Acta Crystallographica Section E
Structure Reports
Online
ISSN 1600-5368
Bis(12-crown-4)lithium(I) bis[(
N
,
N
-diisopropyl-amino)borohydride(1–)]lithium(I)
Franz Dornhaus and Michael Bolte*
Institut fu¨r Anorganische Chemie, J. W. Goethe-Universita¨t Frankfurt, Max-von-Laue-Strasse 7, 60438 Frankfurt/Main, Germany
Correspondence e-mail: bolte@chemie.uni-frankfurt.de
Key indicators
Single-crystal X-ray study T= 173 K
Mean(C–C) = 0.007 A˚ Rfactor = 0.084 wRfactor = 0.176
Data-to-parameter ratio = 19.5
For details of how these key indicators were automatically derived from the article, see http://journals.iucr.org/e.
Received 21 November 2006 Accepted 27 November 2006
The structure of the title compound, [Li(C8H16O4)2
]-[Li(C6H17BN)2], is composed of bis[(N,N
-diisopropyl-amino)borohydride(1 )]lithium(I) anions and Li+ cations which are eight-coordinated by two 12-crown-4 ether mol-ecules.
Comment
The title compound, (I), is a well known hydride transfer reagent with advantageous properties for applications in synthetic organic chemistry (Fisheret al., 1994; Thomaset al., 2001; Pasumansky et al., 2005). In contrast to the often used LiAlH4, which shows comparable hydride transfer activity, it is
not pyrophoric, is thermally stable and does not react violently with protic solvents. Lithium aminoborohydrides are readily available through deprotonation of primary or secondary amine borane adducts withn-butyllithium. The structures of various solvates of lithium (N,N-dimethyl)aminoborohydride, which is the methyl derivative of the title compound, have been published (No¨thet al., 1996). We report here the crystal structure of the 12-crown-4 adduct (I) of LiNiPr2BH3along
with1H and7Li NMR data.
the crown ether molecules with Li—O distances ranging from 2.317 (6) to 2.475 (7) A˚ .
Experimental
All manipulations were carried out under an atmosphere of dry nitrogen using standard Schlenk techniques. Solvents were freshly distilled under argon from sodium/benzophenone (THF, diethyl ether, C6D6) prior to use. LiN
i
Pr2BH3, was obtained following a
literature procedure (Fisher et al., 1994; Thomas et al., 2001; Pasu-mansky et al., 2005). The 12-crown-4 adduct (I) was obtained by adding an equimolar amount of crown ether to a THF solution of LiNiPrBH at room temperature. X-ray quality crystals were grown
Crystal data
[Li(C8H16O4)2][Li(C6H17BN)2]
Mr= 594.33 Triclinic,P1
a= 10.2918 (12) A˚
b= 14.5424 (14) A˚
c= 14.8057 (13) A˚
= 116.790 (7) = 105.314 (8) = 94.850 (9)
V= 1853.7 (4) A˚3
Z= 2
Dx= 1.065 Mg m 3 MoKradiation
= 0.07 mm 1
T= 173 (2) K Block, colourless 0.240.220.19 mm
Data collection
Stoe IPDS-II two-circle diffractometer
!scans
Absorption correction: none 23964 measured reflections
7468 independent reflections 3312 reflections withI> 2(I)
Rint= 0.096
max= 26.4
Refinement
Refinement onF2
R[F2> 2(F2)] = 0.084
wR(F2) = 0.176
S= 0.95 7468 reflections 382 parameters
H-atom parameters constrained
w= 1/[2(F
o2) + (0.0581P)2] whereP= (Fo
2 + 2Fc
2 )/3 (/)max< 0.001
max= 0.49 e A˚ 3 min= 0.32 e A˚ 3
Extinction correction:SHELXL97
Extinction coefficient: 0.0110 (15)
Table 1
Selected geometric parameters (A˚ ,).
Li1—N1 2.049 (8)
Li1—N2 2.053 (8)
Li2—O54 2.317 (6)
Li2—O34 2.338 (6)
Li2—O40 2.360 (6)
Li2—O31 2.374 (6)
Li2—O51 2.383 (7)
Li2—O37 2.417 (7)
Li2—O57 2.433 (6)
Li2—O60 2.475 (7)
N1—Li1—N2 176.4 (4)
H atoms were positioned geometrically (C—H = 0.98–1.00 A˚ , B— H = 0.98 A˚ ) and refined as riding with Uiso(H) = 1.2Ueq(C) or
1.5Ueq(methyl C,B)]. The BH3groups were allowed to rotate but not
to tip.
