Acta Crystallographica Section E
Structure Reports
Online
ISSN 1600-5368
(
SP
-4-1)-Bis{aqua[(1
R
,2
R
)-
N
,
N
000-bis(salicylidene)-cyclohexane-1,2-diamine-
j
4O
,
N
,
N
000,
O
000]mangan-ese(III)}-di-
l
-cyano-
j
4N
:
C
-[dicyanonickel(II)]
Takashiro Akitsu,* Yuiri Takeuchi and Yasuaki Einaga
Department of Chemistry, Faculty of Science and Technology, Keio University, 3-14-1 Hiyoshi, Kohoku-ku, Yokohama 223-8522, Japan
Correspondence e-mail: akitsu@chem.keio.ac.jp
Key indicators
Single-crystal X-ray study
T= 298 K
Mean(C–C) = 0.014 A˚ H-atom completeness 91%
Rfactor = 0.091
wRfactor = 0.290 Data-to-parameter ratio = 8.6
For details of how these key indicators were automatically derived from the article, see http://journals.iucr.org/e.
The title compound, [Mn2Ni(C20H20N2O2)2(CN)4(H2O)2], is
the first chiral trinuclear complex having an MnIII—NC— NiII—CN—MnIIIunit. A pair of thetranssites of [Ni(CN)4]
2
links two MnIIIunits. The MnIIIion has a pseudo-octahedral coordination geometry with the four donor atoms of the tetradentate chiral Schiff base forming the equatorial plane, and with a bridging cyanide and an aqua ligand at the axial positions.
Comment
In recent years, photofunctional and magnetic complexes have been studied widely (Sato, 2003; Satoet al., 2003). One of the important goals is to discover photofunctional molecular magnets such as Prussian blue analogues (Sato et al., 1996), which exhibit photo-induced electron transfer. Furthermore, cyanide-bridged heterometallic assemblies containing Schiff base MnIIIcomplexes (5B1g ground state) have been
devel-oped by using [Ni(CN)4] 2
(Yuan et al., 2002), [Fe(CN)6] 3
(Miyasaka et al., 2003), and [Mo(CN)8] 3
or [W(CN)8] 4
(Przychodzenet al., 2004) precursors. Some trinuclear MnIII— NC—CrIII—CN—MnIII or MnIII—NC—FeIII—CN—MnIII clusters exhibit characteristics of single-molecule magnets (Choiet al., 2004).
Electronic properties due to individual units and crystal structures of the resulting assemblies are important for the design of cyanide-bridged heterometallic complexes. For example, MnIII complexes are employed as catalysts for
& Janda, 2000) and metalloprotein models for photosystem II (Ashmawyet al., 1985; Aurangzebet al., 1994; Bermejoet al., 1996). On the other hand, [Ni(CN)4]2is known as a building
block for various types of cyanide-bridged complexes (Muga
et al., 2004; Shen et al., 2004; Maji et al., 2001; Cˇ erna´k & Abboud, 2002; Cˇ erna´k et al., 1990; Park & Iwamoto, 1992, 1993). For MnIIIcomplexes withOhsymmetry, the
5
Dground state may split into 5T2g and
5
Eg terms, and Jahn–Teller distortion removes the orbital degeneracy of the5Egground state to give an orbital singlet lowest in energy, either the5A1g or 5B1g. Axial elongation results in a
5
B1gground state with negative D (Kennedy & Murray, 1985). The planar [Ni(CN) ]2unit withD symmetry exhibits prominent
low-a2u(px, *
)] andA1g!Eu[eg(dxz,dyz)!a2u(px, *
)], when the planar units are subjected to external perturbation or struc-tural strain (Mantz & Musselman, 2002). We report here the crystal structure of the title compound, (I), which is the first chiral trinuclear MnIII—NC—NiII—CN—MnIIIcluster.
