Original Article
Immunopathogenesis and SETBP1 mutation analysis in
chronic myelomonocytic leukemia
Yan Liang1, Wen-Yi Shen1, Rui-Nan Lu1, Rong Wang1, Chun Qiao1, Jian-Fu Zhang1, Jian-Yong Li1, Su-Jiang
Zhang2, Hua Lu1
1Department of Hematology, The First Affiliated Hospital of Nanjing Medical University, Jiangsu Province Hospital,
Nanjing 210029, China; 2Department of Hematology, Ruijin Hospital North Affiliated with Shanghai Jiao Tong
University School of Medicine, Shanghai, China
Received January 19, 2016; Accepted March 27, 2016; Epub May 1, 2016; Published May 15, 2016
Abstract: To assess the diagnostic value of peripheral blood (PB) and bone marrow (BM) smears, SETBP1 gene mutation analysis, and bone marrow trephine biopsy (BMTB) histology, supplemented by immunohistochemistry in distinguishing chronic myelomonocytic leukemia (CMML) from chronic myeloid leukemia (CML) (chronic phase) and acute monocytic leukemia (AMoL). PB and BM smears were analyzed in 51 CMML patients. Immunostaining of my-eloid- and monocyte-specific markers in 26 CMML patients was compared that observed in 30 CML patients and 30 AMoL patients. SETBP1 mutations were investigated in 28 CMML patients. Most CMML patients presented with leu-kocytosis (median WBC count, 41.90±36.70×109/l) with marked monocytosis (median: CMML-1, 5.04±3.90×109/l;
CMML-2, 10.63±11.60×109/l). BM smears were hypercellular in 44 patients, with increased in granulocytic
prolif-eration and monocytes numbers. In BMTB, CMML was characterized as hypercellular in 84.6% patients, with a mod-erate degree of monocytosis (76.9%). Approximately 34.6% of patients had slightly increased BM fibrosis. Positive immunoreactivity in CMML patients was as follows: MPO, 33.82±6.83%; CD15, 21.97±7.15%; CD34, 4.44±1.98%; CD117, 1.35±0.57%). Monocytic markers, such as CD14, CD56, CD68 (PG-M1) and CD163 were positive in mean 10.30±2.55%, 8.61±2.99%, 13.24±4.64% and 10.50±4.21% of positive cells, respectively. No SETBP1 mutations were detected in 28 CMML patients. Morphological and immunohistochemical features of BMTB samples combined with analysis of PB and BM smears are helpful in distinguishing CMML from CML and AMoL.
Keywords: Chronic myelomonocytic leukemia, immunopathogenesis, SETBP1, molecular mutations
Introduction
Chronic myelomonocytic leukemia (CMML) is a clonal stem cell disorder defined as a myelo-dysplastic syndrome (MDS)/myeloproliferative neoplasm (MPN) by the WHO classification of 2008 [1]. It is characterized by dysplasia, monocytosis, varying degrees of hyperleukocy-tosis, anemia and thrombocytopenia, with an increased risk of transformation to acute myeloid leukemia (AML) [2]. Clinical features include weight loss, malaise, fever, night-sweats and hepatosplenomegaly. CMML, which is a heterogeneous condition, has a highly vari-able clinical course and poor prognosis (2-3 years median survival) [3]. CMML bone marrow (BM) smears are usually hypercellular, and in most patients, show significantly increased granulocytic proliferation with a variable degree
of dysgranulopoiesis, in addition to dysplastic changes in erythroid precursors and megakary-ocytes. Histopathological evaluation of bone marrow trephine biopsy (BMTB) in CMML is often difficult; however, BM immunohistochem-istry, which is a reliable tool for “in situ” identifi-cation of cells, has been shown to improve the accuracy of morphologic assessment. Hence, immunohistochemical evaluation of BMTB can provide an accurate estimate of the proportions of cells expressing particular antigens.
Materials and methods
Patients
Fifty-one CMML patients, 30 chronic myeloid leukemia (CML) patients in chronic phase and 30 acute monocytic leukemia (AMoL) patients were enrolled in this study. All patients were admitted to the First Affiliated Hospital of Nanjing Medical University (China) from January 2008 to January 2014. Written informed con-sent was obtained from all patients.
