Adsorptive Interaction of Chiral Amino Acids on
β-Cyclodextrin Bonded to Silica Particles
Abhijit Banik, Monali Dutta Saikia*
Department of Chemistry, Arya Vidyapeeth College, Guwahati, India Email: *monalisaikia@hotmail.com
Received January 17,2013; revised February 25, 2013; accepted March 3, 2013
ABSTRACT
The adsorption of certain chiral amino acids from aqueous solution onto β-cyclodextrin silica particles (CDS) had been investigated with the aim of in-depth understanding of the host-guest interaction. The adsorption intensity was found to be strongly dependent on the aqueous phase pH and this dependence could be interpreted from a model for neutral spe-cies adsorption in all cases. Adsorption equilibrium data fitted well to the Freundlich isotherm. The adsorption efficien-cies of L-amino acids were found to be higher compared to the corresponding D-isomers. Hydrogen bonding and hy-drophocities of amino acids were responsible for the differences in adsorption, by influencing the strength of interac-tions between the amino acid and CDS. The adsorption rate curves for all the molecules appeared to be typical of the pseudo second-order kinetics. Infrared spectral analysis has been performed to characterize adsorptive interaction. The porous structure of CDS as revealed by scanning electron micrograph thus shown to be promising materials for enanti-oselective separation of amino acids. In addition, molecular modeling studies performed on such molecules were found to correlate very well to the experimental results obtained.
Keywords: Adsorption; Amino Acid; β-Cyclodextrin; Chiral; Molecular Modeling
1. Introduction
Chiral amino acids are one of the most important bio-molecules because of their relevance in nature and their chemical richness. It is well known that in nature amino acids occur in L-forms and they play an important role in the food and pharmaceutical industries. It may be impor- tant to be able to detect the presence of D-amino acids in biological systems [1]. The amount of certain D-amino acids in various types of samples has been found to be of relevance for many aspects of biochemical and molecular biological research [2,3], in the control of food and bev- erages [4], as well as in age determination [5,6]. There- fore, chiral analysis of D/L-amino acids is of increasing importance in biological science. Various chiral separa- tion methods are available for the enantiomeric resolu- tion of amino acids. Methods based on chiral ligand- exchange [7-10] and on the formation of inclusion com- plexes with cyclodextrins have received the most atten- tion [11-13]. Cyclodextrins (CD) were employed in their native form or after modification by methylation, acety- lation or by binding other structural moieties to the hy- droxyl groups of the glucose [14-16]. The unique host- guest chemistry of CDs is highly conducive to the selec- tive uptake of organic molecules from aqueous systems
[17-20]. CDs themselves, are highly water soluble, and must therefore be processed into solid forms before they can be implemented into usable separation technology.
β-Cyclodextrin (β-CD) is a cyclic oligosaccharide with seven glucose units, with its cavity structure, and can form an inclusion complex with certain molecules through a host-guest interaction. The conical shape of
β-CD molecule results in well-defined hydrophobic cav-ity with top and bottom diameters of 6.5 and 6.0 Å that can accommodate the inclusion of various organic mole-cules with suitable geometry and function [21]. Figure 1 shows the chemical structure of β-CD. The chiral nature of the cavity in these molecules makes them useful for enantiomeric separation applications. Polymeric sorbents have been extensively tested for amino acid adsorption [22]. The β-CD polymer has been shown to be a good sorbent for amino acids in our previous study [23]. Tang
et al. [24] have studied the application of cross-linked
β-CD polymer for adsorption of aromatic amino acids. In this paper, we report both experimental and theo-retical results on adsorption behaviour of certain chiral amino acids on β-CD bonded to silica gel with emphasis on understanding the host-guest interaction and such a study will be useful for better understanding of the chiral recognition mechanism of amino acids by β-CD bonded silica gel as a selective sorbent. Also we have character-
O O O O O O O O O O O O O O OH OH OH OH HO HO HO HO HO OH OH OH OH HO HO OH OH HO HO HO OH
Figure 1. Molecular structure of β-cyclodextrin.
ized the amino acid-β-CD complex by UV-Visible (UV- Vis) spectroscopy.
2. Experimental
2.1. Materials
The amino acids used in this study namely D-phenyla- lanine, L-phenylalanine, D-tryptophan, L-tryptophan, D- tyrosine and L-tyrosine (guests) were obtained from Sigma-Aldrich Chemicals Pvt. Ltd., USA. β-CD was obtained from Hi Media Laboratories Pvt. Ltd., Mumbai, India. The silica gel of 60 - 120 mesh size was obtained from SRL Pvt. Ltd., Mumbai, India. We have chosen this particular silica gel because of its highly porous structure and appropriate physical and chemical properties to achieve both adequate loading capacity and high-binding capacities. Moreover, it is an efficient adsorbent for separation of organic compounds by chromatographic methods. 3-Glycidoxypropyltrimethoxysilane was also obtained from Sigma-Aldrich Chemicals Pvt. Ltd., USA. The other reagents used as buffer (sodium phosphate, sodium acetate, sodium carbonate and sodium chloride) were supplied by Qualigens, India (Mumbai) and were of analytical grade.