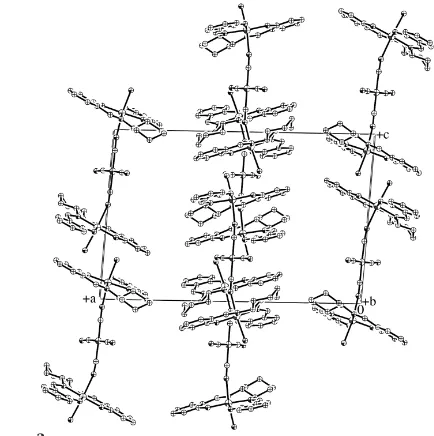
In (I), there are two independent complex molecules in the asymmetric unit, molecules 1 (Ni1 complex, Fig. 1) and 2 (Ni2 complex, Fig. 2). Each molecular structure consists of one [Ni(CN)4]
2
unit and two [Mn{(1R,2R)-salchxn}(H2O)]
units [salchxn is N,N0
-bis(salicylidene)cyclohexane-1,2-diyl-diamine], forming an MnIII—NC—NiII—CN—MnIII trinuc-lear cluster. Terminal water ligands prevent it from forming infinite one-dimensional cyanide-bridged chains. The MnIII ions adopt an elongated octahedral coordination geometry with displacement of Mn1 from the O1/N1/N2/O2 least-squares plane towards the axial cyanide ligand of 0.061 (5) A˚ , Mn2 from O4/N3/N4/O5 of 0.036 (5) A˚ , Mn3 from O7/N9/ N10/O8 of 0.048 (5) A˚ , and Mn4 from O10/N11/N12/O11 of 0.049 (5) A˚ . Both elongated axial sites of the MnIIIion, namely strong-bonding cyanide and water having lone pairs, due to Jahn–Teller distortion are consistent with common high-spin MnIIISchiff base complexes with5B1gground state. Because of the tetradentate Schiff base ligands, the overall molecular shapes are almost planar for the Mn1 and Mn3 units, or slightly stepped for the Mn2 and Mn4 units. The angles between least-squares planes of the aromatic rings are 5.6 (3), 4.3 (3), 4.9 (4) and 5.0 (3)for the Mn1 (C1–C6 and C15–C20),
Mn2 (C21–C26 and C35–C40), Mn3 (C45–C50 and C59–C64), and Mn4 (C65–C70 and C79–C84) units, respectively. The angles between the O1/N1/N2/O2 least-squares plane and N1/ C7/C6/C1/O1 or N2/C14/C15/C20/O2 are 3.0 (3) and 5.9 (4)
in the Mn1 unit, between O4/N3/N4/O5 and N3/C27/C26/C21/ O4 or N4/C34/C35/C40/O5 are 15.0 (3) and 14.7 (4) in Mn2,
between O7/N9/N10/O8 and N9/C51/C50/C45/O7 or N10/C58/ C59/C64/O8 are 2.9 (3) and 10.2 (4) in Mn3, and between
O10/N11/N12/O11 and N11/C71/C70/C65/O10 or N12/C78/ C79/C84/O11 are 10.9 (4) and 7.7 (4) in Mn4, respectively. Besides these, no remarkable geometrical parameters for the related chiral Mn(salchxn) complexes (Nishikoriet al., 1999; Lenobleet al., 1998; Nishikoriet al., 2000; Pospisilet al., 1996) are found in (I).
On the other hand, only two of the four cyanides of [Ni(CN)4]
2
units are involved in bridges, in other words, two cyanides do not form two-dimensional networks. Each cyanide linkage is slightly bent to form wave-like clusters, which may be attributed to bulky ligands and chirality derived from the Mn(salchxn) structural units. The structural features suggest that these trinuclear MnIII—NC—NiII—CN—MnIII clusters exhibit potentially interesting electronic functions. The dihe-dral angles between the least-squares planes of C41/C43/C42/ C44 (Ni1) and O1/N1/N2/O2 (Mn1) or O4/N3/N4/O5 (Mn2) are 72.7 (3) and 86.1 (3), and between those of C85/C87/C86/
C88 (Ni2) and O7/N9/N10/O8 (Mn3) or O10/N11/N12/O11 (Mn4) are 75.3 (3) and 85.8 (4), respectively. The axial water
[image:2.610.56.280.67.279.2]ligands are expected to form one-dimensional hydrogen-bonded chains along the molecular long axis, namely the Figure 1
The structure of molecule 1, showing the atom-labeling scheme. Displacement ellipsoids are drawn at the 30% probability level.