Data collection:X-AREA(Stoe & Cie, 2001); cell refinement: X-AREA; data reduction:X-AREA; program(s) used to solve structure:
SHELXS97(Sheldrick, 1997); program(s) used to refine structure:
SHELXL97 (Sheldrick, 1997); molecular graphics: XP in
SHELXTL-Plus(Sheldrick, 1991); software used to prepare material for publication:SHELXL97andPLATON(Spek, 2003).
References
Allen, F. H. (2002).Acta Cryst.B58, 380–388.
Fisher, G. B., Fuller, J. C., Harrison, J., Alvarez, S. G., Burkhardt, E. R., Goralski, C. T. & Singaram, B. (1994).J. Org. Chem.59, 6378–85. Gottlieb, H. E., Kotlyar, V. & Nudelman, A. (1997).J. Org. Chem.62, 7512–
7515.
No¨th, H., Thomas, S. & Schmidt, M. (1996).Chem. Ber.129, 451–458. Pasumansky, L., Singaram, B. & Goralski, C. T. (2005).Aldrichim. Acta,38,
61–65.
Sheldrick, G. M. (1991).SHELXTL-Plus. Release 4.1. Siemens Analytical X-ray Instruments Inc., Madison, Wisconsin, USA.
Sheldrick, G. M. (1997). SHELXS97 and SHELXL97. University of Go¨ttingen, Germany.
Spek, A. L. (2003).J. Appl. Cryst.36, 7–13.
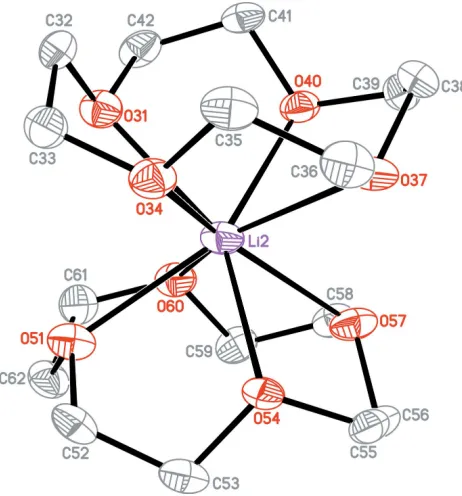
[image:2.610.46.294.73.248.2]Stoe & Cie (2001).X-AREA. Stoe & Cie, Darmstadt, Germany. Figure 1
[image:2.610.54.285.294.542.2]The anion of (I) showing displacement ellipsoids at the 50% probability level; H atoms have been omitted for clarity.
Figure 2
supporting information
Acta Cryst. (2007). E63, m41–m42 [https://doi.org/10.1107/S1600536806051142]
Bis(12-crown-4)lithium(I) bis[(
N
,
N
-diisopropylamino)borohydride(1
–
)]lithium(I)
Franz Dornhaus and Michael Bolte
Bis(12-crown-4)lithium(I) bis[(N,N-diisopropylamino)borohydride(1-)]lithium(I)
Crystal data
[Li(C8H16O4)2][Li(C6H17BN)2]
Mr = 594.33
Triclinic, P1 Hall symbol: -P 1
a = 10.2918 (12) Å
b = 14.5424 (14) Å
c = 14.8057 (13) Å
α = 116.790 (7)°
β = 105.314 (8)°
γ = 94.850 (9)°
V = 1853.7 (4) Å3
Z = 2
F(000) = 656
Dx = 1.065 Mg m−3
Mo Kα radiation, λ = 0.71073 Å Cell parameters from 9247 reflections
θ = 3.6–26.3°
µ = 0.07 mm−1
T = 173 K Block, colourless 0.24 × 0.22 × 0.19 mm
Data collection
Stoe IPDS-II two-circle diffractometer
Radiation source: fine-focus sealed tube Graphite monochromator
ω scans
23964 measured reflections 7468 independent reflections
3312 reflections with I > 2σ(I)
Rint = 0.096
θmax = 26.4°, θmin = 3.6°
h = −12→12
k = −18→18
l = −18→18
Refinement
Refinement on F2
Least-squares matrix: full
R[F2 > 2σ(F2)] = 0.084
wR(F2) = 0.176
S = 0.95 7468 reflections 382 parameters 0 restraints
Primary atom site location: structure-invariant direct methods
Secondary atom site location: difference Fourier map
Hydrogen site location: inferred from neighbouring sites
H-atom parameters constrained
w = 1/[σ2(F
o2) + (0.0581P)2]
where P = (Fo2 + 2Fc2)/3
(Δ/σ)max < 0.001
Δρmax = 0.49 e Å−3
Δρmin = −0.32 e Å−3
Extinction correction: SHELXL97, Fc*=kFc[1+0.001xFc2λ3/sin(2θ)]-1/4
Special details
Experimental. The NMR spectra were recorded on Bruker AV 300 and AV 400 spectrometers. NMR measurements were performed in undeuterated THF solution. C6D6 (approximately 0.1 ml) was added to the sample to provide a lock signal. 1H NMR shifts are reported relative to tetramethylsilane and were referenced against residual solvent peaks (C
6D5H: δ =
7.16; Gottlieb et al., 1997). The 7Li NMR spectrum was referenced against external LiCl in D
2O (ca 9.7 mol kg-1).
