metal-organic papers
m80
Curtiset al. [Ni(C18H36N4)](NCS)2 doi:10.1107/S160053680504078X Acta Cryst.(2006). E62, m80–m82
Acta Crystallographica Section E
Structure Reports
Online
ISSN 1600-5368
C
-
meso
-(3,5,7,7,10,12,12,14-Octamethyl-1,4,8,11-tetraazacyclotetradeca-4,11-diene)nickel(II)
bis(thio-cyanate)
Neil F. Curtis,a* Rebekah Pawleyband Ward T. Robinsonb
a
School of Chemical and Physical Sciences, Victoria University of Wellington, Box 600, Wellington, New Zealand, andbChemistry
Department, University of Canterbury, Christchurch, New Zealand
Correspondence e-mail: neil.curtis@vuw.ac.nz
Key indicators
Single-crystal X-ray study T= 273 K
Mean(C–C) = 0.002 A˚ Rfactor = 0.023 wRfactor = 0.061
Data-to-parameter ratio = 18.0
For details of how these key indicators were automatically derived from the article, see http://journals.iucr.org/e.
#2006 International Union of Crystallography
Printed in Great Britain – all rights reserved
The title complex, (1RS,3SR,8SR,10RS -3,5,7,7,10,12,12,14-octamethyl-1,4,8,11-tetraazacyclotetradeca-4,11-diene-4
N1,4,8,11 )-nickel(II) bis(thiocyanate), [Ni(C18H36N4)](NCS)2, has a
centrosymmetrical square-planar singlet ground state nick-el(II) cation, with Ni—Namine= 1.940 (1) A˚ and Ni—Nimine=
1.921 (1) A˚ . The C-3 methyl substituent is axially oriented. The thiocyanate N atom is hydrogen bonded to the NH group.
Comment
The azamacrocyle cations C-meso- and C-rac-(Me8
[14]-diene)nickel(II) (Me8[14]diene =
3,5,7,7,10,12,12,14-octa-methyl-1,4,8,11-tetraazacyclotetradeca-4,11-diene), formed by reaction of tris(rac-propane-1,2-diamine)nickel(II) complexes with acetone, were separated by fractional crystallization (see scheme; Blight & Curtis, 1962; Curtis, 1973). Compounds of the C-meso isomer can also be prepared from salts [H2
(C-meso-Me8[14]diene)]X2, formed by reaction of
mono-proto-nated salts [H(rac-propane-1,2-diamine)]X,X= ClO4
, Br, NCS,etc, with acetone (Curtis, 1973).
The structure of yellow, singlet ground-state [Ni(C-meso -Me8[14]diene)](CNS)2 is reported here. It consists of a
centrosymmetric square-planar cation [Ni(C-meso-Me8
[14]-diene)]2+with the thiocyanate ion located off the tetragonal axis [with closest approach Ni NNCS = 4.054 (1) A˚ ] and
hydrogen bonded to the N—H group. The closest axial approach to the nickel is by the imine methyl group of another cation with Ni H51B= 3.68 A˚ .
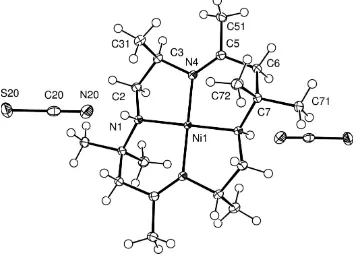
The nickel(II) ion is coordinated by the four N-atoms of the macrocycle, with the Ni—N distance 0.029 (2) A˚ shorter for the imine than the secondary amine N atom (see Fig. 1 and Table 1). The propane-1,2-diamine residue methyl substituent is at ring position 3, adjacent to the imine N atom, axially oriented on the same side of the molecular plane as the N1—H atom, on the same side as the axial component of the gem
dimethyl group, (C72). Displacements of atoms from the NiN4
plane are: C2,0.476 (2); C3, 0.1503 (2); C31, 1.627 (2); C5,
0.068 (2); C51, 0.109 (3); C6, 0.059 (3); C7, 0.703 (2); C71, 0.612 (3); C72, 2.167 (2) A˚ .