2.2. Preparation of β-CD Bonded Silica Stationary Phase (CDS)
CDs are highly soluble in water and so must be to some solid form before implementing them to adsorption technology. CD containing polymers are useful materials
for selective adsorption or separation of organic com-pounds. For that reason, we have synthesized a polymer (i.e. CDS) as reported in our earlier work [23,25] in which β-CD is bonded to silica particles. Briefly, 1.145 gm of β-CD was dissolved in 25 ml of dry dimethyl for-mamide (DMF), to which 0.1 gm of metal sodium was added. The reaction was allowed to occur with stirring at room temperature for about 30 min. After filtration, 0.45 ml of 3-glycidoxypropyltrimethoxysilane was added to the filtrate, which was allowed to react at 90˚C for 5 h. Then, 5.0 gm of silica gel was added, and the mixture was allowed to react for 10 h at 80˚C - 100˚C. The CDS was filtered, and washed with DMF, methanol, doubly distilled water and acetone in sequence. Subsequently, the CDS was dried at 120˚C for 3 h, and kept in a desic-cator before use.
2.3. Equilibrium Isotherms and Adsorption Rate
Equilibrium isotherms were obtained by contacting 25 ml of aqueous amino acid solution with different amounts of CDS in a thermostated shaker bath controlled at 25˚C ± 0.5˚C. The pH of the solution was varied between 3 and 9 using appropriate dose of buffer. The initial concentra- tion of amino acid in the aqueous solution was varied between 5 and 10 mM. The equilibration time was 8 - 10 h. After equilibrium was achieved, the mixture was al- lowed to settle and the supernatant liquor was filtered to remove any particulate matter. The clear solution thus obtained was analyzed by means of a UV-Vis spectro- photometer (Lambda 25 UV/Vis, Perkin Elmer) cali- brated at the wavelength of maximum absorbance (λmax) of the specific amino acid enantiomer. For quantitative spectrophotometric analysis, standard calibration curves of different amino acid enantiomers were prepared at their λmax values such as 289 nm for D- and L-phenyla- lanine; 288 nm for D- and L-tryptophan and 285 nm for D- and L-tyrosine. For the purpose of estimation 0.2 ml of the supernatant liquor was drawn with glass syringe and diluted with the buffer used for preparing the amino acids solution. Absorbance was measured in the spectro- photometer and the concentration was determined from calibration curve. The amount of amino acid per gram of CDS “q” (mmol·g−1) was calculated as q = V∆C/W where,
∆C is the change in solute concentration (mmol·l−1), V is the solution volume (l) and W is the weight of adsorbent (g).
2.4. UV-Vis Spectroscopy
The UV-Vis spectra were recorded with a Varian Cary 50 Bio (Sweden) UV-Vis spectrophotometer using quartz cells, between 200 and 400 nm. In the experiments of guests with β-CD (host), the concentration of the host was varied from 1 × 10−3 M to 12 × 10−3 M while the concentration of the guest was kept constant at 2 × 10−3 M. The mixtures were stirred at 25˚C at a speed of 280 rpm for 4 h and the supernatant was analysed by UV-Vis spectrophotometer.
2.5. Instrumental Studies
FT-IR spectra were obtained by the KBr method using PerkinElmer, model Spectrum One. Morphological fea-tures of samples were obtained with a LEO 1430 VP Scanning Electron Microscope.
2.6. Molecular Modeling Studies
The inclusion process involving the guests and host (β-CD) was investigated by using the Parametric Model 3 (PM3) quantum-mechanical semiempirical method. All theoretical calculations were performed using GAUS-SIAN 09 software package [27]. For all the systems, a full geometry optimization was done at the PM3 level. Using standard bond lengths as the initial input, the car-bon chains or rings in the guest molecule was vertically inserted into the cavity of the hosts along the middle axis from the large rim of hosts in CHEM 3D. Stabilization energy (ΔE) upon complexation between guests and the host were calculated for the minimum energy structure according to Equation (1) [28]
complex guest host
E E E E
(1)
where Ecomplex, Eguest and Ehost represent HF energies (heats of formation) of the complex, the free amino acid and the host, respectively.
3. Results and Discussion
3.1. Adsorption of Amino Acids onto CDS
[image:3.595.307.535.82.355.2]The amino acids studied have molecular structures and pKa valiues shown in Figure 2. The molecular volume and weight of the above enantiomers calculated using Alchemy 2000 software are given in Table 1. The ad-sorption isotherms of the above mentioned molecules are shown in Figures 3(a) and (b). It could be seen from Figure 3 that the adsorption of the amino acids on CDS increased in the following order: D-tyrosine < L-tyrosine < D-phenylalanine < L-phenylalanine < D-tryptophan < L-tryptophan. The pH dependence of the adsorption is of importance as the pH determines the extent of ionisation of the amino acid molecules, thereby affecting the ad-
[image:3.595.310.537.402.526.2]Figure 2. Structures of the amino acids studied.
Table 1. Properties of amino acids studied.
Amino Molecular volume (cm3/mol) (g) Molecular weight acid
L-tryptophan 173.30
D-tryptophan 170.92 196.16
L-phenylalanine 139.83
D-phenylalanine 141.13 157.12
L-tyrosine 153.93
D-tyrosine 154.82 174.13
sorption affinity. The pH dependence could be inter-preted by considering the adsorption affinity, expressed as the slope of the linear region of the isotherm (q/Ce).