Figure 2
[image:2.610.53.284.323.542.2]molecular hydrogen bonds are expected: O3 O4i = 2.92 (1) A˚ , O3 O5i = 3.33 (1) A˚ , O6 O1ii = 2.82 (1) A˚ , O6 O2ii= 3.22 (1) A˚ , O9 O10i= 2.95 (1) A˚ , O9 O11i= 3.24 (1) A˚ , O12 O7ii = 3.00 (1) A˚ and O12 O8ii = 3.15 (1) A˚ [symmetry codes: (i)x,y, z1; (ii)x,y, z+ 1].
Experimental
Under an N2atmosphere at 298 K, treatment of [Et4N]ClO4(1.380 g, 6.01 mmol) and K2[Ni(CN)4] (0.736 g, 3.05 mmol) in methanol (100 ml) for 72 h gave rise to [Et4N]2[Ni(CN)4], a precursor. To a solution of salicylaldehyde (0.065 g, 0.53 mmol) and (1R,2R)-( )-1,2-diaminocyclohexane (0.029 g, 0.25 mmol) in methanol (30 ml), Mn(CH3CO2)32H2O (0.069 g, 0.26 mmol), NaClO4.H2O (0.064 g, 0.45 mmol) and [Et4N]2[Ni(CN)4] were added and stirred for 24 h at 298 K. Brown prismatic crystals suitable for X-ray crystallography were obtained from the resulting brown solution [yield 90%; m.p. 600 K (decomposition)]. IR (KBr, cm1): 1617 (imine), 2135 (cyanide).
Crystal data
[Mn2Ni(C20H20N2O2)2(CN)4
-(H2O)2]
Mr= 949.44 Monoclinic,P21
a= 23.260 (2) A˚
b= 13.872 (2) A˚
c= 15.182 (3) A˚
= 93.79 (4)
V= 4888 (2) A˚3
Z= 4
Dx= 1.290 Mg m 3
Mo Kradiation Cell parameters from 25
reflections
= 10.4–12.6
= 0.94 mm1
T= 298 (2) K Prism, brown 0.600.600.20 mm
Data collection
Rigaku AFC-7Rdiffractometer
!–2scans
Absorption correction: scan (Northet al., 1968)
Tmin= 0.530,Tmax= 0.828
Rint= 0.086
max= 27.6
h= 0!30
k= 0!18
l=19!19
Refinement
Refinement onF2 R[F2> 2(F2)] = 0.091
wR(F2) = 0.290
S= 1.07 8645 reflections 1004 parameters
H-atom parameters constrained
w= 1/[2
(Fo2) + (0.2P)2] whereP= (Fo2+ 2Fc2)/3 (/)max= 0.001
max= 2.49 e A˚
3
min=0.71 e A˚
3
Absolute structure: Flack (1983), no Friedel pairs
[image:3.610.54.271.72.290.2]Flack parameter = 0.01 (3)
Table 1
Selected geometric parameters (A˚ ,).
Ni1—C43 1.859 (15) Ni1—C42 1.860 (11) Ni1—C41 1.869 (12) Ni1—C44 1.900 (15) Ni2—C86 1.854 (12) Ni2—C87 1.853 (14) Ni2—C88 1.860 (14) Ni2—C85 1.888 (12) Mn1—O2 1.863 (9) Mn1—O1 1.876 (8) Mn1—N1 1.965 (10) Mn1—N2 2.001 (10) Mn1—N5 2.285 (8) Mn1—O3 2.406 (9) Mn2—O5 1.857 (9) Mn2—O4 1.882 (8)
Mn2—N3 1.977 (10) Mn2—N4 1.991 (9) Mn2—O6 2.295 (8) Mn2—N6 2.298 (8) Mn3—O7 1.859 (8) Mn3—O8 1.879 (9) Mn3—N9 1.988 (10) Mn3—N10 1.987 (9) Mn3—N13 2.302 (8) Mn3—O9 2.381 (8) Mn4—O11 1.852 (9) Mn4—O10 1.888 (9) Mn4—N11 1.976 (10) Mn4—N12 1.991 (9) Mn4—N14 2.276 (8) Mn4—O12 2.302 (9)