[image:1.610.206.470.402.511.2]The structurally characterized compound of the C-rac
isomeric cation, [Ni(C-rac-Me8[14]diene)](ClO4)2, (Swann,et
al., 1972) has approximate twofold symmetry, with the C3 and C10 methyl substituents axially oriented on the same side of the molecular plane, and with the axially oriented components of the C7 and C14 gem-dimethyl groups oriented towards the other side of the plane, with mean distances Ni—Namine =
1.93 (1) and Ni—Nimine= 1.89 (1) A˚ .
The compound [Cu(C-meso-Me8[14]diene)](ClO4)22H2O
(Hazari et al., 1999) has the same configuration and similar conformation of the macrocycle to the title compound, with
Cu—Namine = 2.010 (2), Cu—Nimine = 1.985 (3)A˚ , with
perchlorate O atoms in approximate axial sites with Cu—O = 2.779 (3) A˚ .
The structures of thiocyanate complexes of (5,7,7,12,12,14-hexamethyl-1,4,8,11-tetraazacyclotetradeca-5,11-diene)nickel(II), [Ni(Me6[14]diene)]
2+
, (Curtis & Curtis, 1966) have been determined. The structure of N-meso-[Ni(Me6
[14]diene)]-(SCN)2(Hanic & Miklos, 1972) is similar to that of the title
complex, with mean distances Ni—Namine = 1.92 and Ni—
Nimine = 1.88 A˚ , while N-rac-[Ni(Me6[14]diene)(NCS)]ClO4
(Shen et al., 1999) has an unusual singlet ground-state five-coordinate structure with mean distances Ni—Namine =
2.045 (8), Ni—Nimine= 2.015 (8) and Ni—NNCS= 2.221 (5) A˚ .
The structure of trans-[Co(C-meso-Me8[14]diene)Cl2]ClO4
(Luet al., 1992) and the space group and cell parameters of [Ni(C-meso-Me8[14]diene)](ClO4)2 (Curtis et al., 1969) have
been reported.
Experimental
Excess of NaCNS was added to a hot saturated aqueous solution of orange [Ni(C-meso-Me8[14]diene)](ClO4)2The yellow thiocyanate
salt was filtered off from the cold solution and recrystallized from methanol.
Crystal data
[Ni(C18H36N4)](NCS)2
Mr= 483.38
Triclinic,P1 a= 7.3383 (6) A˚ b= 8.0955 (6) A˚ c= 10.2042 (8) A˚
= 69.917 (1) = 86.852 (1) = 88.965 (1)
V= 568.48 (8) A˚3
Z= 1
Dx= 1.412 Mg m 3
MoKradiation Cell parameters from 2683
reflections
= 2.8–28.3 = 1.06 mm1
T= 273 (2) K Block, yellow 0.400.200.18 mm
Data collection
Bruker SMART CCD area-detector diffractometer
’and!scans
Absorption correction: none 3335 measured reflections 2472 independent reflections
2393 reflections withI> 2(I) Rint= 0.008
max= 28.4
h=9!9 k=8!10 l=13!13
Refinement
Refinement onF2
R[F2> 2(F2)] = 0.023 wR(F2) = 0.061
S= 1.06 2472 reflections 137 parameters
H-atom parameters constrained
w= 1/[2(F
o2) + (0.0311P)2
+ 0.291P]
whereP= (Fo2+ 2Fc2)/3
(/)max= 0.001
max= 0.38 e A˚
3
min=0.25 e A˚
3
Table 1
Selected geometric parameters (A˚ ,).
Ni1—N4 1.921 (1) Ni1—N1 1.940 (1) N4—C5 1.289 (2)
N20—C20 1.167 (2) C20—S20 1.644 (2)
N4—Ni1—N1 86.05 (5) N4i
—Ni1—N1 93.95 (5) C5—N4—Ni1 129.2 (1)
N4—C5—C6 121.8 (1) N4—C5—C51 124.4 (1) N20—C20—S20 178.9 (1)
Symmetry code: (i)xþ1;yþ1;zþ1.
