings for the 34 molecules is 1.5. On account of
the interest in hydrocarbons and the aromaticity of their aza analogues (Krygowski et al., 2000; Krygowski & Cyranski, 2001), we decided to investigate quantitatively the aromaticity in acridines (Mrozek, Karolak-Wojciechowska, Amiel & Barbe, 2000a,b) by calculating the HOMA index (Krygowski, 1993) for each acridine ring in (I); its values are 0.568 and 0.524 for the carbocyclic rings, and 0.816 for the heterocycle. To con®rm these results, calculations of the HOMA index were performed for all 34 C9-substituted acridines found in the CSD (Set 1, HOMAAV= 0.744 and HOMAA2= 0.510), and
as, an extension of our statistical research, for 18 unsubstituted acridines (Set 2; HOMAAV= 0.810 and HOMAA2= 0.601) also
found in the CSD. For the terminal rings A1 and A3, the results of the HOMA calculations are given as an averaged
HOMA index (HOMAAV), whereas HOMAA2is the value of
the HOMA index for the central heterocyclic ring A2. The aromaticity of the central heterocycle is notably higher than for the terminal carbocyclic rings. A similar effect is observed, not only for the acridine parent molecule anthracene, where the HOMA index values are 0.638 for the terminal rings and 0.763 for the central one, but also for different aza-substituted benzenoids (Krygowski et al., 2000; Krygowski & Cyranski, 2001; Cyranski & Krygowski, 1996). Moreover, the replace-ment of CH by N usually leads to an increase in the aroma-ticity of the system. This conclusion is con®rmed by the fact that the HOMA indices for the central acridine ring are higher or, at least comparable to, those for anthracene.
Experimental
The title compound was synthesized according to the method of Mrozek, Karolak-Wojciechowska, Bsiri & Barbe (2000).
Crystal data
C20H14N2O2S Mr= 346.39 Monoclinic,P21=n a= 9.826 (2) AÊ
b= 7.171 (1) AÊ
c= 23.941 (5) AÊ = 101.57 (3)
V= 1652.7 (5) AÊ3 Z= 4
Dx= 1.392 Mg mÿ3 CuKradiation Cell parameters from 25
re¯ections = 10±35
= 1.87 mmÿ1 T= 293 (2) K Needle, colourless 0.50.20.1 mm
Data collection
KumaKM4 diffractometer !±2scans
Absorption correction: numerical (X-RED; Stoe & Cie, 1999)
Tmin= 0.421,Tmax= 0.872
2670 measured re¯ections 2469 independent re¯ections 1802 re¯ections withI> 2(I)
Rint= 0.063
max= 80.9 h=ÿ8!0
k=ÿ9!0
l=ÿ27!30 2 standard re¯ections
every 100 re¯ections intensity decay: 2%
Re®nement
Re®nement onF2 R[F2> 2(F2)] = 0.058 wR(F2) = 0.166 S= 1.11 2469 re¯ections 228 parameters
H atoms treated by a mixture of independent and constrained re®nement
w= 1/[2(F
o2) + (0.0874P)2] whereP= (Fo2+ 2Fc2)/3 (/)max< 0.001
max= 0.38 e AÊÿ3 min=ÿ0.40 e AÊÿ3
Extinction correction:SHELXL
Extinction coef®cient: 0.0118 (10)
Table 1
Selected geometric parameters (AÊ).
C9ÐC11 1.392 (5) C9ÐC14 1.408 (5) C11ÐC8 1.421 (5) C11ÐC12 1.431 (5) C8ÐC7 1.342 (6) C7ÐC6 1.418 (6) C6ÐC5 1.349 (5) C5ÐC12 1.424 (5)
C12ÐN10 1.342 (4) N10ÐC13 1.339 (5) C13ÐC14 1.428 (5) C13ÐC4 1.434 (5) C4ÐC3 1.342 (5) C3ÐC2 1.408 (6) C2ÐC1 1.359 (5) C1ÐC14 1.429 (5)
As the collected data were relatively weak, there was a large proportion of re¯ections with low intensities, and thus some of the re¯ections were marked as unobserved. This affects the fraction of unique re¯ections observed (to= 80.91), which is equal to 62.6%.