Table 2 shows the variation of q/Ce with pH and in-ferred a strong relationship, the affinity being considera-bly low at high pH under which the amino acids exist in dissociated forms. The behaviour was akin to that ob-served in the case adsorption of the beta-lactams on polymeric resins and activated carbon [29]. Since the concentration of neutral form of the amino acid (Cn) was related to pH and the total concentration by Hendersen- Hasselbach relationship [30]:
1
1 10 e
n pH pKa
C
C
(2)
(a)
(b)
Figure 3. (a) Adsorption isotherms of the L-amino acids in CDS at different pH of aqueous phase. Open, closed and shaded symbols for pH 3, 7 and 9 respectively; (b) Adsorp-tion isotherms of the D-amino acids in CDS at different pH of aqueous phase. Open, closed and shaded symbols for pH 3, 7 and 9 respectively.
model for neutral species adsorption and can well repre-sent the pH variation.
[image:4.595.59.289.79.527.2]The pH dependence of the affinity is more important, in that neutral form of the amino acids could be consid-ered to have a high adsorption affinity. The reduction in adsorption capacity of various amino acids with increase of pH had been interpreted from a model incorporating hydronium ion concentration and dissociation constant [31] attributable to both the carboxylic and amino func-tional groups. At low pH, the amino acids are markedly
[image:4.595.310.537.83.288.2]Figure 4. Dependence of adsorption on the concentration of the neutral form of amino acids in CDS. Open and closed symbols for L- and D-series respectively.
Table 2. Adsorption affinity of amino acids at different pH values on CDS.
Amino acids pH affinity q/Ce (LgAdsorption −1) Interaction energy (∆E) (KJ·mol−1)
3 10.011
7 4.939 L-tryptophan
9 4.174
−52.51
3 7.322
7 4.031 D-tryptophan
9 3.681
−49.88
3 4.317
7 3.599 L-phenylalanine
9 1.986
−47.25
3 3.131
7 2.925 D-phenylalanine
9 1.343
−44.63
3 1.845
7 1.071 L-tyrosine
9 0.767
−44.63
3 1.282
7 0.788 D-tyrosine
9 0.600
[image:4.595.312.538.371.730.2]hydrophobic and the hydrophobic interaction of the un- dissociated amino acids would be greater than the corre-sponding ionic form that exist at high pH. Thus, the ob-servation of high q/Ce at low pH implied the probable role of hydrophobic interaction.
Estimated on the basis of the proposed hydrophobicity scale [32], the hydrophobicity of the beta-lactams was found to increase in the same order: L-tyrosine < L- tryptophan < L-phenylalanine. Thus, the relative differ-ence in adsorption affinity could not be explained only on the basis of hydrophobicity of amino acids as L-tryp- tophan showing higher adsorption is less hydrophobic compared to L-phenylalanine. The difference in adsorp-tion may perhaps be explained on the basis of the forma-tion of the hydrogen bonded network and the suitability in space between the host and guest molecules (i.e. the amino acids and CDS).The adsorption of L-enantiomers are higher than their corresponding D-forms. This dif- ference in adsorption behaviour of CDS for the enanti- omeric species may be due to the number and types of hydrogen bond formed as well as the extent of penetra- tion of amino acids to the torous cavity during the forma- tion of surface supramolecular complex. CD has the chiral binding microenvironment formed by many chiral atoms. That is why β-CD can bind chiral enantiomers. Such inference will further be clear from the discussion on molecular modelling, Fourier Transform Infrared (FTIR) and UV spectroscopic studies to follow.
Freundlich isotherm (Equation (3)) was used to de-scribe equilibrium adsorption data, because different en-ergy adsorption sites are expected in the polymeric net-work [33].
n
e f
q K Ce (3)
where Kf is the Freundlich constant, Lg−1; and 1/n is the Freundlich constant characteristic of adsorption intensity. The fitting parameters for the Freundlich isotherm by non-linear regression analysis are shown in Table 3. The Freundlich isotherm was found to be consistent for ad-sorption of amino acids on cross-linked β-CD polymer [24].
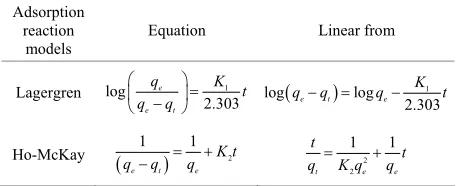
[image:5.595.309.536.113.446.2]Adsorption rate curve for amino acids on CDS were generated at a stirring speed of 800 rpm as shown in Figure 5. Mathematical models of pseudo-first by La- gergren and pseudo-second by Ho-McKay [34] reaction orders have been used to describe kinetic process of physical adsorption of aromatic acids (Table 4). The values of rate constants calculated from the linear plots of pseudo-first and pseudo-second kinetic equations shown in Table 5. It can be seen from Table 5 that the sorption of amino acids is more consistent with kinetic model of pseudo-second order: the correlation coefficient values for pseudo-second order equation are greater than for pseudo-first order one.
Table 3. The values of parameters of the Freundlich ad-sorption isotherms for amino acids on CDS at 25˚C.