C41—Ni1—C42 178.0 (5) C41—Ni1—C43 85.7 (5) C41—Ni1—C44 93.1 (5) C42—Ni1—C43 92.3 (5) C42—Ni1—C44 88.9 (5) C43—Ni1—C44 177.7 (6) C85—Ni2—C86 178.0 (6) C85—Ni2—C87 93.1 (5) C85—Ni2—C88 87.4 (5) C86—Ni2—C87 88.8 (5) C86—Ni2—C88 90.8 (5) C87—Ni2—C88 177.6 (6) O1—Mn1—O2 91.5 (4) O1—Mn1—O3 89.6 (3) O1—Mn1—N1 93.8 (4) O1—Mn1—N2 176.6 (4) O1—Mn1—N5 93.7 (3) O2—Mn1—O3 91.0 (4) O2—Mn1—N1 170.2 (4) O2—Mn1—N2 91.9 (4) O2—Mn1—N5 93.7 (4) O3—Mn1—N1 80.8 (4) O3—Mn1—N2 90.9 (4) O3—Mn1—N5 174.2 (3) N1—Mn1—N2 83.0 (4) N1—Mn1—N5 94.2 (4) N2—Mn1—N5 85.5 (4) O4—Mn2—O5 92.1 (4) O4—Mn2—O6 91.4 (4) O4—Mn2—N3 93.4 (4) O4—Mn2—N4 174.4 (4) O4—Mn2—N6 96.5 (4) O5—Mn2—O6 92.3 (4) O5—Mn2—N3 174.5 (4) O5—Mn2—N4 92.0 (4) O5—Mn2—N6 93.3 (4)
O6—Mn2—N3 87.1 (3) O6—Mn2—N4 84.7 (4) O6—Mn2—N6 170.2 (3) N3—Mn2—N4 82.5 (4) N3—Mn2—N6 86.6 (4) N4—Mn2—N6 87.1 (4) O7—Mn3—O8 91.4 (4) O7—Mn3—O9 91.8 (3) O7—Mn3—N9 93.4 (4) O7—Mn3—N10 175.8 (4) O7—Mn3—N13 94.2 (4) O8—Mn3—O9 89.7 (3) O8—Mn3—N9 172.2 (4) O8—Mn3—N10 92.8 (4) O8—Mn3—N13 93.7 (4) O9—Mn3—N9 84.0 (4) O9—Mn3—N10 88.8 (4) O9—Mn3—N13 173.0 (4) N9—Mn3—N10 82.5 (4) N9—Mn3—N13 92.1 (4) N10—Mn3—N13 85.0 (4) O10—Mn4—O11 90.7 (4) O10—Mn4—O12 91.4 (4) O10—Mn4—N11 94.0 (4) O10—Mn4—N12 173.8 (4) O10—Mn4—N14 96.7 (4) O11—Mn4—O12 90.8 (4) O11—Mn4—N11 175.3 (4) O11—Mn4—N12 92.6 (4) O11—Mn4—N14 92.9 (4) O12—Mn4—N11 88.4 (4) O12—Mn4—N12 83.4 (4) O12—Mn4—N14 171.0 (4) N11—Mn4—N12 82.7 (4) N11—Mn4—N14 87.2 (4) N12—Mn4—N14 88.3 (4)
H atoms bonded to C atoms were placed in calculated positions, with C—H = 0.95 A˚ andUiso(H) = 1.2Ueq(C), and included in the final cycles of refinement using riding-model constraints. H atoms of the aqua ligands were not included, since they could not be observed in
Figure 3
[image:3.610.314.567.188.661.2]C21–C26, C35–C40, C45–C50, C59–C64, C65–C70 and C79–C84) were treated as rigid groups. The maximum residual density is located 2.80 A˚ from H13. There remains a large void space in the crystal. Location of solvent water molecules was unsuccessful in the refine-ment. A void space or flexible region in the crystal structure is also suggested by the remarkable dependence of the IR spectra (cyanide bands) on the temperature,viz. 2135 cm1at 300 K and 2141 cm1at 9 K.
Data collection: WinAFC Diffractometer Control Software
(Rigaku, 1999); cell refinement: WinAFC Diffractometer Control Software; data reduction: TEXSAN (Molecular Structure Corpor-ation, 2001); program(s) used to solve structure:SIR92(Altomareet al., 1994); program(s) used to refine structure: SHELXL97 (Shel-drick, 1997); molecular graphics:ORTEPII(Johnson, 1976); software used to prepare material for publication:TEXSAN.
This work was supported by Grant-in-Aid for the 21st Century COE program ‘KEIO Life Conjugate Chemistry’ from the Ministry of Education, Culture, Sports, Science, and Technology, Japan.