Peripheral blood and bone marrow cytomor -phologic analysis
In addition to complete blood counts (CBC), Wright-Giemsa-stained smears of peripheral blood (PB) and BM were analyzed for all 51 patients. Differential leukocyte counts were based on manual counts of 100 leukocytes in each PB smear sample. BM cytology was exam-ined and cell differential counts were based on 250 nucleated cells. Additional cell morphologi-cal changes were recorded.
Bone marrow biopsy and immunohistochemi -cal analysis
BMTB samples (approximately 1 cm in length) obtained from 26 patients were fixed in acetic acid-zinc-formalin fixative. After approximately 24 h, the specimens were washed in distilled water for 30 min, decalcified in 10% nitric acid, and embedded in paraffin-wax before 2.5-µm- thick sections were cut.
After being deparaffinized, sections were stained with hematoxylin and eosin (H&E) stain
then incubated with the appropriate antigen specific primary detection antibodies for 2 h. Slides were then incubated with biotinylated secondary detection antibodies for 25 min and finally counterstained with hematoxylin. The cells were counted in five randomly selected fields of haemopoietic tissue (×400). A total of 500 nucleated cells were counted and the per-centage of positive cells was recorded. BMTB samples of 30 CML patients and 30 AMoL patients were also analyzed for comparison. Mutation screening
Genomic DNA was extracted from BM cells of 28 CMML patients and amplified by poly-merase chain reaction (PCR). Mutation analysis of the SETBP1 gene was performed by Sanger sequencing [7] of the mutational hotspots with the following primers: Forward 5’-GGAACACG- TGGAGTCAGTTG-3’ and Reverse 5’-GGGGCTC- CTTTGTACCTCC-3’ to cover codons for the amino acid region 232; Forward 5’-ACAAACC- CATGAGCGAGATG-3’ and Reverse 5’-AGATGGT- TCCCCTCTTGCTG-3’ to cover codons for the amino acid region 645; Forward 5’-GGGAC- AGACAACAACAGCAC-3’ and Reverse 5’-CAG- AAACTGGAGGTCATCGC-3’ to cover codons for the amino acid region 858-871.
Diagnosis
[image:2.612.84.532.83.143.2]The diagnosis of CMML was established on the basis of PB monocytosis, morphology and cyto-genetics, in accordance with the guidelines out-lined in the current WHO classification [1]. Patients are subcategorized as CMML-1 (<5% peripheral blasts, <10% marrow blasts) and CMML-2 (5-19% peripheral blasts and 10-19% Table 1. Main hematological features of patients with CMML-1 and CMML-2 (mean ± SD)
WBC (109/l)
(mean ± SD) N (10
9/l)
(mean ± SD) MO (10
9/l)
(mean ± SD) L (10
9/l)
(mean ± SD) EO (10
9/l)
(mean ± SD) BA (10
9/l)
(mean ± SD) (mean ± SD)Hb (g/dl) PLT (10
9/l)
(mean ± SD)
CMML-1 (n=24) 39.19±38.20 25.71±25.22 5.04±3.90 8.01±14.21 0.16±0.21 0.26±0.73 90.21±23.38 62.29±51.83 CMML-2 (n=27) 44.64±35.22 21.99±18.46 10.63±11.60 10.81±16.39 0.12±0.24 0.20±0.31 84.76±24.01 105.38±189.34
P-values 0.668 0.617 0.051 0.605 0.592 0.741 0.511 0.414
CMML, chronic myelomonocytic leukemia; WBC, white blood cell; N, neutrophil; MO, monocytes; L, lymphocytes; EO, eosinophilic granulocyte; BA, basophilic granulocyte; Hb, hemoglobin; PLT, platelet.
Table 2. Peripheral blood smears findings for CMML Myeloblasts
(%) monocytes (%)Immature monocytes (%)Mature CMML-1 (n=24) 0.55±1.19 1.45±2.91 22.95±15.75 CMML-2 (n=27) 3.76±4.95 3.40±2.94 27.60±18.02
P-values 0.004 0.032 0.368
[image:2.612.89.330.193.261.2]marrow blasts), whereas the presence of ≥20% blasts in the PB or BM indicates AML. CML is a myeloproliferative neoplasm that originates in an abnormal BM stem cell and is consistently associated with the BCR-ABL1 fusion gene [1]. AMoL is myeloid leukemia, in which 80% or more of the leukemic cells are promonocytes and monocytes [1].