Amino acids pH Kf 1/n coefficient (RCorrelation 2)
3 5.551 0.2844 0.9900
5 3.871 0.2876 0.9958 L-tryptophan
7 3.288 0.2738 0.9983
3 4.413 0.2020 0.9920
5 3.957 0.3789 0.9753 D-tryptophan
7 3.122 0.2570 0.9903
3 4.318 0.2812 0.9963
5 3.151 0.1913 0.9921 L-phenylalanine
7 2.600 0.1916 0.9857
3 2.810 0.2730 0.9969
5 1.853 0.2403 0.9942 D-phenylalanine
7 1.510 0.3087 0.9941
3 1.845 0.1839 0.9920
5 2.285 0.2472 0.9957 L-tyrosine
7 1.257 0.2656 0.9930
3 1.442 0.1940 0.9934
5 1.160 0.1868 0.9906 D-tyrosine
[image:5.595.309.537.473.566.2]7 1.020 0.1149 0.9934
Table 4. Adsorption kinetic models and their linear forms.
Adsorption reaction
models
Equation Linear from
Lagergren log 1
2.303 e
e t
q K t
q q 1 log log 2.303
e t e
K
q q q t
Ho-McKay 2
1 1
e t e
K t
q q q 2
2
1 1
t e e
t
t q K q q
Table 5. Adsorption parameters and correlation coefficient of kinetic models.
Amino acids K1 (s−1) R2 (gmmol·sK2 −1) R2
L-tryptophan 0.389 0.895 1.811 0.995
D-tryptophan 0.587 0.960 3.119 0.983
L-phenylalanine 0.278 0.987 2.079 0.991
D-phenylalanine 0.631 0.924 1.936 0.943
L-tyrosine 0.686 0.879 1.028 0.907
[image:5.595.310.538.605.732.2]Figure 5. Adsorption rate curves for amino acids in CDS. Open and closed symbols for L- and D-series respectively.
3.2 UV-Vis Spectroscopic Studies
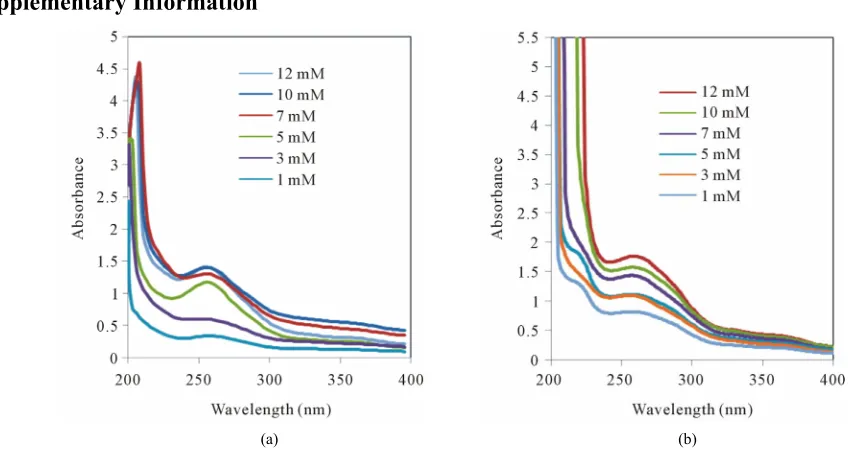
UV-Vis spectrophotometric studies on the interactions of guests with host enabled the determination of stability constants of inclusion complexes when their formation gave rise to appreciable spectral changes. Upon addition of host, to the guests absorptivity increased in each case and the increase was significant with higher added con-centration. The wavelength corresponding to maximum of absorbance (λmax) of free amino acids and their corre-sponding changes upon complexation with β-CD are listed in the Table 6.
The shifts towards lower wavelength in the spectra of the complexes may be attributable to the formation of hydrogen bonds. Because hydrogen bonding lowers the energy of “n” orbitals, a hypsochromic shift (blue shift) was observed. L-phenylalanine-β-CD complex showed blue shift whereas no prominent changes were observed in case of D-phenylalanine. The blue shift was found to be significant in case of L-tryptophan-β-CD complex whereas D-tryptophan did not show any such changes. The L-tyrosine-β-CD complex showed blue shift but red shift have been noticed in case of D-tyrosine-β-CD com- plex. Thus, β-CD was found to be suitable as complexing agent for all the three chiral aromatic amino acids but formed greater number of hydrogen bonds with L-amino acids compared to D-amino acids, which will be more clear from molecular modelling studies performed on such complexes. The lack of selectivity towards phenyla- lanine may be due to lower number of hydrogen bonding sites in this molecule compared to the other two amino acids. Though UV-spectrometry is not conclusive to de-termine selectivity but from the changes we can infer that
β-CD and itsderivatives may be hopeful for chiral
enan-tiomeric separation of molecules like tryptophan and tyrosine (see Figure S1 in the Appendix).
3.3. Determination of Formation Constants of the Inclusion Complexes of Host with Guests
The determination of formation constants (K) of the host to the guests were realized by UV-Vis spectroscopy titra-tion experiments. The K values were calculated by ap-plying least-squares fit to the plots of ([host][guest])/ΔA
versus ([host] + [guest]), according to the modified Benesi-Hildebrand Equation (4) [35,36]:
host guest
1 1
host guestA K
(4)
[image:6.595.308.537.365.707.2]where, [guest] and [host] are the equilibrium concentra- tions of guest and hosts, respectively. Δϵ is the difference between the extinction coefficients of free and com- plexed guest. ΔA is the difference between the absorb- ances of free and complexed guest at the same wave-length. Figure 6 shows the plots of ([host][guest])/ΔA
Table 6. λmax values for free amino acids and their
com-plexes.