Table 2
Hydrogen-bond geometry (A˚ ,).
D—H A D—H H A D A D—H A
N1—H1 N20 0.91 2.07 2.968 (2) 171
All H atoms were placed in calculated positions, with C—H = 0.96 A˚ , and were included in the least squares refinement as riding on their carrier atoms, withUiso(H) = 1.2Ueqof the corresponding carrier
atom.
Data collection:SMART(Siemens, 1995); cell refinement:SAINT
(Siemens, 1995); data reduction:SAINT; program(s) used to solve structure:SHELXS97(Sheldrick, 1990); program(s) used to refine structure: SHELXL97 (Sheldrick, 1997); molecular graphics:
ORTEP-3.2(Farrugia, 1997); software used to prepare material for publication:SHELXL97.
References
Blight, M. M. & Curtis, N. F. (1962).J. Chem. Soc.pp. 1204–1207. Curtis, N. F. (1973).J. Chem. Soc. Dalton Trans.pp. 863–866. Curtis, N. F. & Curtis, Y. M. (1966).J. Chem. Soc. A, pp. 1653–1656. Curtis, N. F., Swann, D. A., Waters, T. N. & Maxwell, I. E. (1969).J. Am. Chem.
Soc.91, 4588–4589.
Farrugia, L. J. (1997).ORTEP3.2. J. Appl. Cryst.30, 565. Hanic, F. & Miklos, D. (1972).J. Cryst. Mol. Struct.2, 107–116.
metal-organic papers
Acta Cryst.(2006). E62, m80–m82 Curtiset al. [Ni(C
[image:2.610.44.297.70.258.2]18H36N4)](NCS)2
m81
Figure 1[Ni(C-meso-Me8[14]diene))(NCS)2drawn with displacement ellipsoids at
Hazari, S. K. S., Roy, T. G., Dey, B. K., Chakrabarti, S. & Tiekink, E. R. T. (1999).Z. Kristallogr. New Cryst. Struct.214, 51–52.
Lu, T. H., Chen, B. H. & Chung, C. S. (1992).J. Chin. Chem. Soc. (Taipei),39, 343–345.
Sheldrick, G. M. (1990).Acta Cryst.A46, 467–473.
Sheldrick, G. M. (1997).SHELXTL. University of Gottingen, Germany.
Shen, H.-Y., Liao, D.-Z., Jiang Z.-H. & Yan, S. P. (1999).Trans. Metal Chem.
24, 581–583.
Siemens (1995).SMARTandSAINT. Bruker AXS Inc., Madison, Wisconsin, USA.
Swann, D. A., Waters, T. N. & Curtis, N. F. (1972).J. Chem. Soc. Dalton Trans. pp. 1115–1120.
metal-organic papers
m82
Curtiset al. [Ni(Csupporting information
sup-1
Acta Cryst. (2006). E62, m80–m82supporting information
Acta Cryst. (2006). E62, m80–m82 [doi:10.1107/S160053680504078X]
C-meso-(3,5,7,7,10,12,12,14-Octamethyl-1,4,8,11-tetraazacyclotetradeca-4,11-diene)nickel(II) bis(thiocyanate)
Neil F. Curtis, Rebekah Pawley and Ward T. Robinson
S1. Comment
The azamacrocyle cations C-meso- and C-rac-(Me8[14]diene)nickel(II) (Me8[14]diene =
3,5,7,7,10,12,12,14-octa-methyl-1,4,8,11- tetraazacyclotetradeca-4,11-diene), formed by reaction of tris(rac-propane-1,2-diamine)nickel(II)
compounds with acetone, were separated by fractional crystallization, see the Scheme (Blight & Curtis, 1962; Curtis,
1973). Compounds of the C-meso isomer can also be prepared from salts [H2(C-meso-Me8[14]diene)]X2, formed by
reaction of mono-protonated salts [H(rac-propane-1,2-diamine)]X, X− = ClO
4−, Br−, NCS−, etc, with acetone (Curtis,
1973).