Statistical analysis
The Statistical Package for the Social Sciences (SPSS) version 19.0 was used for all statistical analysis. All experimental data were analyzed for normality, and the data were shown as mean ± SD. PB results, PB and BM smears were analyzed by using Student’s t-tests, and significance was defined by P-values < 0.05. The differences in immunohistochemistry between the cohorts were evaluated using one-way analysis of variance (ANOVA) with signifi-cance defined by P-values < 0.001.
Results
Clinical characteristics
Of the 51 patients enrolled in this study, 35 (71%) were male and 16 (29%) were female, with a median age of 65 y (range, 16-84 y). Twenty-four patients were subclassified as CMML-1, while the other 27 patients were CMML-2. Nine patients underwent transforma-tion to AML. According to the CMML-specific prognostic scoring system (CPSS) [8], six patients (12%) were classified as low risk, 20 patients (39%) as intermediate-1, 21 patients (41%) as intermediate-2, and 4 patients (8%) were classified as high risk.
CBC results
CBC results are summarized in Table 1. At the initial examination, the majority of the patients showed mild-to-moderate anemia, and almost all patients showed significant leukocytosis
Peripheral blood and bone marrow smears findings
PB and BM smear results are summarized in Tables 2 and 3. In 45 patients, PB smears showed markedly increased mature cytes, while myeloblasts and immature mono-cytes were more obviously increased in CMML-2 patients than in CMML-1 patients. As ex- pected, BM smears of 44 patients showed hypercellularity. Increased granulocytic prolif-eration was consistently observed in 41 pati- ents, and dysgranulopoiesis was also observed in 34 patients, with 18 patients exhibiting nuclear-cytoplasm asynchrony, nine showing nuclear hypolobation (pseudo-Pelger-Huet), four showing hypogranularity, and three show-ing binucleate granulocytes; however, no Auer rods were found in these patients. In addition, 35 (68.6%) patients exhibited mature monocy-tosis, including 13 CMML-1 and 22 CMML-2. In CMML-2, the percentage of myeloblasts was significantly higher than CMML-1 (P < 0.05).
Bone marrow pathology and immunohisto
-chemistry results
[image:3.612.92.369.86.162.2]BMTB of CMML patients revealed several char-acteristic features, although none was specific enough to confirm the diagnosis. Hypercellularity was the most prominent feature of BM histolo-gy in 22 CMML patients (84.6%). Increased granulocytic proliferation and dysgranulopoie-sis were present in the majority of the patients, and the myeloid: erythroid (M:E) ratios were sig-nificantly raised. Monocytic cells were increased in 20 patients (76.9%) appearing as diffuse cells, clusters or nodules that were often very difficult to discern. Blast counts were increased, especially in CMML-2 and CMML in acute trans-formation, and abnormal localization of imma-ture precursor (ALIP) was found in 23.1% (6/26) of patients. Furthermore, 14 patients (53.8%) exhibited dysmegakaryopoiesis characterized by the presence of micromegakaryocytes or Table 3. Bone marrow smears findings for CMML
Myeloblasts
(%) Granulocytic (%)
Immature monocytes
(%)
Mature monocytes
(%) CMML-1 (n=24) 2.64±2.96 66.04±17.57 3.12±6.32 9.34±8.60 CMML-2 (n=27) 8.49±8.12 63.71±16.97 4.42±3.15 10.79±10.51
P-values 0.002 0.636 0.368 0.621
hypolobated nuclei. Nine patients (34.6%) had a slight increase in BM fibrosis and approxi-mately 25.0% of the patients exhibited dys-erythropoiesis, particularly in the form of multi-nuclearity and megaloblastoid changes (Figure 1).
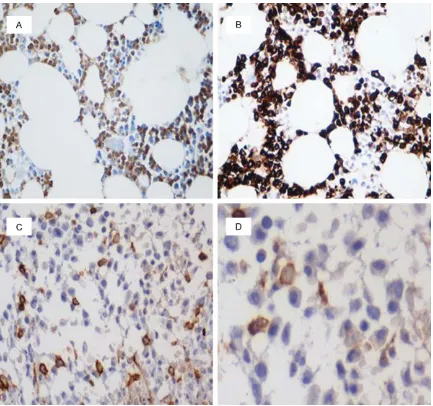
The granulocytic lineage expresses CD15 and MPO, myeloblasts typically express CD34 and CD117, and monocytes were investigated by CD14, CD56, CD68 (PG-M1) and CD163 ex- pression (Figures 2 and 3). Our results demon-strated some differences between the three groups (Table 4).