Guests λmax (nm) λmaxof the guest with (nm) (upon complexationβ-CD)
L-phenylalanine 211 196 - 206 (bs)*
D-phenylalanine 208 208 - 211
L-tryptophan 220, 280, 287 200 - 206, 253 - 258 (bs)
D-tryptophan 280, 287 280 - 285, 285 - 288
L-tyrosine 202, 211, 270, 277 200 - 206, 251 - 261 (bs)
D-tyrosine 200, 222, 274 222 - 225, 280 - 282 (rs)**
*Blue shift; **Red shift.
versus ([host] + [guest]). The initial concentration of amino acids were kept at 2.0 × 10−3 M, while the concen-trations of the host were in the range from 1 to 12 × 10−3 M. A linear least-square fitted to the plots. The K values of inclusion complexes of host with guests were deter-mined from the slopes and intercepts of the linear plots based on the Equation (4). The calculated K values of the inclusion complexes are summarized in Table 7. For all the four inclusion systems, the dependencies were linear in the investigated concentration range, confirming that the stoichiometries of the inclusion complexes in solution were 1:1 [37,38].
For the amino acids studied, the changes in K values reflected the same order as adsorption affinity, which suggests that the equilibrium of adsorption is a strong function of the strength of the solute-sorbent binding interaction. It is now apparent that the observed differ-ences in adsorption affinity for various amino acids on CDS cannot be merely explained on the basis of hydro-phobicity.
3.4. Characterisation of Unloaded and Amino Acids/CDS Loaded Adsorbent
3.4.1. FTIR Spectroscopic Studies
FTIR analysis permits spectrophotometric observation of the adsorbent surface in the range 450 - 4000 cm−1, and serves as a direct means for identification of the func-tional groups on the surface. An examination of the ad-sorbent surface after adsorption reaction possibly pro-vides information regarding the surface groups that might have participated in the adsorption reaction and also in-dicates the surface sites on which adsorption has taken place.
[image:7.595.59.286.611.730.2]The FTIR spectra of polymer showed a characteristic absorption band at 3481 cm−1 due to O-H stretching vi-bration. The absorption peak at 1645 cm−1 possibly due to the presence of hydroxyl groups that were part of an aromatic system; vibration bands of O-H were also visi-ble at 1088 cm−1. Upon adsorption of amino acids on CDS the characteristics absorption peaks have been shifted from their position indicating interactions of the
Table 7. Calculated K values of the complexes. Complex K in (M) R2
L-tryptophan-β-CD 6.076 0.983 D-tryptophan-β-CD 4.435 0.978 L-phenylalanine-β-CD 2.496 0.948 D-phenylalanine-β-CD 1.551 0.977
L-tyrosine-β-CD 1.138 0.989 D-tyrosine-β-CD 1.087 0.828
amino acid molecules with silica-bonded surface of β-CD molecules. The adsorption complexes showed a lowering of frequency of the O-H stretching vibration thereby confirming complexation through hydrogen bonding and the lowering have been found more in case of complexes of L-amino acid molecules compared to that of D-amino acid molecules which may be due to stronger hydrogen bonding of L-series of molecules. In case of D-tyrosine, complexation caused a slight increase in O-H stretching frequency which is possibly due to cleavage of hydrogen bonding during complexation. Additionally, disappear-ance of N-H peak in any of the complexes clearly dem-onstrated complexation (see Figures S2 and S3 in the Appendix).
3.4.2. Scanning Electron Micrographic (SEM) Study The scanning electron micrographs for the CDS, L- tryptophan and L-phenylalanine adsorbed on CDS are presented in Figure 7. SEM analysis of polymer showed aggregates of irregularly shaped polycrystalline samples (Figure 7(a)) and the SEM of internal structure of the CDS (Figure 7(b)) showed porous structure which should significantly increase the available surface area of the CDS and, therefore, increase the adsorption capacity. L-tryptophan-CDS complex (Figure 7(c)) showed poly-crystalline structures in which some threadlike structures have been found to be embedded onto the polymer sur-face and that of L-phenylalanine-CDS complex (Figure 7(d)) different from the morphology of the polymer in-dicating adsorption of amino acid molecules onto the porous surface of CDS.
3.5. Molecular Modeling Studies
Intensive theoretical works have been performed over the past few years on CDs owing to their conformational flexibility and large size. Parametric Model 3 (PM3) has been chosen to study host-guest complexes between
β-CD and three important chiral amino acid molecules: phenylalanine, tryptophan and tyrosine. Due to the mo-lecular size, PM3 is a powerful technique which can be currently applied and performs better than Austin model 1 (AM1) in biochemical systems due to its improved description of the interactions between non bonded atoms, e.g. hydrogen bond and steric effects [39]. The PM3 op-timized structures of these complexes are schematically illustrated in Figure 8.
(a) (b)
[image:8.595.58.541.82.495.2]
(c) (d)
Figure 7. Scanning electron micrographs for (a) CDS; (b) Internal structure of CDS; (c) L-tryptophan-CDS complex and (d) L-phenylalanine-CDS complex.