Figure 1
All H atoms were placed in calculated positions and treated as riding on the adjacent C atom. The methyl group was allowed to rotate about its local threefold axis.
Data collection: KM4 Software (Kuma, 1993); cell re®nement:
KM4Software; data reduction:DATAPROC(Gaødeckiet al., 1998); program(s) used to solve structure:SHELXS97 (Sheldrick, 1990a); program(s) used to re®ne structure:SHELXL97 (Sheldrick, 1997); molecular graphics: XP in SHELXTL/PC (Sheldrick, 1990b)
ORTEP-3 (Farrugia, 1997); software used to prepare material for publication:SHELXL97.
References
Allen, F. R. & Kennard, O. (1993).Chem. Des. Autom. News,8, 1, 31±37. Cyranski, M. & Krygowski, T. M. (1996).Tetrahedron,52, 13795±13802. Durchheimer, W., Raether, W., Seliger, H. & Seidenath, H. (1980).Arzneim.
Forsch. Drug. Res.30, 1041±1046. Farrugia, L. J. (1997).J. Appl. Cryst.30, 565.
Gaødecki, Z., Kowalski, A. & Uszynski, I. (1998). DATAPROC. Version 10.0.4. Kuma Diffraction, Wrocøaw, Poland.
Kuma (1993).KM4Software. Kuma Diffraction, Wrocøaw, Poland.
Karolak-Wojciechowska, J., Mrozek, A., Amiel, P., Brouant, P.& Barbe, J. (1996).Acta Cryst.C52, 2939±2941.
Karolak-Wojciechowska, J., Mrozek, A., Trzezwinska, B., Morel, S., Galy, J. P. & Barbe, J. (1998).Acta Cryst.C54, 1689±1690.
Krygowski, T. M. (1993).J. Chem. Inf. Comput. Sci.33, 70.
Krygowski, T. M., Cyranski, M., Czarnocki, Z., Hafelinger, G. & Katritzky, A. R. (2000).Tetrahedron,56, 1783±1796.
Krygowski, T. M. & Cyranski, M. (2001).Chem. Rev.101, 1385±1419. Mesa-Valle, C., Castilla-Calvente, J. J. & Sanchez-Moreno, M. (1996).
Antimicrob. Agents Chemother.40, 684.
Mrozek, A., Karolak-Wojciechowska, J., Amiel, P. & Barbe, J. (2000a).J. Mol. Struct.524, 151±157.
Mrozek, A., Karolak-Wojciechowska, J., Amiel, P. & Barbe, J. (2000b).J. Mol. Struct.524, 159±167.
Mrozek, A., Karolak-Wojciechowska, J., Bsiri, N. & Barbe, J. (2000).Acta Pol. Pharm. Drug. Res.,57, 345±351.
Ngadi, L., Bsiri, N., Mahamoud, A., Galy, A. M., Galy, J. P., Soyfer, J. C., Barbe, J., Placidi, M., Rodriguez-Santiago, J. J., Mesa-Valle, C., Lombardo, R. Mascaro, C. & Osuna, A. (1993).Arzneim. Forsch. Drug. Res.43, 480. Sheldrick, G. M. (1990a).Acta Cryst.A46, 467±473.
Sheldrick, G. M. (1990b).SHELXTL/PC. Siemens Analytical X-ray Instru-ments Inc., Madison, Wisconsin, USA.
Sheldrick, G. M. (1997).SHELXL97. University of GoÈttingen, Germany. Stoe & Cie (1999).X-RED. Version 1.18. Stoe & Cie GmbH, Darmstadt,
Germany.
