organic papers
Acta Cryst.(2005). E61, o1139–o1140 doi:10.1107/S1600536805006501 Zhu, Huang and Pan C
17H22O3
o1139
Acta Crystallographica Section EStructure Reports Online
ISSN 1600-5368
9-Butyl-3,4,5,6,7,9-hexahydro-2
H
-xanthene-1,8-dione
Yu-Lin Zhu, Shen-Lin Huang and Yuan-Jiang Pan*
Department of Chemistry, Zhejiang University, Hangzhou 310027, People’s Republic of China
Correspondence e-mail: cheyjpan@zju.edu.cn
Key indicators
Single-crystal X-ray study
T= 298 K
Mean(C–C) = 0.005 A˚
Rfactor = 0.039
wRfactor = 0.089 Data-to-parameter ratio = 9.6
For details of how these key indicators were automatically derived from the article, see http://journals.iucr.org/e.
#2005 International Union of Crystallography Printed in Great Britain – all rights reserved
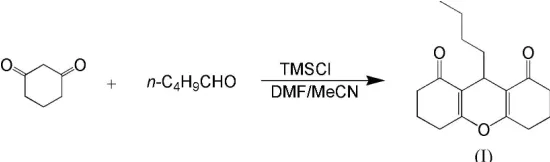
The title compound, C17H22O3, which was synthesized by the
condensation of cyclohexane-1,3-dione and n-valeraldehyde, includes a partially hydrogenated xanthene ring system. The molecule has crystallographic mirror symmetry. The central ring adopts a very shallow boat conformation while the symmetry-related outer six-membered rings have sofa confor-mations. Molecules form extended tapes in thec-axis direction though weak C—H O interactions.
Comment
[image:1.610.206.481.371.452.2]The synthesis of 9-butyl-3,4,5,6,7,9-hexahydro-2H -xanthene-1,8-dione, (I), was initially reported in 1962 (Hellmann & Schroeder, 1962). In our experiment, the reaction of cyclo-hexane-1,3-dione and n-valeraldehyde in the presence of tri-methylsilyl chloride (TMSCl) affords (I) in 78% yield. Fig. 1 shows the molecular structure of (I).
The central ring is in a very shallow boat conformation, with atoms C1, C6, C1i and C6i [symmetry code: (i) 1x, y, z] exactly coplanar by symmetry and atoms O1 and C7 0.156 (4) and 0.263 (6) A˚ from this plane. Atoms O1 and C7–C11 lie on a crystallographic mirror plane at x = 12. The two outer
symmetry-related six-membered rings are each in a sofa conformation, with atoms C1, C2, C4, C5 and C6 forming a plane (r.m.s. deviation = 0.024 A˚ ) and atom C3 0.595 (4) A˚ from this plane. The bond lengths and angles in (I) are normal. The O2—C5—C6—C1 torsion angle of 176.2 (2) and the C5—C6 bond length of 1.458 (4) A˚ indicate conjugation between the O2 C5 and C6 C1 bonds. The torsion angle of 61.8 (2) for C6—C7—C8—C9 defines the orientation of the n-butyl group. Molecules are linked by weak C—H O interactions [H3A O2ii = 2.59 A˚ , C3 O2ii = 3.316 (4) A˚ and C3—H3A O2i = 132, symmetry code: (ii) x, 1y,
1
2+z], forming tapes in thec-axis direction (see Fig. 2).
Experimental
Cyclohexane-1,3-dione (10 mmol), n-valeraldehyde (10 mmol) and dimethylformamide–acetonitrile (1:2v/v9 ml) were mixed in a 25 ml flask. TMSCl (10 mmol) was then added dropwise at room
temperature. The resulting reaction mixture was stirred at 353 K for 3 h, cooled to room temperature and precipitation was observed. The precipitate was isolated by filtering through a Buchner funnel, washed with ethanol and dried to give the crystalline powder. The powder was further purified by recrystallization from C2H5OH. The
crystalline product was dissolved in a DMF solution and single crystals suitable for X-ray structure analysis were obtained by slow evaporation of the solution at room temperature.