The structure of yellow, singlet ground state, [Ni(C-meso-Me8[14]diene)](CNS)2, is reported here. It is comprised of a
centrosymmetrical square-planar cation [Ni(C-meso-Me8[14]diene)]2+ with the thiocyanate ion located off the tetragonal
axis (with closest approach Ni—NNCS = 4.054 Å) and hydrogen bonded to the N—H group. The closest axial approach to
the nickel is by the imine methyl group of another cation with Ni···H51B = 3.68 Å.
The nickel(II) ion is coordinated by the four N-atoms of the macrocycle, with the Ni—N distance 0.029 (2) Å shorter
for the imine than the secondary amine N atom, see Fig. 1 and Table 1. The propane-1,2-diamine residue methyl
substituent is at ring position 3, adjacent to the imine N atom, axially oriented on the same side of the molecular plane as
the N1—H atom, on the opposite side to the axial component of the gem dimethyl group, (C72). Displacements of atoms
from the NiN4 plane are: C2, −0.476 (2); C3, 0.1503 (2); C31, 1.627 (2); C5, −0.068 (2); C51, −0.109 (3); C6, −0.059 (3);
C7, 0.703 (2); C71, 0.612 (3); C72, 2.167 (2) Å.
The structurally characterized compound of the C-rac isomeric cation, [Ni(C-rac-Me8[14]diene)](ClO4)2, (Swann, et al.,
1972) has approximate twofold symmetry, with the C3 and C10 methyl substituents axially oriented on the same side of
the molecular plane, with the axially oriented components of the C7 and C14 gem-dimethyl groups oriented towards the
other side of the plane, with mean distances Ni—Namine = 1.93 (1) and Ni—Nimine = 1.89 (1) Å.
The compound [Cu(C-meso-Me8[14]diene)](ClO4)2·2H2O (Hazari et al., 1999) has the same configuration and similar
conformation of the macrocycle to the title compound, with Cu—Namine = 2.010 (2), Cu—Nimine = 1.985 (3) Å, with
perchlorate oxygen atoms in approximate axial sites with Cu—O = 2.779 (3) Å.
The structures of thiocyanate complexes of (5,7,7,12,12,14-hexamethyl-1,4,8,11-tetraazacyclotetradeca-5,11-
diene)nickel(II), [Ni(Me6[14]diene)]2+, (Curtis & Curtis, 1966) have been determined. The structure of
N-meso-[Ni(Me6[14]diene)](SCN)2 (Hanic & Miklos, 1972) is similar to that of the title complex, with mean distances Ni—
Namine = 1.92 and Ni—Nimine = 1.88 Å, while N-rac-[Ni(Me6[14]diene)(NCS)]ClO4 (Shen et al., 1999) has an unusual
singlet ground state five-coordinate structure with mean distances Ni—Namine = 2.045 (8), Ni—Nimine = 2.015 (8) and Ni—
supporting information
sup-2
Acta Cryst. (2006). E62, m80–m82The structure of trans-[Co(C-meso-Me8[14]diene)Cl2]ClO4 (Lu et al., 1992) and the space group and cell parameters of
[Ni(C-meso-Me8[14]diene)](ClO4)2 (Curtis et al., 1969) have been reported.
S2. Experimental
Excess of NaCNS was added to a hot saturated aqueous solution of orange [Ni(C-meso-L)](ClO4)2. (What is L?) The
yellow thiocyanate salt was filtered off from the cold solution and recrystallized from methanol.
S3. Refinement
All H atoms were placed in calculated positions, with C—H = 0.96 Å and were included in the least squares refinement
[image:5.610.128.486.218.476.2]as riding on their carrier atoms, with Uiso(H) = 1.2Ueq of the corresponding carrier atom.
Figure 1
[Ni(C-meso-Me8[14]diene))(NCS)2 drawn with displacement ellipsoids at 50% probability level, with H atoms shown as
circles of arbitrary radii.