supporting information
sup-1
Acta Cryst. (2002). E58, o1065–o1067supporting information
Acta Cryst. (2002). E58, o1065–o1067 [doi:10.1107/S1600536802015763]
9-(Thio-2
′
-methyl-4
′
-nitrophenyl) acridine
Agnieszka Mrozek, Janina Karolak-Wojciechowska, Pascale Amiel and Jacques Barbe
S1. Comment
Acridine derivatives are currently delivered to animals as antihelminthics agents (Durchheimer et al., 1980). In addition, acridine derivatives have shown in vitro activity against protozoan-like Trypanosoma cruzi (Ngadi et al., 1993) and Leishmania donovani (Messa-Valle et al., 1996). This work has been undertaken in the context of our studies on acridine derivatives with potential pharmacological properties (Karolak-Wojciechowska et al., 1996; Karolak-Wojciechowska et al., 1998). It was especially important to perform an X-ray analysis on single crystals of 9-(thio-2-methyl-4-nitrophenyl) acridine, (I), to obtain atomic coordinates which could be used as a starting point for further molecular modelling. The
molecular geometry of(I) is presented in Fig. 1. The molecule consists of two cyclic moieties, an acridine and a phenyl
ring. The rings are joined via a sulfur bridge at C9 (S1—C9 = 1.782 (3) and S1—C1′ = 1.764 (4) Å). The phenyl ring in (I) is nearly perpendicular to the acridine moiety, with a dihedral of 75.01 (7)°. The molecules of (I) stack head-to-tail
along the y-axis, with a distance between molecules of about 4.381 Å. All bond distances and angles have typical values. The acridine skeleton is slightly bent, with dihedral angles between the two terminal carborings A1 and A3 and the
heterocycle A2 1.2 (1) and 0.5 (1)°, respectively. As has been shown for various hydrocarbon systems, such small
deviations from planarity of an aromatic species does not affect the aromaticity significantly (Krygowski et al., 2000; Krygowski & Cyranski, 2001). It should also be noted that such a slight deformation of the acridine skeleton is in
accordance with data for 34 C9-substituted acridines retrieved from the Cambridge Structural Database (Allen &
Kennard, 1993). Most of the molecules found include an acridine moiety with a very slight boat conformation. Only two
structures found in the CSD contain the acridine in a chair conformation. The average value of the dihedral angles
between the external and internal rings for the 34 molecules is 1.5°. Taking into consideration the interest in
hydro-carbons and the aromaticity of their aza analogues (Krygowski et al., 2000; Krygowski & Cyranski, 2001) we decided to investigate quantitatively the aromaticity in acridines (Mrozek,Karolak-Wojciechowska, Amiel & Barbe, 2000a,b) by calculating the HOMA index (Krygowski, 1993) for each acridine ring in (I); its values are 0.568 and 0.524 for the
carborings, and 0.816 for the heterocycle. To confirm these results, calculations of the HOMA index were performed for
all 34 C9-substituted acridines found in the CSD (Set 1; HOMAAV = 0.744 and HOMAA2 = 0.510) and as an extension of
our statistical research for 18 unsubstituted acridines (Set 2; HOMAAV = 0.810 and HOMAA2 = 0.601) retrieved
additionally. For the terminal rings A1 and A3, the results of the HOMA calculations are given as an averaged HOMA
index (HOMAAV) whereas HOMAA2 is the value of HOMA index for internal heterocyclic ring A2. The aromaticity of the
internal heterocycle is notably higher than for the external homorings. A similar effect is observed not only for the
acridine parent molecule anthracene, where the HOMA index values are 0.638 for the external rings and 0.763 for the
internal one, but also for different aza-substitued benzoides (Krygowski et al., 2000; Krygowski & Cyranski, 2001; Cyranski & Krygowski, 1996) and may be attributed to topological conditions. Moreover, the replacement of CH by N
usually leads to an increase in the aromaticity of the system. This conclusion is confirmed by the fact that the HOMA
supporting information
sup-2
Acta Cryst. (2002). E58, o1065–o1067S2. Experimental
The title compound was synthesized according to the method of Mrozek, Karolak-Wojciechowska, Bsiri & Barbe (2000).
S3. Refinement
As the collected data were relatively weak, there was a large proportion of reflections with low intensities, and thus some
of the reflections were marked as unobserved. This affects the fraction of unique reflections observed (out to θ=80.91°), which is equal to 62.6%. All hydrogen atoms were placed in calculated positions and treated as riding on the adjacent
[image:5.610.128.483.195.606.2]carbon atom. The methyl group was allowed to rotate about its local threefold axis.