The percentage of CD15 positive cells de- creased in CMML (21.97±7.15%) compared to
CML (40.44±12.60%) and even compared to AMoL (6.29±3.54%). MPO-positive myeloid cells were found in CMML and CML but not in AMoL. Immunostaining with MPO showed sig-nificantly decreased numbers of granulocytes in CMML patients (33.82±6.83%) compared to CML patients (54.78±12.33%).
[image:4.612.89.526.70.476.2]CD34 reactivity was observed in all groups, with increased blast counts in patients with AMoL (5.92±3.84%) and CMML (4.44±1.98%) in comparison with those with CML (0.64± 0.42%). The immunohistochemical positivity for CD117 was weak and variable. CD117 posi-tive percentages were 4.83±2.56% for AMoL, 1.35±0.57% for CMML, but no immunoreactiv-ity for CD117 was seen in CML.
Monocytes are difficult to identify morphologi-cally although their identification is greatly facil-itated by immunohistochemical staining of anti-bodies. CD14, which is considered to be a mature monocytic marker, was detected in 10.30±2.55% of CMML patients but only in 5.31±1.38% of AMoL and 0.97±0.47% of CML. CD56 positive percentages were 10.15±2.52% for AMoL, 8.61±2.99% for CMML and 0.68± 0.40% for CML. The percentage of CD68 (PG-M1) positive cells was increased in AMoL (20.89±9.25%) as compared with CMML (13.24±4.64%) and CML (0.78±0.33%). CD163 positive percentages were 23.39±6.02% for AMoL, 10.50±4.21% for CMML and 2.57± 1.36% for CML.
Cytogenetic results
Although SETBP1 mutation is not disease-spe-cific, the frequencies of mutations in this gene are typically low in CMML. No SETBP1 muta-tions were detected in any of the 28 patients. Discussion
[image:5.612.91.524.69.475.2]CMML is a rare clonal hematologic disorder that is characterized by absolute peripheral monocytosis with myelodysplastic and myelo-proliferative overlap features. According to the WHO guidelines, CMML patients are classified into two categories, CMML-1 and CMML-2, defined by the proportion of blasts in the PB and BM [1].
In this study, the monocyte (MO) counts in PB smears were increased and higher than the WHO diagnostic criteria (MO ≥1×109/l). No
sig-nificant differences in clinical, hematologic and cytogenetic features were found between CMML-1 and CMML-2. The WBC and MO counts in CMML-1 were lower than those in CMML-2, but these differences did not reach the level of statistical significance.
Blast counts in the PB and BM smears were higher, and the proportion of immature mono-cytes greater in CMML-2 than in CMML-1. Compared with CMML-1, CMML-2 patients have a greater risk of transformation to AML [9]. Thus, blast counts are one of the most
impor-tant prognostic factors for CMML patients. In our study, increased granulocytic proliferation was consistently found, and dysgranulopoieis and dysmegakaryocytopoiesis were frequently observed, although these findings were not found to be of specific value for diagnosis.
[image:6.612.90.523.71.472.2]In the present study, BMTB was performed to provide supplementary information for BM smears. This technique facilitates assessment of the overall bone marrow architecture and cellularity, with trephine sections also providing greater sensitivity for the assessment of focal lesions, patchy infiltrates and the presence of fibrosis [10]. Based on these investigations in a small series of patients, the utility of BMTB was
confirmed as an aid to the diagnosis of CMML. Almost all of the patients exhibited hypercellu-larity and showed a significant increase in gran-ulocytic proliferation. A high proportion of pa- tients had monocytosis, and ALIP was clearly observed in CMML. When BM smears contain few megakaryocytes, BMTB is essential for the accurate assessment of megakaryocyte dysplasia.