Crystal data
C17H22O3 Mr= 274.35
Orthorhombic,Cmc21 a= 15.121 (4) A˚ b= 8.872 (2) A˚ c= 11.025 (3) A˚ V= 1479.0 (7) A˚3 Z= 4
Dx= 1.232 Mg m 3
MoKradiation Cell parameters from 40
reflections = 4.6–15.0
= 0.08 mm1 T= 298 (2) K Block, colorless 0.500.260.26 mm
Data collection
SiemensP4 diffractometer !scans
Absorption correction: none 1011 measured reflections 955 independent reflections 638 reflections withI> 2(I) Rint= 0.010
max= 27.8
h= 0!19 k= 0!11 l=14!1 3 standard reflections
every 97 reflections intensity decay: 3.2%
Refinement
Refinement onF2 R[F2> 2(F2)] = 0.039 wR(F2) = 0.089 S= 0.88 955 reflections 100 parameters
H-atom parameters constrained w= 1/[2
(Fo2) + (0.0465P)2]
whereP= (Fo2+ 2Fc2)/3
(/)max< 0.001
max= 0.15 e A˚3
min=0.12 e A˚3
H atoms were placed in calculated positions, with C—H = 0.96– 0.98 A˚ , and refined in riding-model approximation with Uiso(H) values set equal to 1.2Ueq(carrier atom). In the absence of significant
anomalous dispersion effects, Friedel pairs were merged.
Data collection: XSCANS (Siemens, 1991); cell refinement:
XSCANS; data reduction: SHELXTL/PC (Siemens, 1994); program(s) used to solve structure: SHELXS97 (Sheldrick, 1997); program(s) used to refine structure:SHELXL97(Sheldrick, 1997); molecular graphics:SHELXTL/PC; software used to prepare mate-rial for publication:SHELXTL/PC.
This research was funded by NSFC of China (No. 20375036).
References
Hellmann, H. & Schroeder, M. (1962).Gerany Ann.656, 85–89.
Sheldrick, G. M. (1997). SHELXS97 and SHELXL97. University of Go¨ttingen, Germany.
Siemens (1991).XSCANS.Version 2.10b. Siemens Analytical X-ray Instru-ments Inc., Madison, Wisconsin, USA.
Siemens (1994). SHELXTL/PC. Version 4.2. Siemens Analytical X-ray Instruments Inc., Madison, Wisconsin, USA.
[image:2.610.313.562.68.382.2]Spek, A. L. (2003).J. Appl. Cryst.36, 7–13. Figure 1
View of the title compound, showing the atom-labeling scheme. Displacement ellipsoids are drawn at the 50% probability level and H atoms are shown as small spheres of arbitrary radii. Unlabeled atoms are related by the symmetry code (1x, y, z).
Figure 2
[image:2.610.46.298.73.291.2]supporting information
sup-1 Acta Cryst. (2005). E61, o1139–o1140
supporting information
Acta Cryst. (2005). E61, o1139–o1140 [https://doi.org/10.1107/S1600536805006501]
9-Butyl-3,4,5,6,7,9-hexahydro-2
H
-xanthene-1,8-dione
Yu-Lin Zhu, Shen-Lin Huang and Yuan-Jiang Pan
9-Butyl-3,4,5,6,7,9-hexahydro-2H-xanthene-1,8-dione
Crystal data
C17H22O3
Mr = 274.35
Orthorhombic, Cmc21
Hall symbol: C 2c -2
a = 15.121 (4) Å
b = 8.872 (2) Å
c = 11.025 (3) Å
V = 1479.0 (7) Å3
Z = 4
F(000) = 592
Dx = 1.232 Mg m−3
Mo Kα radiation, λ = 0.71073 Å Cell parameters from 40 reflections
θ = 4.6–15.0°
µ = 0.08 mm−1
T = 298 K Block, colorless 0.50 × 0.26 × 0.26 mm
Data collection
Siemens P4 diffractometer
Radiation source: fine-focus sealed tube Graphite monochromator
ω scans
1011 measured reflections 955 independent reflections 638 reflections with I > 2σ(I)
Rint = 0.010
θmax = 27.8°, θmin = 2.7°
h = 0→19
k = 0→11
l = −14→1
3 standard reflections every 97 reflections intensity decay: 3.2%
Refinement
Refinement on F2
Least-squares matrix: full
R[F2 > 2σ(F2)] = 0.039
wR(F2) = 0.089
S = 0.88 955 reflections 100 parameters 1 restraint
Primary atom site location: structure-invariant direct methods
Secondary atom site location: difference Fourier map
Hydrogen site location: inferred from neighbouring sites
H-atom parameters constrained
w = 1/[σ2(F
o2) + (0.0465P)2]
where P = (Fo2 + 2Fc2)/3
(Δ/σ)max < 0.001
Δρmax = 0.15 e Å−3
Δρmin = −0.12 e Å−3
Special details
Refinement. Refinement of F2 against ALL reflections. The weighted R-factor wR and goodness of fit S are based on F2,
conventional R-factors R are based on F, with F set to zero for negative F2. The threshold expression of F2 > σ(F2) is used
only for calculating R-factors(gt) etc. and is not relevant to the choice of reflections for refinement. R-factors based on F2
are statistically about twice as large as those based on F, and R- factors based on ALL data will be even larger.