Figure 1
supporting information
sup-3
Acta Cryst. (2002). E58, o1065–o10679-thio-2-methyl-4-nitrophenyl) acridine
Crystal data
C20H14N2O2S
Mr = 346.39
Monoclinic, P21/n
a = 9.826 (2) Å
b = 7.171 (1) Å
c = 23.941 (5) Å
β = 101.57 (3)°
V = 1652.7 (5) Å3
Z = 4
F(000) = 720
Dx = 1.392 Mg m−3
Cu Kα radiation, λ = 1.54178 Å Cell parameters from 25 reflections
θ = 10–35°
µ = 1.87 mm−1
T = 293 K Needle, colourless 0.5 × 0.2 × 0.1 mm
Data collection
Kuma KM4 diffractometer
Radiation source: fine-focus sealed tube Graphite monochromator
ω–2θ scans
Absorption correction: numerical
X-RED (Stoe & Cie, 1999)
Tmin = 0.421, Tmax = 0.872
2670 measured reflections
2469 independent reflections 1802 reflections with I > 2σ(I)
Rint = 0.063
θmax = 80.9°, θmin = 3.8°
h = −8→0
k = −9→0
l = −27→30
2 standard reflections every 100 reflections intensity decay: 2%
Refinement
Refinement on F2
Least-squares matrix: full
R[F2 > 2σ(F2)] = 0.058
wR(F2) = 0.166
S = 1.11 2469 reflections 228 parameters 0 restraints
Primary atom site location: structure-invariant direct methods
Secondary atom site location: structure-invariant direct methods
Hydrogen site location: inferred from neighbouring sites
H atoms treated by a mixture of independent and constrained refinement
w = 1/[σ2(F
o2) + (0.0874P)2]
where P = (Fo2 + 2Fc2)/3
(Δ/σ)max < 0.001
Δρmax = 0.38 e Å−3
Δρmin = −0.40 e Å−3
Extinction correction: SHELXL, Fc*=kFc[1+0.001xFc2λ3/sin(2θ)]-1/4
Extinction coefficient: 0.0118 (10)
Special details
Geometry. All e.s.d.'s (except the e.s.d. in the dihedral angle between two l.s. planes) are estimated using the full covariance matrix. The cell e.s.d.'s are taken into account individually in the estimation of e.s.d.'s in distances, angles and torsion angles; correlations between e.s.d.'s in cell parameters are only used when they are defined by crystal symmetry. An approximate (isotropic) treatment of cell e.s.d.'s is used for estimating e.s.d.'s involving l.s. planes.
Refinement. Refinement of F2 against ALL reflections. The weighted R-factor wR and goodness of fit S are based on F2,
conventional R-factors R are based on F, with F set to zero for negative F2. The threshold expression of F2 > σ(F2) is used
only for calculating R-factors(gt) etc. and is not relevant to the choice of reflections for refinement. R-factors based on F2
are statistically about twice as large as those based on F, and R- factors based on ALL data will be even larger.
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
x y z Uiso*/Ueq
supporting information
sup-4