Fewer CD15-positive cells were detected in AMoL compared with those in CML and CMML; however, in CMML, there was a slight decrease in the numbers of granulocytes expressing CD15 compared with CML. Compared with CML, a slight decrease in the number of myeloid MPO-positive cells was detected in CMML. In addition, the absence of MPO-immunoreactivity is often observed in AMoL [11]. Generally, this is due to the absence of erythroblastic hypopla-sia in CMML, and the decreased numbers of polymorphonuclear granulocytes and mono-cytes from the granulocyte lineage [12]. In the current study, fewer granulocytes expressing MPO and CD15 were observed in CMML com-pared to CML, which is consistent with the report of CD15-staining by Qubaja et al [12], which suggested that this was related to dysgranulopoiesis.
CD34 immunostaining facilitates the reliable identification of patients with an increased number of blasts, based on CD34-positive cells representing 1% of cells in normal bone. Orazi et al [13] examined the use of a CD34-specific antibody as an immunohistochemical tool to distinguish CMML-1, CMML-2 and CMML in acute transformation to AML, and showed that increased numbers of blasts are associated with a poorer prognosis. These results also indicated that the presence of CD34-positive cell clusters is a risk factor for progression to
AML. In our study, CD34-positive blasts were observed in some AMoL and CMML patients, with no significant difference between the two populations, largely because monoblasts are frequently negative for CD34 [14]; however, CD34-positive blasts were rarely seen in CML patients. In addition, CMML patients have some marrow blasts, making them distinguish-able from CMML and AMoL on the basis of CD117 immunostaining. The study reported by Villeneuve et al [15] showed that CD34 and CD117 were detected in only 29.9% and 11.1% of the AMoL patients, respectively.
[image:7.612.83.527.95.177.2]Mature monocytes strongly express CD14, while most immature leukemic monocytes lack CD14 expression. In our study, CD14 expres-sion by monocytes in CMML appeared to be stronger than in AMoL, which is consistent with previous reports that most AMoL patients are CD14-negative [16]. Furthermore, the percent-age of CD14-positive cells in CMML was higher than in CML, which provides further evidence in support of the proposal by Qubaja et al [12] that CD14 expression is helpful in distinguish-ing CMML and CML. Thus, CD14 is useful in distinguishing CMML from AMoL and CML. CD56 expression was weak and very homoge-neous on monocytes. CD56 expression com-bined with underexpression of a myeloid mark-er is unique to CMML monocytes [17]. Fur- thermore, aberrant CD56 expression in mono-cytopoiesis has been reported to be highly characteristic of CMML and AMoL [18]. Wolf-gang et al [19] showed aberrant expression of CD56 by monocytopoietic cells in 100% of AMoL patients and 81.9% of CMML patients. This also showed that monocytes from patients of CMML bear multiple immunophenotypic anomalies, including underexpression of CD15 and aberrant expression of CD56 [20].
Table 4. Percentage of CD15, MPO, CD14, CD56, CD68 (PG-M1), CD163, CD34 and CD117 positive cells in bone marrow trephine biopsies among cases of CMML, AMoL and CML
CD15 (%)
(mean ± SD) (mean ± SD)MPO (%) (mean ± SD)CD14 (%) (mean ± SD)CD56 (%) (mean ± SD)CD68 (%) (mean ± SD)CD163 (%) (mean ± SD)CD34 (%) (mean ± SD)CD117 (%)
CMML (n=26) 21.97±7.15 33.82±6.83 10.30±2.55 8.61±2.99 13.24±4.64 10.50±4.21 4.44±1.98 1.35±0.57 CML (n=30) 40.44±12.6 54.78±12.33 0.97±0.47 0.68±0.40 0.78±0.33 2.57±1.36 0.64±0.42 0 AMoL (n=30) 6.29±3.54 0 5.31±1.38 10.15±2.52 20.89±9.25 23.39±6.02 5.92±3.84 4.83±2.56
P1-values <0.001 <0.001 <0.001 <0.001 <0.001 <0.001 <0.001 <0.001
P2-values <0.001 <0.001 <0.001 0.071 <0.001 <0.001 0.007 <0.001
In our study, the percentage of CD68 (PG-M1)-positive cells, which was highest in AMoL, was higher in CMML than in CML. Similarly, Ngo et al [21] reported significantly higher levels of CD68 (PG-M1)-positive cells in CMML (20.7±6.1%) compared to CML. Furthermore, Marian et al [22] observed significantly increased CD68 (PG-M1)-positive cells were observed in most AMoL patients, but rarely in CMML patients. However, Orazi [13] and Qubaja [12] et al reported that there were no significant differ-ences in the percentages of CD68 (PG-M1)- and CD163-positive cells between CMML and CML patients. In CMML, a greater proportion of monocytes were positive for CD68 (PG-M1) as compared to CD163. In contrast, in AMoL, a higher proportion of monocytes are positive for CD163 as compared to CD68 (PG-M1). Naresh et al [23] also reported that the proportion of CD163-positive cells exceeded that of CD68 (PG-M1) in AMoL, while Ngo et al [21] demon-strated that CD163 was less helpful than CD68 (PG-M1) in identifying monocytes and pro-monocytes in CMML. The results of these stud-ies indicate that CD163 is a more reliable mark-er of early monocytic cells, and is useful in AMoL, where the majority of the cells are atypi-cal or blastic. Based on previous data, CD163 is a more specific marker of disorders of mono-cyte-origin than CD68 (PG-M1) [24]. Moreover, Rollins-Raval et al [22] showed that the increased percentage of CD68 (PG-M1)-posi- tive cells is more sensitive for diagnosing AMoL than CD14.