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
x y z Uiso*/Ueq Occ. (<1)
O1 0.5000 0.7222 (3) 0.3745 (2) 0.0439 (7) O2 0.33006 (12) 0.5093 (3) 0.0610 (2) 0.0674 (6) C1 0.42210 (15) 0.6886 (3) 0.3153 (2) 0.0367 (6) C2 0.34659 (17) 0.7700 (3) 0.3713 (3) 0.0479 (7) H2A 0.3484 0.8752 0.3473 0.058* H2B 0.3516 0.7654 0.4589 0.058* C3 0.25933 (19) 0.7014 (4) 0.3323 (3) 0.0598 (9) H3A 0.2500 0.6076 0.3757 0.072* H3B 0.2115 0.7695 0.3533 0.072* C4 0.2575 (2) 0.6716 (3) 0.1984 (3) 0.0581 (8) H4A 0.2551 0.7672 0.1559 0.070* H4B 0.2040 0.6164 0.1790 0.070* C5 0.33572 (18) 0.5839 (3) 0.1528 (3) 0.0447 (7) C6 0.41886 (15) 0.5978 (2) 0.2187 (2) 0.0355 (6) C7 0.5000 0.5174 (4) 0.1727 (3) 0.0390 (9) H7 0.5000 0.5256 0.0840 0.047* C8 0.5000 0.3482 (4) 0.2047 (4) 0.0439 (9)
H8A 0.4483 0.3022 0.1681 0.053* 0.50 H8B 0.5517 0.3022 0.1681 0.053* 0.50 C9 0.5000 0.3111 (4) 0.3377 (3) 0.0414 (9)
H9A 0.4482 0.3561 0.3749 0.050* 0.50 H9B 0.5518 0.3561 0.3749 0.050* 0.50 C10 0.5000 0.1437 (4) 0.3638 (4) 0.0526 (11)
H10A 0.4482 0.0990 0.3262 0.063* 0.50 H10B 0.5518 0.0990 0.3262 0.063* 0.50 C11 0.5000 0.1040 (5) 0.4965 (4) 0.0817 (16)
H11A 0.5000 −0.0037 0.5054 0.123*
H11B 0.4482 0.1451 0.5343 0.123* 0.50 H11C 0.5518 0.1451 0.5343 0.123* 0.50
Atomic displacement parameters (Å2)
U11 U22 U33 U12 U13 U23
supporting information
sup-3 Acta Cryst. (2005). E61, o1139–o1140
C6 0.0367 (13) 0.0325 (12) 0.0372 (13) −0.0023 (10) −0.0017 (11) 0.0023 (12) C7 0.0452 (19) 0.0371 (19) 0.035 (2) 0.000 0.000 −0.0053 (17) C8 0.045 (2) 0.0375 (19) 0.049 (2) 0.000 0.000 −0.0094 (19) C9 0.043 (2) 0.0325 (18) 0.048 (2) 0.000 0.000 0.0001 (17) C10 0.058 (2) 0.0355 (19) 0.065 (3) 0.000 0.000 −0.001 (2) C11 0.130 (5) 0.047 (3) 0.067 (3) 0.000 0.000 0.013 (3)
Geometric parameters (Å, º)
O1—C1 1.379 (3) C7—C6i 1.508 (3)
O1—C1i 1.379 (3) C7—C8 1.542 (5)
O2—C5 1.213 (4) C7—H7 0.9800