Abbreviations: s = singlet, d = dublet, m = multiplet, n.o. = not observed. 1H NMR (THF, 400.13 MHz): δ n.o. (BH 3),
0.99 (d, 3J
HH = 6.6 Hz, 6 H, CH3), 2.83 (m, 2 H, CH) p.p.m. 7Li{1H} NMR (THF, 116.60 MHz): δ = -0.52 (s) p.p.m.
Geometry. All e.s.d.'s (except the e.s.d. in the dihedral angle between two l.s. planes) are estimated using the full covariance matrix. The cell e.s.d.'s are taken into account individually in the estimation of e.s.d.'s in distances, angles and torsion angles; correlations between e.s.d.'s in cell parameters are only used when they are defined by crystal symmetry. An approximate (isotropic) treatment of cell e.s.d.'s is used for estimating e.s.d.'s involving l.s. planes.
Refinement. Refinement of F2 against ALL reflections. The weighted R-factor wR and goodness of fit S are based on F2,
conventional R-factors R are based on F, with F set to zero for negative F2. The threshold expression of F2 > σ(F2) is used
only for calculating R-factors(gt) etc. and is not relevant to the choice of reflections for refinement. R-factors based on F2
are statistically about twice as large as those based on F, and R- factors based on ALL data will be even larger.
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
x y z Uiso*/Ueq
Li1 0.7647 (8) 0.2792 (6) 0.2620 (5) 0.0380 (17) N1 0.6404 (3) 0.3817 (3) 0.2600 (2) 0.0297 (8) B1 0.7083 (5) 0.3750 (4) 0.1764 (3) 0.0298 (10)
H1A 0.6728 0.3042 0.1129 0.045*
H1B 0.8090 0.3884 0.2080 0.045*
H1C 0.6859 0.4282 0.1553 0.045*
N2 0.8854 (3) 0.1723 (2) 0.2538 (2) 0.0259 (7) B2 0.8339 (7) 0.1848 (6) 0.3478 (4) 0.0568 (17)
H2A 0.8909 0.1563 0.3887 0.085*
H2B 0.7369 0.1458 0.3184 0.085*
H2C 0.8417 0.2600 0.3953 0.085*
C11 0.6959 (7) 0.4881 (4) 0.3601 (3) 0.0610 (16)
H11 0.7967 0.5028 0.3695 0.073*
C12 0.6990 (9) 0.4915 (4) 0.4568 (4) 0.104 (3)
H12A 0.7304 0.4304 0.4588 0.155*
H12B 0.6058 0.4893 0.4618 0.155*
H12C 0.7629 0.5571 0.5176 0.155*
C13 0.6519 (6) 0.5800 (4) 0.3486 (4) 0.0525 (13)
H13A 0.6569 0.5737 0.2809 0.079*
H13B 0.7138 0.6468 0.4091 0.079*
H13C 0.5566 0.5791 0.3485 0.079*
C14 0.4901 (5) 0.3462 (4) 0.2238 (4) 0.0536 (13)
H14 0.4630 0.3903 0.2874 0.064*
C15 0.4501 (6) 0.2356 (4) 0.2010 (6) 0.084 (2)
H15A 0.5062 0.2283 0.2611 0.125*
H15B 0.4659 0.1872 0.1350 0.125*
H15C 0.3518 0.2181 0.1919 0.125*
C16 0.4034 (5) 0.3609 (5) 0.1333 (4) 0.0671 (16)