(a) (b)
Figure 8. Optimised structures computed for (a) L-trypto- phan-β-CD complex and (b) D-tryptophan-β-CD complex.
the cavity of the CD molecule compared to its D-enan- tiomer thereby enhancing the interaction sites to come in a suitable position for interaction. Hydrogen bonding and hydrophobic interactions were found to play a significant
[image:8.595.58.289.532.646.2]a more suitable position for interaction.
4. Conclusion
The results of the investigation revealed some important insights for the adsorption mechanism of certain amino acid enantiomers on β-CD bonded to silica particles. The adsorption equilibrium may well characterized by the Freundlich isotherm. The rates of adsorption appeared to follow pseudo second order model under the experimen-tal conditions used in the study. The adsorption intensity was strongly dependent on the aqueous phase pH and this dependence was typical of the behaviour predicted by a neutral species for all the amino acids on CDS. Adsorp-tion of amino acids on CDS increased in the following order: tyrosine < phenylalanine < tryptophan. Hydropho-bicity as well as hydrogen bonding interactions were mainly responsible for such differences in adsorption. Spectroscopic and theoretical studies supported such interactions and indicated that β-CD can bind the amino acids to form supramolecular complexes. Separation of chiral amino acids using CDS depended on the formation of an inclusion complex which was due to the hydropho-bic effect, hydrogen bonding interactions between the molecule and secondary hydroxyl groups of the β-CD. This work further suggests that the host-guest interaction which can be postulated from the chemistry of binding mechanism may be used to adsorb amino acid enanti-omers selectively onto CDS.
5. Acknowledgements
The authors gratefully acknowledge the award of a re-search Project under FAST TRACK SCHEME from Department of Science and Technology, New Delhi.
REFERENCES
[1] D. W. Armstrong, J. M. Duncan and S. H. Lee, “Evalua-tion of D-Ammino Acid Levels in Human Urine and in Commercial L-Amino Samples,” Journal of Amino Acids, Vol. 95, No. 1, 1991, pp. 97-106.
[2] G. C. Barret, “Chemistry and Biochemistry of Amino Acids,” Chapman and Hall, New York, 1985.
doi:10.1007/978-94-009-4832-7
[3] K. H. Schlefier and E. Stackebrandt, “Molecular Sys-tematics of Prokaryotes,” Annual Review of Microbiology, Vol. 37, 1983, pp. 143-187.
doi:10.1146/annurev.mi.37.100183.001043
[4] M. Langer, R. Wittner, P. Jack, H. Godel and H. Bruckner, “Strategies for Food Quality Control and Analytical Methods in Europe,” In: W. Baltes, T. Eklund, R. Fen-wick, W. Pfannhauser, A. Ruiter and H. P. Their, Eds.,
Proceedings of the 6th European Conference on Food Chemistry, Hamburg, 22-26 September 1991, pp. 385- 390.
[5] S. Ohtani and K. Yamamoto, “Age Estimation Using the
Racemization of Amino Acid in Human Dentin,” Journal of Forensic Sciences, Vol. 36, No. 3, 1991, pp. 792-800. [6] V. R. Meyer, “Chiral Separations by Liquid
Chromatog-raphy,” In: S. Ahuja, Ed., ACS Symposium Series, American Chemical Society, Washington DC, Vol. 471, 1991, pp. 217-227. doi:10.1021/bk-1991-0471.ch013 [7] V. A. Davankov, “Resolution of Racemates by Ligand
Exchange-Exchange Chromatography,” Advances in Chromatography, Vol. 18, 1980, pp. 139-142.
[8] Y. Tapuhi, N. Miller and B. L. Karger, “Practical Con-siderations in the Chiral Separation of DNS-Amino Acids by Reversed-Phase Liquid Chromatography Using Metal Chelated Additives,” Journal of Chromatography A, Vol. 205, No. 2, 1981, pp. 325-337.
doi:10.1016/S0021-9673(00)82660-6
[9] N. Oi, H. Kitahara and R. Kira, “Direct Separation of Enatiomers by High-Performance Liquid Chromatogra-phy on a New Chiral Ligand-Exchange Phase,” Journal of Chromatography A, Vol. 592, No. 1-2, 1992, pp. 291- 296. doi:10.1016/0021-9673(92)85098-E
[10] S. Lam, F. Chow and A. Karmen, “Reversed-Phase High- Performance Liquid Chromatographic Resolution of D- and D-DNS-Amino Acids by Mixed Chelate Complexa-tion,” Journal of Chromatography A, Vol. 199, 1980, pp. 295-305. doi:10.1016/S0021-9673(01)91381-0
[11] W. L. Hinze, T. E. Riehl, D. W. Armstrong, E. De Mond, A. Alak and T.Ward, “Liquid Chromatographic Separa-tion of Enantiomers Using a Chiral, β-Cyclodextrin- Bonded Stationary Phase and Conventional Aqueous- Organic Mobile Phases,” Analytical Chemistry, Vol. 57, No. 1, 1985, pp. 237-242. doi:10.1021/ac00279a055 [12] S. Li and W. C. Purdy, “From Inclusion Chemistry to
Water Purifying Technology,” Journal of Chromatogra-phy A, Vol. 543, No. 1, 1991, pp. 105-112.