References
Altomare, A., Cascarano, G., Giacovazzo, C., Guagliardi, A., Burla, M. C., Polidori, G. & Camalli, M. (1994).J. Appl. Cryst.27, 435.
Ashmawy, F. M., McAuliffe, C. A., Parish, R. V. & Tames, J. (1985).J. Chem. Soc. Dalton Trans.pp. 1391–1397.
Aurangzeb, N., Hulme, C. E., McAuliffe, C. A., Pritchard, R. G., Watkinson, M., Bermejo, M. R., Garcia-Deibe, A., Rey, M., Sanmartin, J. & Sousa, A. (1994).J. Chem. Soc. Chem. Commun.pp. 1153–1155.
Bermejo, M. R., Castineiras, A., Garcia-Monteagudo, J. C., Ray, M., Sousa, A., Watkinson, M., McAuliffe, C. A., Pritchard, R. G. & Beddoes, R. L. (1996).
J. Chem. Soc. Dalton Trans.pp. 2935–2944.
Cˇ erna´k, J. & Abboud, K. A. (2002).Acta Cryst.C58, m167–m170.
Cˇ erna´k, J., Chomic, J., Domiano, P., Ori, O. & Andreetti, D. (1990).Acta Cryst.
C46, 2103–2107.
Choi, H. J., Sokol, J. J. & Long, J. R. (2004).Inorg. Chem.43, 1606–1608.
Flack, H. D. (1983).Acta Cryst.A39, 876–881.
Johnson, C. K. (1976).ORTEPII. Report ORNL-5138. Oak Ridge National Laboratory, Tennessee, USA.
Kennedy, B. J. & Murray, K. S. (1985).Inorg. Chem.24, 1552–1557. Korendovych, I. V. & Rybak-Akimova, E. V. (2004).Acta Cryst. C60, m82–
m84.
Lenoble, G., Lacroix, P. G., Daren, J. C., Di Bella, S. & Nakatani, K. (1998).
Inorg. Chem.37, 2158–2165.
Maji, T. K., Mukherjee, P. S., Mostafa, G., Zangrando, E. & Chaudhuri, N. R. (2001).Chem. Commun.pp. 1368–1369.
Mantz, Y. A. & Musselman, R. L. (2002).Inorg. Chem.41, 5770–5777. Martı´nez, D., Motevalli, M. & Watkinson, M. (2002).Acta Cryst.C58, m258–
m260.
Miyasaka, H., Ieda, H., Matsumoto, N., Sugiura, K. & Yamashita, M. (2003).
Inorg. Chem.42, 3509–3515.
Molecular Structure Corporation (2001).TEXSAN.Version 1.11. MSC, 9009 New Trails Drive, The Woodlands, TX 77381-5209, USA.
Muga, I., Gutierrez-Zorrilla, J. M., Vitoria, P., Roman, P., Lezama, L. & Beitia, J. I. (2004).Eur. J. Inorg. Chem.pp. 1886–1893.
Nishikori, H., Ohta, C. & Katsuki, T. (2000).Synlett, pp. 1557–1560. Nishikori, H., Ohta, C., Oberlin, E., Irie, R. & Katsuki, T. (1999).Tetrahedron,
55, 13937–13946.
North, A. C. T., Phillips, D. C. & Mathews, F. S. (1968).Acta Cryst.A24, 351– 359.
Park, K.-M. & Iwamoto, T. (1992).J. Chem. Soc. Chem. Commun.pp. 72–74. Park, K.-M. & Iwamoto, T. (1993).J. Chem. Soc. Dalton. Trans.pp. 1875–1881. Pospisil, P. J., Carsten, D. H. & Jacobsen, E. M. (1996).Chem. Eur. J.2, 974–
980.
Przychodzen, P., Lewinski, K., Balanda, M., Pelka, R., Rams, M., Wasiutynski, T., Guyard-Duhayon, C. & Sieklucka, B. (2004).Inorg. Chem. 43, 2967– 2974.
Reger, T. S. & Janda, K. D. (2000).J. Am. Chem. Soc.122, 6929–6934. Rigaku (1999).WinAFC Diffractometer Control Software. Rigaku
Corpor-ation, 3-9-12 Akishima, Tokyo, Japan. Sato, O. (2003).Acc. Chem. Res.36, 692–700.