H16A 0.4302 0.4349 0.1509 0.101*
H16C 0.4189 0.3144 0.0665 0.101* C21 0.8442 (4) 0.0657 (3) 0.1569 (3) 0.0380 (10)
H21 0.9094 0.0644 0.1170 0.046*
C22 0.8514 (6) −0.0279 (4) 0.1804 (5) 0.0703 (17)
H22A 0.9453 −0.0170 0.2273 0.105*
H22B 0.8286 −0.0944 0.1127 0.105*
H22C 0.7850 −0.0312 0.2163 0.105*
C23 0.6979 (5) 0.0473 (4) 0.0821 (3) 0.0449 (12)
H23A 0.6933 0.1056 0.0661 0.067*
H23B 0.6307 0.0442 0.1174 0.067*
H23C 0.6763 −0.0198 0.0150 0.067*
C24 1.0326 (4) 0.2283 (3) 0.2924 (3) 0.0324 (10)
H24 1.0452 0.2976 0.3576 0.039*
C25 1.1394 (5) 0.1730 (4) 0.3290 (4) 0.0434 (11)
H25A 1.1163 0.1564 0.3809 0.065*
H25B 1.2323 0.2201 0.3628 0.065*
H25C 1.1376 0.1072 0.2665 0.065*
C26 1.0695 (5) 0.2553 (4) 0.2125 (4) 0.0419 (11)
H26A 1.0022 0.2913 0.1900 0.063*
H26B 1.0673 0.1900 0.1494 0.063*
H26C 1.1626 0.3021 0.2467 0.063*
Li2 0.7601 (7) 0.2453 (5) 0.7488 (4) 0.0262 (14) O31 0.6061 (3) 0.26003 (19) 0.60775 (19) 0.0283 (6) C32 0.6668 (4) 0.3320 (3) 0.5803 (3) 0.0279 (9)
H32A 0.5944 0.3592 0.5474 0.033*
H32B 0.7139 0.2957 0.5283 0.033*
C33 0.7695 (4) 0.4215 (3) 0.6828 (3) 0.0301 (9)
H33A 0.8167 0.4715 0.6666 0.036*
H33B 0.7200 0.4605 0.7317 0.036*
O34 0.8714 (3) 0.3832 (2) 0.73530 (19) 0.0286 (6) C35 0.9808 (4) 0.3613 (3) 0.6909 (3) 0.0307 (10)
H35A 1.0424 0.4282 0.7103 0.037*
H35B 0.9419 0.3180 0.6116 0.037*
C36 1.0603 (4) 0.3016 (3) 0.7378 (3) 0.0324 (10)
H36A 1.1355 0.2832 0.7076 0.039*
H36B 1.1033 0.3475 0.8167 0.039*
O37 0.9715 (3) 0.2064 (2) 0.71465 (18) 0.0304 (7) C38 0.9517 (4) 0.1182 (3) 0.6117 (3) 0.0301 (9)
H38A 1.0332 0.0866 0.6141 0.036*
H38B 0.9388 0.1409 0.5569 0.036*
C39 0.8233 (4) 0.0384 (3) 0.5847 (3) 0.0293 (9)
H39A 0.8036 −0.0228 0.5122 0.035*
H39B 0.8410 0.0121 0.6369 0.035*
O40 0.7050 (3) 0.0833 (2) 0.58723 (18) 0.0266 (6) C41 0.6396 (4) 0.0903 (3) 0.4923 (3) 0.0263 (9)
H41A 0.5914 0.0189 0.4304 0.032*
H41B 0.7096 0.1231 0.4740 0.032*
H42A 0.4906 0.1641 0.4544 0.035*
H42B 0.4675 0.1224 0.5337 0.035*
O51 0.6631 (3) 0.3805 (2) 0.85411 (18) 0.0281 (6) C52 0.7740 (4) 0.4584 (3) 0.9503 (3) 0.0282 (9)
H52A 0.7345 0.5071 1.0022 0.034*
H52B 0.8320 0.5008 0.9321 0.034*
C53 0.8625 (4) 0.4053 (3) 1.0009 (3) 0.0300 (9)
H53A 0.9387 0.4590 1.0666 0.036*
H53B 0.8060 0.3640 1.0210 0.036*
O54 0.9179 (3) 0.33607 (19) 0.92266 (17) 0.0257 (6) C55 0.9812 (4) 0.2650 (3) 0.9516 (3) 0.0246 (9)
H55A 1.0380 0.3039 1.0290 0.030*
