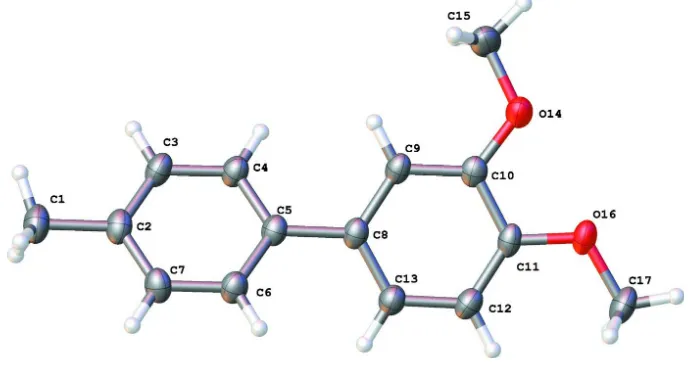
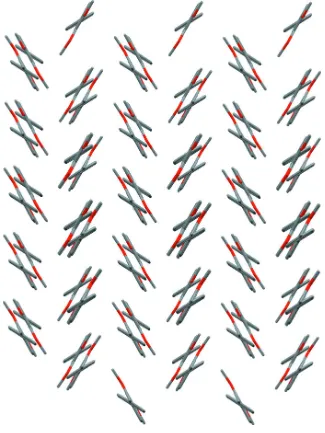
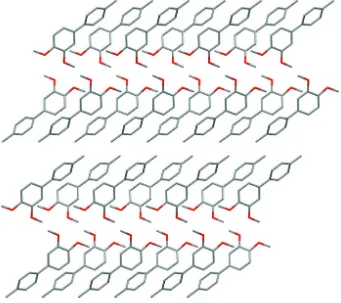
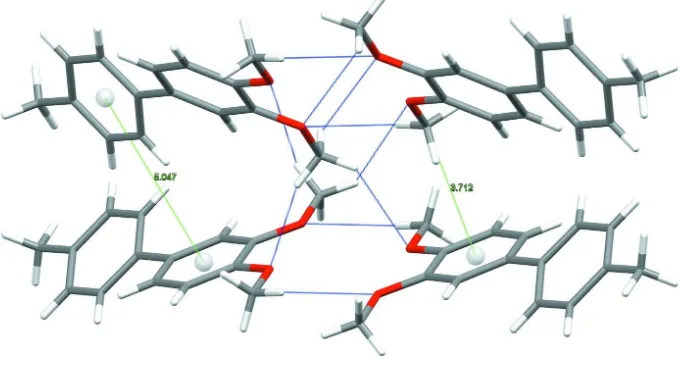
3,4 Dimethoxy 4′ methylbiphenyl
Full text
Figure
Related documents
During the thesis work, I measured six different parameters: the number of emergency processes, hash table entry number, caching replacement policy, cache entry
Make changes to section on agreements to reflect emphasis of agreements in new
• For a home purchase loan, a refinancing, or a dwelling-secured home improvement loan that you originated, report the spread (difference) between the annual percentage rate
An examination of trends by race/ethnicity indicate that Asian/Native American, Hispanic, and White students in charter schools all show greater percentages of proficient or
ter mean to the prototypes computed from the true labels of all the samples. Similar to the semi-supervised scenario, we use a PN trained in the episodic mode as the feature
Although total labor earnings increase with the unskilled unions’ bargaining power, we can say nothing when the increase in production is due to stronger skilled unions, since
We also deal with the question whether the inferiority of the polluter pays principle in comparison to the cheapest cost avoider principle can be compensated
A metadata volume contains information specific to a VPLEX Cluster such as virtual-to-physical mapping information, data about the devices and virtual volumes, system