C1—C6 1.336 (3) C8—C9 1.502 (5)
C1—C2 1.485 (3) C8—H8A 0.9700
C2—C3 1.515 (4) C8—H8B 0.9700
C2—H2A 0.9700 C9—C10 1.513 (5)
C2—H2B 0.9700 C9—H9A 0.9700
C3—C4 1.500 (5) C9—H9B 0.9700
C3—H3A 0.9700 C10—C11 1.504 (6)
C3—H3B 0.9700 C10—H10A 0.9700
C4—C5 1.502 (4) C10—H10B 0.9700
C4—H4A 0.9700 C11—H11A 0.9600
C4—H4B 0.9700 C11—H11B 0.9600
C5—C6 1.458 (4) C11—H11C 0.9600
C6—C7 1.508 (3)
C1—O1—C1i 117.3 (3) C6i—C7—C8 112.6 (2)
C6—C1—O1 122.6 (2) C6—C7—C8 112.6 (2) C6—C1—C2 126.6 (2) C6i—C7—H7 107.5
O1—C1—C2 110.8 (2) C6—C7—H7 107.5
C1—C2—C3 110.9 (2) C8—C7—H7 107.5
C1—C2—H2A 109.5 C9—C8—C7 115.9 (3)
C3—C2—H2A 109.5 C9—C8—H8A 108.3
C1—C2—H2B 109.5 C7—C8—H8A 108.3
C3—C2—H2B 109.5 C9—C8—H8B 108.3
H2A—C2—H2B 108.0 C7—C8—H8B 108.3
C4—C3—C2 111.4 (3) H8A—C8—H8B 107.4 C4—C3—H3A 109.3 C8—C9—C10 113.6 (3)
C2—C3—H3A 109.3 C8—C9—H9A 108.8
C4—C3—H3B 109.3 C10—C9—H9A 108.8
C2—C3—H3B 109.3 C8—C9—H9B 108.8
H3A—C3—H3B 108.0 C10—C9—H9B 108.8
C3—C4—C5 114.0 (3) H9A—C9—H9B 107.7 C3—C4—H4A 108.8 C11—C10—C9 114.5 (4) C5—C4—H4A 108.8 C11—C10—H10A 108.6
C3—C4—H4B 108.8 C9—C10—H10A 108.6
O2—C5—C6 121.6 (3) H10A—C10—H10B 107.6 O2—C5—C4 120.5 (3) C10—C11—H11A 109.5 C6—C5—C4 117.9 (3) C10—C11—H11B 109.5 C1—C6—C5 118.7 (2) H11A—C11—H11B 109.5 C1—C6—C7 121.6 (2) C10—C11—H11C 109.5 C5—C6—C7 119.6 (2) H11A—C11—H11C 109.5 C6i—C7—C6 108.9 (3) H11B—C11—H11C 109.5
C1i—O1—C1—C6 15.0 (4) O2—C5—C6—C1 176.2 (2)
C1i—O1—C1—C2 −163.21 (18) C4—C5—C6—C1 −0.8 (4)
C6—C1—C2—C3 18.3 (4) O2—C5—C6—C7 −0.2 (4) O1—C1—C2—C3 −163.6 (2) C4—C5—C6—C7 −177.2 (2) C1—C2—C3—C4 −45.4 (3) C1—C6—C7—C6i −20.6 (4)
C2—C3—C4—C5 51.3 (3) C5—C6—C7—C6i 155.73 (19)
C3—C4—C5—O2 154.9 (3) C1—C6—C7—C8 104.9 (3) C3—C4—C5—C6 −28.1 (4) C5—C6—C7—C8 −78.7 (3) O1—C1—C6—C5 −172.3 (2) C6i—C7—C8—C9 61.8 (2)
C2—C1—C6—C5 5.6 (4) C6—C7—C8—C9 −61.8 (2) O1—C1—C6—C7 4.1 (4) C7—C8—C9—C10 180.0 C2—C1—C6—C7 −178.0 (3) C8—C9—C10—C11 180.0