H55B 1.0437 0.2377 0.9101 0.030*
C56 0.8742 (4) 0.1727 (3) 0.9299 (3) 0.0298 (9)
H56A 0.9202 0.1220 0.9458 0.036*
H56B 0.8159 0.1982 0.9755 0.036*
O57 0.7906 (3) 0.1224 (2) 0.81807 (18) 0.0315 (7) C58 0.6627 (4) 0.0541 (3) 0.7907 (3) 0.0318 (10)
H58A 0.6766 0.0167 0.8330 0.038*
H58B 0.6316 0.0001 0.7136 0.038*
C59 0.5526 (4) 0.1135 (3) 0.8121 (3) 0.0301 (10)
H59A 0.4640 0.0640 0.7907 0.036*
H59B 0.5801 0.1658 0.8896 0.036*
O60 0.5373 (3) 0.1667 (2) 0.74892 (19) 0.0312 (7) C61 0.4586 (4) 0.2457 (3) 0.7762 (3) 0.0311 (9)
H61A 0.3775 0.2193 0.7893 0.037*
H61B 0.4238 0.2587 0.7150 0.037*
C62 0.5440 (4) 0.3481 (3) 0.8747 (3) 0.0306 (9)
H62A 0.4895 0.4029 0.8891 0.037*
H62B 0.5727 0.3378 0.9381 0.037*
Atomic displacement parameters (Å2)
U11 U22 U33 U12 U13 U23
C24 0.030 (2) 0.040 (3) 0.032 (2) 0.010 (2) 0.0098 (18) 0.021 (2) C25 0.034 (3) 0.048 (3) 0.051 (3) 0.014 (2) 0.004 (2) 0.032 (2) C26 0.034 (3) 0.053 (3) 0.056 (3) 0.013 (2) 0.018 (2) 0.039 (2) Li2 0.031 (4) 0.024 (3) 0.018 (3) 0.002 (3) 0.007 (3) 0.007 (3) O31 0.0329 (16) 0.0251 (15) 0.0275 (13) 0.0028 (12) 0.0120 (12) 0.0133 (12) C32 0.037 (2) 0.027 (2) 0.028 (2) 0.0117 (19) 0.0131 (18) 0.0184 (18) C33 0.042 (3) 0.024 (2) 0.031 (2) 0.0092 (19) 0.0173 (19) 0.0154 (18) O34 0.0296 (16) 0.0341 (16) 0.0273 (13) 0.0067 (13) 0.0135 (12) 0.0173 (13) C35 0.028 (2) 0.035 (2) 0.030 (2) −0.0021 (19) 0.0133 (18) 0.0155 (19) C36 0.023 (2) 0.038 (2) 0.029 (2) −0.0003 (19) 0.0078 (17) 0.0118 (19) O37 0.0368 (17) 0.0313 (16) 0.0184 (12) 0.0021 (13) 0.0102 (12) 0.0090 (12) C38 0.034 (2) 0.034 (2) 0.0241 (19) 0.0140 (19) 0.0152 (18) 0.0117 (18) C39 0.042 (3) 0.020 (2) 0.0241 (19) 0.0119 (19) 0.0115 (18) 0.0088 (17) O40 0.0289 (16) 0.0333 (15) 0.0224 (12) 0.0073 (12) 0.0104 (11) 0.0166 (12) C41 0.033 (2) 0.023 (2) 0.0164 (17) −0.0011 (18) 0.0040 (16) 0.0089 (17) C42 0.032 (2) 0.023 (2) 0.0198 (18) −0.0025 (18) −0.0038 (17) 0.0079 (17) O51 0.0225 (15) 0.0326 (16) 0.0234 (13) −0.0015 (12) 0.0087 (11) 0.0101 (12) C52 0.025 (2) 0.023 (2) 0.0246 (19) 0.0018 (17) 0.0105 (17) 0.0017 (17) C53 0.031 (2) 0.032 (2) 0.0185 (18) 0.0021 (19) 0.0089 (17) 0.0059 (18) O54 0.0303 (16) 0.0315 (15) 0.0189 (12) 0.0100 (12) 0.0132 (11) 0.0123 (12) C55 0.022 (2) 0.035 (2) 0.0202 (17) 0.0070 (18) 0.0064 (16) 0.0157 (17) C56 0.030 (2) 0.037 (2) 0.0213 (18) 0.0049 (19) 0.0028 (17) 0.0168 (18) O57 0.0260 (16) 0.0411 (17) 0.0194 (12) −0.0010 (13) 0.0019 (11) 0.0132 (12) C58 0.038 (3) 0.027 (2) 0.0230 (19) 0.0002 (19) 0.0028 (18) 0.0118 (18) C59 0.030 (2) 0.032 (2) 0.032 (2) 0.0006 (19) 0.0102 (18) 0.0214 (19) O60 0.0371 (17) 0.0292 (16) 0.0243 (13) 0.0043 (13) 0.0086 (12) 0.0124 (12) C61 0.021 (2) 0.039 (3) 0.033 (2) 0.0075 (19) 0.0095 (18) 0.017 (2) C62 0.032 (2) 0.035 (2) 0.030 (2) 0.0112 (19) 0.0191 (19) 0.0155 (19)
Geometric parameters (Å, º)
Li1—N1 2.049 (8) Li2—O60 2.475 (7)
Li1—N2 2.053 (8) O31—C42 1.426 (4)
N1—C14 1.457 (5) O31—C32 1.436 (4)
N1—C11 1.501 (6) C32—C33 1.501 (5)
N1—B1 1.545 (5) C32—H32A 0.9900
B1—H1A 0.9800 C32—H32B 0.9900
B1—H1B 0.9800 C33—O34 1.440 (4)
B1—H1C 0.9800 C33—H33A 0.9900
N2—C24 1.484 (5) C33—H33B 0.9900
N2—C21 1.485 (5) O34—C35 1.437 (5)
N2—B2 1.557 (6) C35—C36 1.506 (6)
B2—H2A 0.9800 C35—H35A 0.9900
B2—H2B 0.9800 C35—H35B 0.9900
B2—H2C 0.9800 C36—O37 1.436 (4)
C11—C12 1.403 (7) C36—H36A 0.9900
C11—C13 1.512 (6) C36—H36B 0.9900
C12—H12A 0.9800 C38—C39 1.514 (6)
C12—H12B 0.9800 C38—H38A 0.9900
C12—H12C 0.9800 C38—H38B 0.9900
C13—H13A 0.9800 C39—O40 1.432 (5)
C13—H13B 0.9800 C39—H39A 0.9900
C13—H13C 0.9800 C39—H39B 0.9900
C14—C15 1.482 (7) O40—C41 1.442 (4)
C14—C16 1.516 (6) C41—C42 1.491 (6)
C14—H14 1.0000 C41—H41A 0.9900
C15—H15A 0.9800 C41—H41B 0.9900
C15—H15B 0.9800 C42—H42A 0.9900
C15—H15C 0.9800 C42—H42B 0.9900
C16—H16A 0.9800 O51—C62 1.430 (4)
C16—H16B 0.9800 O51—C52 1.443 (4)
C16—H16C 0.9800 C52—C53 1.506 (5)
C21—C23 1.532 (6) C52—H52A 0.9900
C21—C22 1.552 (6) C52—H52B 0.9900
C21—H21 1.0000 C53—O54 1.441 (4)
C22—H22A 0.9800 C53—H53A 0.9900
C22—H22B 0.9800 C53—H53B 0.9900
C22—H22C 0.9800 O54—C55 1.429 (4)
C23—H23A 0.9800 C55—C56 1.514 (5)
C23—H23B 0.9800 C55—H55A 0.9900
C23—H23C 0.9800 C55—H55B 0.9900
C24—C26 1.527 (5) C56—O57 1.437 (4)
C24—C25 1.541 (5) C56—H56A 0.9900
C24—H24 1.0000 C56—H56B 0.9900
C25—H25A 0.9800 O57—C58 1.421 (5)
C25—H25B 0.9800 C58—C59 1.496 (6)
C25—H25C 0.9800 C58—H58A 0.9900
C26—H26A 0.9800 C58—H58B 0.9900
C26—H26B 0.9800 C59—O60 1.447 (4)
C26—H26C 0.9800 C59—H59A 0.9900
Li2—O54 2.317 (6) C59—H59B 0.9900
Li2—O34 2.338 (6) O60—C61 1.432 (5)
Li2—O40 2.360 (6) C61—C62 1.503 (5)
Li2—O31 2.374 (6) C61—H61A 0.9900
Li2—O51 2.383 (7) C61—H61B 0.9900
Li2—O37 2.417 (7) C62—H62A 0.9900
Li2—O57 2.433 (6) C62—H62B 0.9900