Sato, O., Hayami, S., Einaga, Y. & Gu, Z.-Z. (2003).Bull. Chem. Soc. Jpn,76, 443–470.
Sato, O., Iyoda, T., Fujishima, A. & Hashimoto, K. (1996).Science,272, 704– 705.
Sheldrick, G. M. (1997).SHELXL97. University of Go¨ttingen, Germany. Shen, Z.-P., Xu, Z., Yuan, A.-H. & Huang, Z.-X. (2004).Transition Met. Chem.
29, 100–106.
supporting information
Acta Cryst. (2005). E61, m502–m505 [https://doi.org/10.1107/S160053680500379X]
(
SP
-4-1)-Bis{aqua[(1
R
,2
R
)-
N
,
N
′
-bis(salicylidene)cyclohexane-1,2-diamine-κ
4O
,
N
,
N
′
,
O
′
]manganese(III)}-di-
µ
-cyano-
κ
4N
:
C
-[dicyanonickel(II)]
Takashiro Akitsu, Yuiri Takeuchi and Yasuaki Einaga
(I)
Crystal data
[Mn2Ni(C20H20N2O2)2(CN)4(H2O)2]
Mr = 949.44 Monoclinic, P21
Hall symbol: P 2yb a = 23.260 (2) Å b = 13.872 (2) Å c = 15.182 (3) Å β = 93.79 (4)° V = 4888 (2) Å3
Z = 4
F(000) = 1960.0 Dx = 1.290 Mg m−3
Mo Kα radiation, λ = 0.7107 Å Cell parameters from 25 reflections θ = 10.4–12.6°
µ = 0.94 mm−1
T = 298 K Prismatic, brown 0.60 × 0.60 × 0.20 mm
Data collection Rigaku AFC-7R
diffractometer ω–2θ scans
Absorption correction: ψ scan (North et al., 1968)
Tmin = 0.530, Tmax = 0.828
12714 measured reflections 11688 independent reflections
8645 reflections with I > 2σ(I) Rint = 0.086
θmax = 27.6°
h = 0→30 k = 0→18 l = −19→19
3 standard reflections every 150 reflections intensity decay: 6.2%
Refinement Refinement on F2
R[F2 > 2σ(F2)] = 0.091
wR(F2) = 0.290
S = 1.07 8645 reflections 1004 parameters
H-atom parameters constrained
w = 1/[σ2(F
o2) + (0.2P)2]
where P = (Fo2 + 2Fc2)/3
(Δ/σ)max = 0.001
Δρmax = 2.49 e Å−3
Δρmin = −0.71 e Å−3
Absolute structure: Flack (1983), no Friedel pair Absolute structure parameter: 0.01 (3)
Special details
Refinement. Refinement using reflections with F2 > 0.0 σ(F2). The weighted R-factor (wR), goodness of fit (S) and R
-factor (gt) are based on F, with F set to zero for negative F. The threshold expression of F2 > 2.0 σ(F2) is used only for
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
x y z Uiso*/Ueq
H68 0.5540 −0.1592 0.4686 0.0728* H69 0.6470 −0.2157 0.4509 0.0790* H70 0.6349 −0.1227 0.3962 0.0790* H71 0.6986 −0.1286 0.5589 0.0770* H72 0.7247 −0.1090 0.4692 0.0770* H73 0.7127 0.0379 0.5469 0.0767* H74 0.6768 0.0395 0.4570 0.0767* H75 0.6319 −0.0065 0.6175 0.0550* H76 0.6837 0.1804 0.5690 0.0561* H77 0.7130 0.3449 0.5772 0.0824* H78 0.6968 0.5046 0.6046 0.0850* H79 0.6098 0.5544 0.6423 0.1084* H80 0.5338 0.4460 0.6557 0.0696*