(1RS,3SR,8SR,10RS-3,5,7,7,10,12,12,14-octamethyl-1,4,8,11- tetraazacyclotetradeca-4,11-diene-κ4N1,4,8,11)nickel(II) bis(thiocyanate)
Crystal data
[Ni(C18H36N4)](NCS)2
Mr = 483.38 Triclinic, P1 Hall symbol: -P 1
a = 7.3383 (6) Å
b = 8.0955 (6) Å
c = 10.2042 (8) Å
α = 69.917 (1)°
β = 86.852 (1)°
γ = 88.965 (1)°
V = 568.48 (8) Å3
Z = 1
F(000) = 258
Dx = 1.412 Mg m−3
Mo Kα radiation, λ = 0.71073 Å Cell parameters from 2683 reflections
θ = 2.8–28.3°
µ = 1.06 mm−1
T = 273 K Block, yellow
supporting information
sup-3
Acta Cryst. (2006). E62, m80–m82Data collection
Bruker SMART CCD area-detector diffractometer
Radiation source: fine-focus sealed tube Graphite monochromator
φ and ω scans
3335 measured reflections 2472 independent reflections
2393 reflections with I > 2σ(I)
Rint = 0.008
θmax = 28.4°, θmin = 2.1°
h = −9→9
k = −8→10
l = −13→13
Refinement
Refinement on F2 Least-squares matrix: full
R[F2 > 2σ(F2)] = 0.023
wR(F2) = 0.061
S = 1.06 2472 reflections 137 parameters 0 restraints
Primary atom site location: structure-invariant direct methods
Secondary atom site location: difference Fourier map
Hydrogen site location: inferred from neighbouring sites
H-atom parameters constrained
w = 1/[σ2(F
o2) + (0.0311P)2 + 0.291P] where P = (Fo2 + 2Fc2)/3
(Δ/σ)max = 0.001 Δρmax = 0.38 e Å−3 Δρmin = −0.25 e Å−3
Special details
Geometry. All e.s.d.'s (except the e.s.d. in the dihedral angle between two l.s. planes) are estimated using the full covariance matrix. The cell e.s.d.'s are taken into account individually in the estimation of e.s.d.'s in distances, angles and torsion angles; correlations between e.s.d.'s in cell parameters are only used when they are defined by crystal symmetry. An approximate (isotropic) treatment of cell e.s.d.'s is used for estimating e.s.d.'s involving l.s. planes
Refinement. Refinement of F2 against ALL reflections. The weighted R-factor wR and goodness of fit S are based on F2, conventional R-factors R are based on F, with F set to zero for negative F2. The threshold expression of F2 > σ(F2) is used only for calculating R-factors(gt) etc. and is not relevant to the choice of reflections for refinement. R-factors based on F2 are statistically about twice as large as those based on F, and R- factors based on ALL data will be even larger.
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
x y z Uiso*/Ueq
Ni1 0.5000 0.5000 0.5000 0.00971 (8)
N1 0.50725 (14) 0.47718 (14) 0.69517 (11) 0.0120 (2)