N1—Li1—N2 176.4 (4) O37—Li2—O60 143.5 (3)
C14—N1—C11 112.7 (4) O57—Li2—O60 69.00 (18)
C14—N1—B1 118.3 (3) C42—O31—C32 113.5 (3)
C11—N1—B1 111.0 (3) C42—O31—Li2 110.1 (2)
C14—N1—Li1 120.7 (3) C32—O31—Li2 114.5 (3) C11—N1—Li1 112.1 (3) O31—C32—C33 107.0 (3)
N1—B1—H1A 109.5 C33—C32—H32A 110.3
N1—B1—H1B 109.5 O31—C32—H32B 110.3
H1A—B1—H1B 109.5 C33—C32—H32B 110.3
N1—B1—H1C 109.5 H32A—C32—H32B 108.6
H1A—B1—H1C 109.5 O34—C33—C32 111.3 (3)
H1B—B1—H1C 109.5 O34—C33—H33A 109.4
C24—N2—C21 115.0 (3) C32—C33—H33A 109.4
C24—N2—B2 112.4 (3) O34—C33—H33B 109.4
C21—N2—B2 117.6 (4) C32—C33—H33B 109.4
C24—N2—Li1 108.5 (3) H33A—C33—H33B 108.0
C21—N2—Li1 120.2 (3) C35—O34—C33 113.5 (3)
B2—N2—Li1 77.6 (3) C35—O34—Li2 115.5 (3)
N2—B2—H2A 109.5 C33—O34—Li2 109.6 (3)
N2—B2—H2B 109.5 O34—C35—C36 106.8 (3)
H2A—B2—H2B 109.5 O34—C35—H35A 110.4
N2—B2—H2C 109.5 C36—C35—H35A 110.4
H2A—B2—H2C 109.5 O34—C35—H35B 110.4
H2B—B2—H2C 109.5 C36—C35—H35B 110.4
C12—C11—N1 116.9 (4) H35A—C35—H35B 108.6
C12—C11—C13 114.5 (4) O37—C36—C35 111.3 (3)
N1—C11—C13 115.6 (4) O37—C36—H36A 109.4
C12—C11—H11 102.2 C35—C36—H36A 109.4
N1—C11—H11 102.2 O37—C36—H36B 109.4
C13—C11—H11 102.2 C35—C36—H36B 109.4
C11—C12—H12A 109.5 H36A—C36—H36B 108.0
C11—C12—H12B 109.5 C38—O37—C36 113.8 (3)
H12A—C12—H12B 109.5 C38—O37—Li2 114.8 (3)
C11—C12—H12C 109.5 C36—O37—Li2 109.3 (3)
H12A—C12—H12C 109.5 O37—C38—C39 106.8 (3)
H12B—C12—H12C 109.5 O37—C38—H38A 110.4
C11—C13—H13A 109.5 C39—C38—H38A 110.4
C11—C13—H13B 109.5 O37—C38—H38B 110.4
H13A—C13—H13B 109.5 C39—C38—H38B 110.4
C11—C13—H13C 109.5 H38A—C38—H38B 108.6
H13A—C13—H13C 109.5 O40—C39—C38 112.0 (3)
H13B—C13—H13C 109.5 O40—C39—H39A 109.2
N1—C14—C15 110.5 (4) C38—C39—H39A 109.2
N1—C14—C16 118.1 (4) O40—C39—H39B 109.2
C15—C14—C16 111.0 (5) C38—C39—H39B 109.2
N1—C14—H14 105.4 H39A—C39—H39B 107.9
C15—C14—H14 105.4 C39—O40—C41 114.3 (3)
C16—C14—H14 105.4 C39—O40—Li2 110.2 (3)
C14—C15—H15A 109.5 C41—O40—Li2 113.5 (2)
C14—C15—H15B 109.5 O40—C41—C42 106.6 (3)
H15A—C15—H15B 109.5 O40—C41—H41A 110.4
C14—C15—H15C 109.5 C42—C41—H41A 110.4
H15A—C15—H15C 109.5 O40—C41—H41B 110.4
C14—C16—H16A 109.5 H41A—C41—H41B 108.6
C14—C16—H16B 109.5 O31—C42—C41 111.1 (3)
H16A—C16—H16B 109.5 O31—C42—H42A 109.4
C14—C16—H16C 109.5 C41—C42—H42A 109.4
H16A—C16—H16C 109.5 O31—C42—H42B 109.4
H16B—C16—H16C 109.5 C41—C42—H42B 109.4
N2—C21—C23 110.4 (3) H42A—C42—H42B 108.0