Atomic displacement parameters (Å2)
U11 U22 U33 U12 U13 U23
C46 0.058 (7) 0.076 (10) 0.062 (8) −0.002 (7) 0.024 (6) 0.009 (7) C47 0.077 (10) 0.09 (1) 0.08 (1) −0.016 (9) 0.039 (8) 0.015 (10) C48 0.09 (1) 0.08 (1) 0.11 (1) −0.022 (10) 0.05 (1) −0.01 (1) C49 0.062 (8) 0.09 (1) 0.074 (10) −0.025 (8) 0.017 (7) 0.004 (9) C50 0.054 (6) 0.052 (7) 0.043 (6) −0.007 (5) 0.003 (5) 0.001 (5) C51 0.052 (6) 0.043 (6) 0.043 (6) −0.002 (5) −0.001 (5) −0.001 (5) C52 0.047 (6) 0.043 (6) 0.042 (5) 0.008 (5) 0.005 (4) −0.005 (5) C53 0.055 (7) 0.049 (7) 0.073 (9) −0.001 (6) 0.016 (6) −0.012 (6) C54 0.067 (8) 0.049 (7) 0.08 (1) 0.014 (7) 0.006 (7) −0.009 (7) C55 0.050 (6) 0.077 (9) 0.046 (6) 0.008 (6) 0.004 (5) −0.009 (6) C56 0.043 (5) 0.073 (9) 0.046 (6) 0.005 (6) 0.008 (5) 0.001 (6) C57 0.040 (5) 0.049 (6) 0.037 (5) 0.010 (5) 0.000 (4) −0.005 (5) C58 0.046 (6) 0.056 (7) 0.055 (7) −0.001 (5) 0.007 (5) −0.009 (6) C59 0.056 (7) 0.051 (7) 0.051 (6) −0.012 (6) 0.007 (5) −0.013 (6) C60 0.061 (8) 0.08 (1) 0.11 (1) −0.012 (8) 0.025 (9) −0.03 (1) C61 0.10 (1) 0.07 (1) 0.16 (2) −0.05 (1) 0.02 (1) −0.04 (1) C62 0.082 (10) 0.059 (9) 0.09 (1) −0.017 (8) 0.011 (8) −0.014 (9) C63 0.073 (8) 0.056 (8) 0.047 (7) −0.002 (7) −0.004 (6) −0.009 (6) C64 0.061 (7) 0.060 (8) 0.031 (5) −0.011 (6) −0.005 (5) −0.001 (5) C65 0.046 (6) 0.056 (7) 0.042 (6) 0.002 (5) 0.007 (5) 0.005 (5) C66 0.047 (6) 0.068 (9) 0.074 (9) −0.003 (6) 0.004 (6) −0.003 (7) C67 0.039 (6) 0.10 (1) 0.09 (1) −0.009 (8) 0.020 (7) 0.00 (1) C68 0.052 (7) 0.09 (1) 0.09 (1) −0.027 (8) 0.010 (7) −0.004 (10) C69 0.069 (8) 0.054 (8) 0.058 (7) −0.010 (7) 0.006 (6) −0.007 (6) C70 0.049 (6) 0.071 (9) 0.036 (5) −0.018 (6) −0.003 (4) 0.003 (6) C71 0.059 (7) 0.047 (7) 0.038 (5) 0.007 (5) 0.000 (5) 0.002 (5) C72 0.047 (5) 0.057 (7) 0.031 (5) 0.002 (5) 0.004 (4) −0.001 (5) C73 0.070 (8) 0.055 (8) 0.057 (7) 0.005 (6) 0.009 (6) −0.010 (6) C74 0.073 (8) 0.058 (8) 0.067 (8) −0.005 (7) 0.008 (7) −0.014 (7) C75 0.065 (8) 0.049 (7) 0.083 (10) 0.009 (6) 0.019 (7) −0.014 (7) C76 0.051 (7) 0.059 (8) 0.09 (1) 0.007 (6) 0.024 (7) −0.011 (8) C77 0.043 (5) 0.050 (7) 0.044 (6) 0.006 (5) 0.008 (4) −0.001 (5) C78 0.051 (6) 0.053 (7) 0.036 (5) 0.006 (5) 0.008 (4) 0.008 (5) C79 0.055 (6) 0.053 (7) 0.038 (5) −0.004 (6) 0.002 (5) 0.001 (5) C80 0.070 (8) 0.057 (8) 0.077 (9) −0.017 (7) 0.016 (7) −0.010 (7) C81 0.077 (9) 0.065 (9) 0.073 (10) −0.021 (8) 