H1 0.5779 0.5674 0.6971 0.014*
N4 0.27883 (15) 0.63089 (14) 0.49926 (11) 0.0128 (2)
C5 0.16839 (17) 0.69536 (17) 0.40027 (14) 0.0136 (2)
C6 0.20809 (18) 0.68678 (18) 0.25715 (14) 0.0153 (3)
H6A 0.1484 0.7858 0.1906 0.018*
H6B 0.1526 0.5805 0.2538 0.018*
C2 0.32061 (18) 0.51070 (18) 0.74570 (14) 0.0155 (3)
H2A 0.3268 0.5375 0.8310 0.019*
H2B 0.2448 0.4072 0.7654 0.019*
C7 0.40883 (18) 0.68814 (17) 0.20850 (13) 0.0135 (3)
C51 −0.00540 (18) 0.79061 (19) 0.41374 (14) 0.0180 (3)
H51A −0.0539 0.7461 0.5087 0.027*
H51B −0.0926 0.7725 0.3532 0.027*
H51C 0.0192 0.9141 0.3880 0.027*
supporting information
sup-4
Acta Cryst. (2006). E62, m80–m82H31A 0.4514 0.8367 0.6102 0.029*
H31B 0.2893 0.8649 0.7070 0.029*
H31C 0.2731 0.9317 0.5445 0.029*
C72 0.5068 (2) 0.85158 (18) 0.21209 (15) 0.0180 (3)
H72A 0.5170 0.8441 0.3074 0.027*
H72B 0.4385 0.9547 0.1636 0.027*
H72C 0.6265 0.8585 0.1676 0.027*
C71 0.41348 (19) 0.68588 (19) 0.05837 (13) 0.0172 (3)
H71A 0.5373 0.6976 0.0208 0.026*
H71B 0.3422 0.7818 0.0017 0.026*
H71C 0.3639 0.5768 0.0588 0.026*
C3 0.24005 (18) 0.66384 (17) 0.63368 (13) 0.0147 (3)
H3 0.1077 0.6660 0.6521 0.018*
N20 0.76296 (17) 0.74108 (17) 0.72386 (13) 0.0221 (3)
C20 0.83366 (18) 0.77659 (18) 0.81043 (14) 0.0164 (3)
S20 0.93355 (5) 0.83066 (5) 0.93031 (4) 0.02264 (10)
Atomic displacement parameters (Å2)
U11 U22 U33 U12 U13 U23
Ni1 0.01007 (12) 0.01126 (12) 0.00886 (12) 0.00289 (8) −0.00114 (8) −0.00484 (9)
N1 0.0118 (5) 0.0135 (5) 0.0116 (5) 0.0027 (4) −0.0011 (4) −0.0054 (4)
N4 0.0131 (5) 0.0148 (5) 0.0115 (5) 0.0025 (4) −0.0003 (4) −0.0060 (4)
C5 0.0132 (6) 0.0133 (6) 0.0147 (6) 0.0012 (5) −0.0004 (5) −0.0054 (5)
C6 0.0143 (6) 0.0197 (6) 0.0130 (6) 0.0059 (5) −0.0037 (5) −0.0069 (5)
C2 0.0134 (6) 0.0207 (7) 0.0128 (6) 0.0037 (5) 0.0006 (5) −0.0067 (5)
C7 0.0152 (6) 0.0152 (6) 0.0102 (6) 0.0039 (5) −0.0023 (5) −0.0044 (5)
C51 0.0152 (6) 0.0232 (7) 0.0153 (6) 0.0071 (5) −0.0023 (5) −0.0065 (5)
C31 0.0221 (7) 0.0176 (7) 0.0213 (7) 0.0045 (5) −0.0029 (6) −0.0108 (6)
C72 0.0221 (7) 0.0141 (6) 0.0173 (6) 0.0009 (5) −0.0006 (5) −0.0047 (5)
C71 0.0198 (6) 0.0212 (7) 0.0105 (6) 0.0049 (5) −0.0018 (5) −0.0052 (5)
C3 0.0145 (6) 0.0177 (6) 0.0133 (6) 0.0031 (5) 0.0008 (5) −0.0075 (5)
N20 0.0209 (6) 0.0253 (6) 0.0234 (6) −0.0027 (5) 0.0022 (5) −0.0127 (5)
C20 0.0136 (6) 0.0151 (6) 0.0186 (6) 0.0012 (5) 0.0033 (5) −0.0043 (5)
S20 0.02041 (18) 0.0299 (2) 0.01789 (18) −0.00037 (14) −0.00455 (14) −0.00796 (15)
Geometric parameters (Å, º)