N2—C21—C22 114.9 (4) C62—O51—C52 113.4 (3) C23—C21—C22 109.2 (4) C62—O51—Li2 117.7 (3)
N2—C21—H21 107.4 C52—O51—Li2 108.0 (3)
C23—C21—H21 107.4 O51—C52—C53 110.5 (3)
C22—C21—H21 107.4 O51—C52—H52A 109.5
C21—C22—H22A 109.5 C53—C52—H52A 109.5
C21—C22—H22B 109.5 O51—C52—H52B 109.5
H22A—C22—H22B 109.5 C53—C52—H52B 109.5
C21—C22—H22C 109.5 H52A—C52—H52B 108.1
H22A—C22—H22C 109.5 O54—C53—C52 107.3 (3)
H22B—C22—H22C 109.5 O54—C53—H53A 110.3
C21—C23—H23A 109.5 C52—C53—H53A 110.3
C21—C23—H23B 109.5 O54—C53—H53B 110.3
H23A—C23—H23B 109.5 C52—C53—H53B 110.3
C21—C23—H23C 109.5 H53A—C53—H53B 108.5
H23A—C23—H23C 109.5 C55—O54—C53 113.2 (3)
H23B—C23—H23C 109.5 C55—O54—Li2 110.9 (3)
N2—C24—C26 113.2 (3) C53—O54—Li2 113.7 (3) N2—C24—C25 115.2 (3) O54—C55—C56 111.6 (3)
C26—C24—C25 109.5 (4) O54—C55—H55A 109.3
N2—C24—H24 106.1 C56—C55—H55A 109.3
C26—C24—H24 106.1 O54—C55—H55B 109.3
C25—C24—H24 106.1 C56—C55—H55B 109.3
C24—C25—H25A 109.5 H55A—C55—H55B 108.0
C24—C25—H25B 109.5 O57—C56—C55 106.9 (3)
H25A—C25—H25B 109.5 O57—C56—H56A 110.3
C24—C25—H25C 109.5 C55—C56—H56A 110.3
H25A—C25—H25C 109.5 O57—C56—H56B 110.3
H25B—C25—H25C 109.5 C55—C56—H56B 110.3
C24—C26—H26A 109.5 H56A—C56—H56B 108.6
C24—C26—H26B 109.5 C58—O57—C56 114.1 (3)
H26A—C26—H26B 109.5 C58—O57—Li2 112.0 (3)
C24—C26—H26C 109.5 C56—O57—Li2 113.5 (3)
H26A—C26—H26C 109.5 O57—C58—C59 112.0 (3)
H26B—C26—H26C 109.5 O57—C58—H58A 109.2
O54—Li2—O34 82.7 (2) C59—C58—H58A 109.2
O54—Li2—O40 140.7 (3) O57—C58—H58B 109.2
O34—Li2—O40 110.4 (2) C59—C58—H58B 109.2
O54—Li2—O51 72.85 (19) C58—C59—H59A 110.4
O34—Li2—O51 82.7 (2) O60—C59—H59B 110.4
O40—Li2—O51 143.4 (3) C58—C59—H59B 110.4
O31—Li2—O51 81.6 (2) H59A—C59—H59B 108.6
O54—Li2—O37 79.6 (2) C61—O60—C59 114.7 (3) O34—Li2—O37 71.2 (2) C61—O60—Li2 108.8 (3) O40—Li2—O37 70.9 (2) C59—O60—Li2 113.7 (3) O31—Li2—O37 111.7 (2) O60—C61—C62 111.7 (3)
O51—Li2—O37 144.1 (3) O60—C61—H61A 109.3
O54—Li2—O57 71.28 (18) C62—C61—H61A 109.3
O34—Li2—O57 145.6 (3) O60—C61—H61B 109.3
O40—Li2—O57 79.3 (2) C62—C61—H61B 109.3
O31—Li2—O57 140.3 (3) H61A—C61—H61B 107.9 O51—Li2—O57 109.6 (2) O51—C62—C61 107.3 (3)
O37—Li2—O57 81.9 (2) O51—C62—H62A 110.3
O54—Li2—O60 109.7 (2) C61—C62—H62A 110.3
O34—Li2—O60 143.4 (3) O51—C62—H62B 110.3
O40—Li2—O60 82.1 (2) C61—C62—H62B 110.3
O31—Li2—O60 80.8 (2) H62A—C62—H62B 108.5
O51—Li2—O60 69.4 (2)