Acta Cryst. (2002). E58, o1065–o1067C11 −0.0543 (4) 0.8047 (4) 0.09442 (14) 0.0518 (9) C8 −0.1123 (5) 0.8445 (5) 0.14292 (17) 0.0698 (11)
H8 −0.0556 0.8436 0.1790 0.084*
C7 −0.2480 (6) 0.8833 (6) 0.1371 (2) 0.0825 (13)
H7 −0.2839 0.9081 0.1695 0.099*
C6 −0.3380 (5) 0.8874 (6) 0.0828 (2) 0.0801 (13)
H6 −0.4318 0.9142 0.0798 0.096*
C5 −0.2872 (4) 0.8522 (5) 0.03560 (18) 0.0655 (10)
H5 −0.3464 0.8557 0.0001 0.079*
C12 −0.1440 (4) 0.8098 (4) 0.03940 (15) 0.0517 (9) N10 −0.1001 (3) 0.7748 (3) −0.00914 (12) 0.0535 (7) C13 0.0342 (4) 0.7332 (4) −0.00580 (14) 0.0525 (9) C4 0.0800 (5) 0.6944 (5) −0.05782 (16) 0.0670 (11)
H4 0.0165 0.6997 −0.0923 0.080*
C3 0.2129 (5) 0.6505 (5) −0.0577 (2) 0.0756 (12)
H3 0.2402 0.6245 −0.0919 0.091*
C2 0.3111 (5) 0.6435 (5) −0.0061 (2) 0.0784 (12)
H2 0.4035 0.6174 −0.0068 0.094*
C1 0.2728 (4) 0.6745 (5) 0.04453 (19) 0.0658 (11)
H1 0.3388 0.6654 0.0782 0.079*
C14 0.1326 (4) 0.7209 (4) 0.04678 (15) 0.0525 (9) C1′ 0.3072 (4) 0.9238 (4) 0.17037 (13) 0.0516 (8) C6′ 0.2824 (4) 1.0684 (4) 0.13049 (14) 0.0554 (9)
H6′ 0.2051 1.0636 0.1009 0.067*
C5′ 0.3716 (4) 1.2177 (4) 0.13475 (14) 0.0540 (9)
H5′ 0.3558 1.3141 0.1082 0.065*
C4′ 0.4844 (4) 1.2213 (5) 0.17896 (14) 0.0517 (8) N8′ 0.5826 (4) 1.3758 (5) 0.18233 (15) 0.0695 (9) O1 0.5573 (3) 1.5005 (4) 0.14802 (14) 0.0989 (11) O2 0.6870 (4) 1.3714 (5) 0.21948 (15) 0.1162 (13) C3′ 0.5085 (3) 1.0822 (5) 0.21942 (13) 0.0513 (9)
H3′ 0.5852 1.0903 0.2492 0.062*
C2′ 0.4203 (4) 0.9308 (5) 0.21634 (13) 0.0514 (8) C7′ 0.4442 (4) 0.7806 (5) 0.26112 (15) 0.0713 (11)
H71′ 0.5313 0.8019 0.2867 0.107*
H72′ 0.4460 0.6612 0.2431 0.107*
H73′ 0.3705 0.7830 0.2821 0.107*
Atomic displacement parameters (Å2)
U11 U22 U33 U12 U13 U23
supporting information
sup-5
Acta Cryst. (2002). E58, o1065–o1067C12 0.051 (3) 0.0386 (15) 0.062 (2) −0.0002 (14) 0.0035 (17) 0.0037 (13) N10 0.054 (2) 0.0443 (13) 0.0581 (17) −0.0095 (13) 0.0028 (14) 0.0032 (11) C13 0.062 (3) 0.0405 (15) 0.0542 (19) −0.0080 (15) 0.0086 (17) 0.0014 (13) C4 0.086 (3) 0.054 (2) 0.061 (2) 0.004 (2) 0.016 (2) 0.0016 (16) C3 0.085 (4) 0.054 (2) 0.096 (3) −0.003 (2) 0.037 (3) −0.004 (2) C2 0.063 (3) 0.059 (2) 0.118 (4) 0.000 (2) 0.032 (3) 0.001 (2) C1 0.057 (3) 0.0474 (18) 0.091 (3) −0.0066 (17) 0.009 (2) 0.0094 (17) C14 0.049 (3) 0.0412 (14) 0.065 (2) −0.0047 (14) 0.0052 (17) 0.0069 (14) C1′ 0.051 (2) 0.0544 (17) 0.0473 (18) 0.0044 (15) 0.0045 (16) 0.0064 (13) C6′ 0.053 (2) 0.0532 (17) 0.0532 (19) −0.0020 (16) −0.0063 (17) 0.0042 (14) C5′ 0.059 (2) 0.0443 (15) 0.0547 (19) −0.0054 (15) 0.0015 (17) 