Ni1—N4 1.921 (1) C7—C72 1.5297 (18)
Ni1—N4i 1.9212 (11) C7—C71 1.5368 (17)
Ni1—N1 1.940 (1) C51—H51A 0.9600
Ni1—N1i 1.9395 (11) C51—H51B 0.9600
N1—C2 1.4901 (16) C51—H51C 0.9600
N1—C7i 1.5066 (16) C31—C3 1.5263 (19)
N1—H1 0.9100 C31—H31A 0.9600
N4—C5 1.289 (2) C31—H31B 0.9600
N4—C3 1.4963 (16) C31—H31C 0.9600
supporting information
sup-5
Acta Cryst. (2006). E62, m80–m82C5—C51 1.5027 (18) C72—H72B 0.9600
C6—C7 1.5275 (18) C72—H72C 0.9600
C6—H6A 0.9700 C71—H71A 0.9600
C6—H6B 0.9700 C71—H71B 0.9600
C2—C3 1.5058 (18) C71—H71C 0.9600
C2—H2A 0.9700 C3—H3 0.9800
C2—H2B 0.9700 N20—C20 1.167 (2)
C7—N1i 1.5066 (16) C20—S20 1.644 (2)
N4—Ni1—N4i 180.00 (7) C6—C7—C71 106.91 (10)
N4—Ni1—N1 86.05 (5) C72—C7—C71 110.20 (11)
N4i—Ni1—N1 93.95 (5) C5—C51—H51A 109.5
N4—Ni1—N1i 93.95 (5) C5—C51—H51B 109.5
N4i—Ni1—N1i 86.05 (5) H51A—C51—H51B 109.5
N1—Ni1—N1i 180.0 C5—C51—H51C 109.5
C2—N1—C7i 112.68 (10) H51A—C51—H51C 109.5
C2—N1—Ni1 108.42 (8) H51B—C51—H51C 109.5
C7i—N1—Ni1 117.01 (8) C3—C31—H31A 109.5
C2—N1—H1 106.0 C3—C31—H31B 109.5
C7i—N1—H1 106.0 H31A—C31—H31B 109.5
Ni1—N1—H1 106.0 C3—C31—H31C 109.5
C5—N4—C3 118.29 (11) H31A—C31—H31C 109.5
C5—N4—Ni1 129.2 (1) H31B—C31—H31C 109.5
C3—N4—Ni1 112.44 (8) C7—C72—H72A 109.5
N4—C5—C6 121.8 (1) C7—C72—H72B 109.5
N4—C5—C51 124.4 (1) H72A—C72—H72B 109.5
C6—C5—C51 113.72 (11) C7—C72—H72C 109.5
C5—C6—C7 116.80 (11) H72A—C72—H72C 109.5
C5—C6—H6A 108.1 H72B—C72—H72C 109.5
C7—C6—H6A 108.1 C7—C71—H71A 109.5
C5—C6—H6B 108.1 C7—C71—H71B 109.5
C7—C6—H6B 108.1 H71A—C71—H71B 109.5
H6A—C6—H6B 107.3 C7—C71—H71C 109.5
N1—C2—C3 108.32 (10) H71A—C71—H71C 109.5
N1—C2—H2A 110.0 H71B—C71—H71C 109.5
C3—C2—H2A 110.0 N4—C3—C2 106.12 (10)
N1—C2—H2B 110.0 N4—C3—C31 109.63 (11)
C3—C2—H2B 110.0 C2—C3—C31 113.20 (11)
H2A—C2—H2B 108.4 N4—C3—H3 109.3
N1i—C7—C6 106.76 (10) C2—C3—H3 109.3
N1i—C7—C72 110.97 (11) C31—C3—H3 109.3
C6—C7—C72 111.31 (11) N20—C20—S20 178.9 (1)
N1i—C7—C71 110.57 (10)
supporting information
sup-6
Acta Cryst. (2006). E62, m80–m82Hydrogen-bond geometry (Å, º)
D—H···A D—H H···A D···A D—H···A

![Figure 1[Ni(C-meso-Me8[14]diene))(NCS)2 drawn with displacement ellipsoids at50% probability level, with H atoms shown as circles of arbitrary radii.Unlabelled atom are related to labelled atoms by the symmetry operation(1 � x, 1 � y, 1 � z).](https://thumb-us.123doks.com/thumbv2/123dok_us/684768.571404/2.610.44.297.70.258/displacement-ellipsoids-probability-arbitrary-unlabelled-labelled-symmetry-operation.webp)