doi:10.1016/S0021-9673(01)95758-9
[13] N. Thuaud, B. Sebille, A. Deratani and G. Lelievre, “Re-tention Behavior and Chiral Recognition of β-Cyclodextrin Derivative Polymer Adsorbed on Silica for Warfarin, Structurally Related Compounds and DNS-Amino Ac-ids,” Journal of Chromatography A, Vol. 555, No. 1-2, 1991, pp. 53-64. doi:10.1016/S0021-9673(01)87166-1 [14] S. H. Lee, A. Berthod and D. W. Armstrong, “Systematic
Study on the Resolution of Derivatized Amino Acids Enantiomers on Different Cyclodextrin-Bonded Station-ary Phases,” Journal of Chromatography A, Vol. 603, No. 1-2, 1992, pp. 83-93.
doi:10.1016/0021-9673(92)85348-W
[15] J. Zukowska, M. Pawlowska and D. W. Armstrong, “Ef-ficient Enantioselective Separation and Determination of Trace Impurities in Secondary Amino Acids (i.e., Imino Acids),” Journal of Chromatography A, Vol. 623, No. 1, 1992, pp. 33-41. doi:10.1016/0021-9673(92)85295-5 [16] M. Pawlowska, S. Chen and D. W. Armstrong,
“Enanti-omeric Separation of Fluorescent, 6-Aminoquinolyl-N- hydroxysuccinimidyl Carbamate, Tagged Amino Acids,”
Journal of Chromatography A, Vol. 641, No. 2, 1993, pp. 257-265. doi:10.1016/0021-9673(93)80142-U
“Sorp-tion of Aromatic Compounds in Water Using Insoluble Cyclodextrin Polymers,” Journal of Applied Polymer Science, Vol. 68, No. 12, 1998, pp. 1973-1978.
doi:10.1002/(SICI)1097-4628(19980620)68:12<1973::AI D-APP11>3.0.CO;2-T
[18] G. Crini, L. Janus, M. Morcellet, G. Torri, A. Naggi, S. Bertini and C. Vecchi, “Macroporous Polyamines Con-taining Cyclodextrin: Synthesis, Characterization, and Sorption Properties,” Journal of Applied Polymer Science, Vol. 69, No. 7, 1998, pp. 1419-1427.
doi:10.1002/(SICI)1097-4628(19980815)69:7<1419::AID -APP17>3.0.CO;2-O
[19] D. Li and M. Ma, “Nanosponges: From Inclusion Chem-istry to Water Purifying Technology,” Clean Products and Processes, Vol. 2, No. 2, 2000, pp. 112-116.
[20] M. Ma and D. Li, “New Organic Nanoporous Polymers and Their Inclusion Complexes,” Chemistry of Materials, Vol. 11, No. 4, 1999, pp. 872-874.
doi:10.1021/cm981090y
[21] M. V. Rekharsky and Y. Inoue, “Complexation Thermo-dynamics of Cyclodextrins,” Chemical Reviews, Vol. 98, No. 5, 1998, pp. 1875-1918. doi:10.1021/cr970015o [22] D. Doulia, F. Rigas and C. Gimouhopoulos, “Removal of
Amino Acids from Water by Adsorption on Polystyrene Resins,”Journal of Chemical Technology & Biotechnol-ogy, Vol. 76, No. 1, 2001, pp. 83-89.
doi:10.1002/1097-4660(200101)76:1<83::AID-JCTB345 >3.0.CO;2-N
[23] M. D. Saikia, “Studies on Adsorption of Amino Acids on
β-Cyclodextrin Bonded to Silica Particles,” Colloids and Surfaces A, Vol. 329, No. 3, 2008, pp. 177-183.
doi:10.1016/j.colsurfa.2008.07.007
[24] S. Tang, L. Kong, J. Ou, Y. Liu, X. Li and H. Zou, “ Ap-plication of Cross-Linked Beta-Cyclodextrin Polymer for Adsorption of Aromatic Amino Acids,”Journal of Mo-lecular Recognition, Vol. 19, No.1, 2006, pp. 39-48. doi:10.1002/jmr.756
[25] A. Banik, P. Gogoi and M. D. Saikia, “Interaction of Naproxen with β-Cyclodextrin and Its Derivatives/Poly- mer: Experimental and Molecular Modeling Studies,”
Journal of Inclusion Phenomena and Macrocyclic Chem-istry, Vol. 72, No. 3-4, 2011, pp. 449-458.
doi:10.1007/s10847-011-0014-7
[26] M. Dutta, R. Baruah, N. N. Dutta and A. C. Ghosh, “The Adsorption of Certain Semisynthetic Cephalosporins on Activated Carbon,” Colloids and Surfaces A, Vol. 127, No. 1-3, 1997, pp. 25-37.
doi:10.1016/S0927-7757(97)00062-9
[27] M. J. Frisch, G. W. Trucks, H. B. Schlegel, G. E. Scuseria, M. A. Robb, J. R. Cheeseman, G. Scalmani, V. Barons, B. Mennucci, G. A. Peterson, H. Nakatsuji, M. Li, Y. Car-cicato, H. P. Hratchian, A. Izmaylov, J. Bloino, G. Zheng, J. L. Sonnenberg, M. Hada, M. Ehara, K. Toyota, R. Fu-kuda, J. Hasegawa, M. Ishida, T. Nakajima, Y. Honda, O. Kitao, H. Nakai, T. Vreven, J. A. Montgomery Jr., J. E. Peratta, F. Ogliaro, M. Bearpark, J. J. Heyd, E. Brothers, N. Kudin, V. N. Staroveroy, T. Keith, R. Kobayashi, J. Normand, K. Raghavachari, A. Rendell, J. C. Burant, S. S. Iyengar, J. Tomasi, M. Cossi, N. Rega, J. M. Millan, M.