0.0016 (13) C4′ 0.048 (2) 0.0537 (17) 0.0536 (19) −0.0051 (15) 0.0101 (17) −0.0062 (14) N8′ 0.063 (2) 0.070 (2) 0.072 (2) −0.0147 (17) 0.0056 (19) −0.0083 (16) O1 0.092 (2) 0.078 (2) 0.117 (3) −0.0264 (18) 0.000 (2) 0.0229 (18) O2 0.085 (3) 0.140 (3) 0.105 (3) −0.046 (2) −0.027 (2) 0.012 (2) C3′ 0.041 (2) 0.0633 (19) 0.0454 (18) 0.0024 (16) −0.0010 (15) −0.0095 (14) C2′ 0.052 (2) 0.0571 (18) 0.0420 (17) 0.0103 (16) 0.0031 (16) 0.0019 (13) C7′ 0.079 (3) 0.073 (2) 0.054 (2) 0.004 (2) −0.006 (2) 0.0160 (17)
Geometric parameters (Å, º)
S—C1′ 1.764 (4) C3—H3 0.9300
S—C9 1.782 (3) C2—C1 1.359 (5)
C9—C11 1.392 (5) C2—H2 0.9300
C9—C14 1.408 (5) C1—C14 1.429 (5)
C11—C8 1.421 (5) C1—H1 0.9300
C11—C12 1.431 (5) C1′—C6′ 1.397 (4)
C8—C7 1.342 (6) C1′—C2′ 1.399 (4)
C8—H8 0.9300 C6′—C5′ 1.374 (4)
C7—C6 1.418 (6) C6′—H6′ 0.9300
C7—H7 0.9300 C5′—C4′ 1.370 (5)
C6—C5 1.349 (5) C5′—H5′ 0.9300
C6—H6 0.9300 C4′—C3′ 1.377 (4)
C5—C12 1.424 (5) C4′—N8′ 1.461 (4)
C5—H5 0.9300 N8′—O1 1.206 (4)
C12—N10 1.342 (4) N8′—O2 1.216 (4)
N10—C13 1.339 (5) C3′—C2′ 1.382 (5)
C13—C14 1.428 (5) C3′—H3′ 0.9300
C13—C4 1.434 (5) C2′—C7′ 1.504 (4)
C4—C3 1.342 (5) C7′—H71′ 0.9600
C4—H4 0.9300 C7′—H72′ 0.9600
C3—C2 1.408 (6) C7′—H73′ 0.9600
C1′—S—C9 103.54 (14) C3—C2—H2 119.6
C11—C9—C14 119.9 (3) C2—C1—C14 120.9 (4)
C11—C9—S 119.8 (3) C2—C1—H1 119.5
C14—C9—S 120.2 (3) C14—C1—H1 119.5
supporting information
sup-6
Acta Cryst. (2002). E58, o1065–o1067C9—C11—C12 117.8 (3) C9—C14—C1 124.7 (3)
C8—C11—C12 118.2 (4) C13—C14—C1 117.9 (3)
C7—C8—C11 120.7 (4) C6′—C1′—C2′ 120.9 (3)
C7—C8—H8 119.7 C6′—C1′—S 121.4 (3)
C11—C8—H8 119.7 C2′—C1′—S 117.8 (2)
C8—C7—C6 121.5 (4) C5′—C6′—C1′ 120.4 (3)
C8—C7—H7 119.2 C5′—C6′—H6′ 119.8
C6—C7—H7 119.2 C1′—C6′—H6′ 119.8
C5—C6—C7 119.8 (4) C4′—C5′—C6′ 118.5 (3)
C5—C6—H6 120.1 C4′—C5′—H5′ 120.8
C7—C6—H6 120.1 C6′—C5′—H5′ 120.8
C6—C5—C12 120.9 (4) C5′—C4′—C3′ 121.9 (3)
C6—C5—H5 119.5 C5′—C4′—N8′ 118.7 (3)
C12—C5—H5 119.5 C3′—C4′—N8′ 119.4 (3)
N10—C12—C5 118.0 (3) O1—N8′—O2 123.0 (4)
N10—C12—C11 123.1 (3) O1—N8′—C4′ 118.8 (3)
C5—C12—C11 118.9 (4) O2—N8′—C4′ 118.2 (3)
C13—N10—C12 118.3 (3) C4′—C3′—C2′ 120.9 (3)
N10—C13—C14 123.4 (3) C4′—C3′—H3′ 119.5
N10—C13—C4 117.9 (3) C2′—C3′—H3′ 119.5
C14—C13—C4 118.6 (4) C3′—C2′—C1′ 117.4 (3)
C3—C4—C13 121.2 (4) C3′—C2′—C7′ 121.2 (3)
C3—C4—H4 119.4 C1′—C2′—C7′ 121.4 (3)
C13—C4—H4 119.4 C2′—C7′—H71′ 109.5
C4—C3—C2 120.4 (4) C2′—C7′—H72′ 109.5
C4—C3—H3 119.8 H71′—C7′—H72′ 109.5
C2—C3—H3 119.8 C2′—C7′—H73′ 109.5
C1—C2—C3 120.9 (4) H71′—C7′—H73′ 109.5
C1—C2—H2 119.6 H72′—C7′—H73′ 109.5