0.012 (8) −0.013 (8) C82 0.13 (2) 0.033 (7) 0.11 (1) −0.019 (9) 0.01 (1) −0.023 (8) C83 0.063 (8) 0.058 (8) 0.055 (7) −0.009 (6) 0.006 (6) −0.008 (6) C84 0.059 (6) 0.047 (6) 0.029 (5) −0.006 (5) −0.003 (4) −0.003 (4) C85 0.066 (7) 0.042 (6) 0.039 (6) 0.005 (6) 0.000 (5) −0.011 (5) C86 0.059 (6) 0.035 (6) 0.046 (6) 0.002 (5) 0.011 (5) −0.004 (5) C87 0.072 (8) 0.051 (7) 0.026 (5) 0.002 (6) 0.008 (5) 0.003 (5) C88 0.075 (8) 0.051 (7) 0.022 (4) 0.000 (6) −0.004 (5) 0.000 (4)
Geometric parameters (Å, º)
C17—C18 1.39 (1) C76—H74 0.946 C17—H18 0.944 C77—H75 0.950 C18—C19 1.39 (1) C78—C79 1.47 (1) C18—H19 0.932 C78—H76 0.951 C19—C20 1.39 (1) C79—C80 1.39 (1) C19—H20 0.957 C79—C84 1.39 (1) C21—C22 1.390 (10) C80—C81 1.39 (1) C21—C26 1.390 (10) C80—H77 0.926 C22—C23 1.39 (1) C81—C82 1.39 (1) C22—H21 0.952 C81—H78 0.939 C23—C24 1.39 (1) C82—C83 1.39 (1) C23—H22 0.944 C82—H79 0.906 C24—C25 1.39 (1) C83—C84 1.39 (1) C24—H23 0.974 C83—H80 0.923
C17—C18—C19 120.0 (7) C75—C76—C77 109 (1) C17—C18—H19 119.5 C75—C76—H73 109.2 C19—C18—H19 120.5 C75—C76—H74 109.5 C18—C19—C20 120.0 (7) C77—C76—H73 109.4 C18—C19—H20 118.4 C77—C76—H74 109.7 C20—C19—H20 121.6 H73—C76—H74 109.9 O2—C20—C15 123.9 (7) N12—C77—C72 107.9 (9) O2—C20—C19 116.1 (7) N12—C77—C76 115 (1) C15—C20—C19 120.0 (6) N12—C77—H75 107.0 O4—C21—C22 117.2 (6) C72—C77—C76 111.1 (10) O4—C21—C26 122.7 (6) C72—C77—H75 106.8 C22—C21—C26 120.0 (6) C76—C77—H75 107.7 C21—C22—C23 120.0 (6) N12—C78—C79 126.9 (10) C21—C22—H21 121.3 N12—C78—H76 117.2 C23—C22—H21 118.6 C79—C78—H76 115.9 C22—C23—C24 120.0 (6) C78—C79—C80 117.8 (8) C22—C23—H22 119.0 C78—C79—C84 122.2 (7) C24—C23—H22 121.0 C80—C79—C84 120.0 (7) C23—C24—C25 120.0 (6) C79—C80—C81 120.0 (7) C23—C24—H23 119.9 C79—C80—H77 122.2 C25—C24—H23 120.1 C81—C80—H77 117.8 C24—C25—C26 120.0 (6) C80—C81—C82 120.0 (7) C24—C25—H24 120.4 C80—C81—H78 119.9 C26—C25—H24 119.5 C82—C81—H78 120.1 C21—C26—C25 120.0 (6) C81—C82—C83 120.0 (7) C21—C26—C27 124.1 (7) C81—C82—H79 115.2 C25—C26—C27 115.8 (7) C83—C82—H79 124.8 N3—C27—C26 125.2 (10) C82—C83—C84 120.0 (7) N3—C27—H25 117.5 C82—C83—H80 117.9 C26—C27—H25 117.3 C84—C83—H80 122.1 N3—C28—C29 117.8 (9) O11—C84—C79 123.1 (7) N3—C28—C33 104.5 (8) O11—C84—C83 116.9 (7) N3—C28—H26 107.5 C79—C84—C83 120.0 (6) C29—C28—C33 111.7 (10) Ni2—C85—N13 179 (1) C29—C28—H26 107.6 Ni2—C86—N14 176 (1) C33—C28—H26 107.3 Ni2—C87—N15 176 (1) C28—C29—C30 110 (1) Ni2—C88—N16 175 (1)