Klene, J. E. Knox, J. B. Cross, V. Bakken, C. Adamo, J. Jarmillo, R. Gomperts, R. E. Stratmann, O. Yazev, A. J. Austin, R. Cammi, C. Pommeli, J. W. Ochierski, R. L. Martin, K. Morokuma, V. G. Zakrzewski, G. A. Voth, P.Salvador, J. J. Dannenberg, S. Dapprich, A. D. Daniels, O. Farkas, J. B. Foresman, J. V. Ortiz, J. Ciolowski and D. J. Fox, “Gaussian 09, Revision B.01,” Gaussian, Inc., Wallingford, 2010.
[28] G. Piel, G. Dive, B. Evrard, T. Van Hees, S. H. de Has-sonville and L. Delattre, “Molecular Modeling Study of Beta- and Gamma-Cyclodextrin Complexes with Mi-conazole,” European Journal of Pharmaceutical Sciences, Vol. 13, No. 3, 2001, pp. 271-279.
doi:10.1016/S0928-0987(01)00113-0
[29] M. Dutta, N. N. Dutta and K. G. Bhattacharya, “Aqueous Phase Adsorption of Certain Beta-Lactam Antibiotics onto Polymeric Resins and Activated Carbon,” Separa-tion and PurificaSepara-tion Technology, Vol. 16, No. 3, 1999, pp. 213-224. doi:10.1016/S1383-5866(99)00011-8 [30] M. V. Chaubal, G. F. Payne, C. H. Reynolds and R. L.
Albright, “Equilibria for the Adsorption of Antibiotics onto Neutral Polymeric Sorbents: Experimental and Modeling Studies,” Biotechnology and Bioengineering, Vol. 47, No. 2, 1995, pp. 215-226.
doi:10.1002/bit.260470213
[31] F. Salto and J. G. Prieto, “Interactions of and Penicillins with Nonpolar Macroporous Styrenedivinylbenzene Co-polymers,” Journal of Pharmaceutical Sciences, Vol. 70, No. 9, 1981, pp. 994-998. doi:10.1002/jps.2600700906 [32] Y. Nozaki and C. Tanford, “The Solubility of Amino
Acids and Two Glycine Peptides in Aqueous Ethanol and Dioxane Solutions. Establishment of a hydrophobicity Scale,” The Journal of Biological Chemistry, Vol. 246, No. 7, 1971, pp. 2211-2217.
[33] A. Romo, F. J. Penas and J. R. Isasi, “Sorption of Diben-zofuran Derivatives from Aqueous Solutions by β-Cyclo- dextrin Polymers: An Isosteric Heat Approach,” Journal of Colloid and Interface Science, Vol. 279, No. 1, 2004, pp. 55-60. doi:10.1016/j.jcis.2004.06.043
[34] Y. S. Ho and G. McKay, “Pseudo-Second Order Model for Sorption Processes,” Process Biochemistry, Vol. 34, No. 5, 1999, pp. 451-465.
doi:10.1016/S0032-9592(98)00112-5
[35] K. Kano and H. Hasegawa, “Chiral Recognition of Heli-cal Metal Complexes by Modified Cyclodextrins,” Jour-nal of the American Chemical Society, Vol. 123, No. 43, 2001, pp. 10616-10627. doi:10.1021/ja0112644
[36] H. A. Benesi and J. H. Hildebrand, “A Spectrophotomet-ric Investigation of the Interaction of Iodine with Aro-matic Hydrocarbons,” Journal of the American Chemical Society, Vol. 71, No. 8, 1949, pp. 2703-2707.
doi:10.1021/ja01176a030
[37] L. X. Song, H. M. Wang, P. Xu, Z. Q. Zhang and Q. Q. Liu, “Formation, Structure, and Stability of α- and β- Cyclodextrin Inclusion Complexes of Phenol and Benzoic Acid Derivatives in Vacuo and in Water,” Bulletin of the Chemical Society of Japan, Vol. 80, No. 12, 2007, pp. 2313-2322. doi:10.1246/bcsj.80.2313
Titration and Quantum Calculation for the Inclusion Complexes of Styrene and α-Methyl Styrene with α, β- and γ-Cyclodextrins,” Journal of Molecular Structure, Vol. 660, No. 1-3, 2003, pp. 73-80.
doi:10.1016/j.molstruc.2003.07.011
Supplementary Information
[image:12.595.72.497.90.315.2]
(a) (b)
Figure S1. UV absorption spectra of (a) L-Tryptophan and (b) D-Tryptophan in presence of various concentration of β-CD.
[image:12.595.60.539.337.706.2]