SETBP1 mutations are found not only in CMML, but also in aCML, secondary acute myeloid leu-kemia, and MDS/MPN [4, 25], with different mutational frequencies in these diseases reported in the range of 4.5-15% [4, 5, 7, 26]. Although there are no significant differences in SETBP1 mutations between CMML-1 and CMML-2 [25], some reports have indicated that patients with SETBP1 mutations have a signifi-cantly worse overall survival (OS) and leukemia-free survival compared with patients with wild-type SETBP1 [4, 7, 27, 28]. Patients with SETBP1 mutations also have significantly high-er WBC counts and lowhigh-er PLT and Hb levels than patients with wild-type SETBP1 [29], sug-gesting that SETBP1 mutations play a role in disease progression [30].
In summary, while the diagnosis of CMML is still based largely on PB and BM smears, we showed
that BMTB investigations, particularly, immuno-histochemical analysis, provides important supplementary information that can aid in the diagnosis of CMML. It is hoped that this approach will improve the diagnostic accuracy of CMML.
Acknowledgements
This study was supported by a project funded by the Priority Academic Programme Deve- lopment of Jiangsu Higher Education Institute (JX10231801), the National Natural Science Foundation of China (Grant No. 81170490), the National Public Health Grand Research Foundation (201202017), the Priority Academic Programme Development of Province Health (Z201402), and the Youth Fund of the National Natural Science Foundation of China (814- 00079). All authors have contributed to, criti-cally reviewed and approved this article. None of the authors has any conflict of interest to declare.
Disclosure of conflict of interest
None.
Address correspondence to: Dr. Hua Lu, Department of Hematology, The First Affiliated Hospital of Nanjing Medical University, Jiangsu Province Hos- pital, Nanjing, China. Tel: +86 25 68135682; Fax: +86 25 83781120; E-mail: luhua1956@aliyun.com; Dr. Su-Jiang Zhang, Department of Hematology, Ruijin Hospital North Affiliated with Shanghai Jiao Tong University School of Medicine, Shanghai, China. Tel: +86 25 68136091; Fax: +86 25 83781120; E-mail: zbruce.zhang@gmail.com
References
[1] Vardiman JW, Thiele J, Arber DA, Brunning RD, Borowitz MJ, Porwit A, Harris NL, Le Beau MM. The 2008 revision of the World Health Organi-zation (WHO) classification of myeloid neo -plasms and acute leukemia: rationale and im-portant changes. Blood 2009; 114: 937-51. [2] Parikh SA, Tefferi A. Chronic myelomonocytic
leukemia: 2012 update on diagnosis, risk stratification, and management. Am J Hematol 2012; 87: 610-9.
[3] Germing U, Kündgen A, Gattermann N. Risk as-sessment in chronic myelomonocytic leukemia (CMML). Leuk Lymphoma 2004; 45: 1311-8. [4] Damm F, Itzykson R, Kosmider O, Droin N,
myelodysplastic syndromes, chronic myelo-monocytic leukemia and secondary acute my-eloid leukemias. Leukemia 2013; 27: 1401-3. [5] Makishima H, Yoshida K, Nguyen N, Przy-chodzen B, Sanada M, Okuno Y, Ng KP, Gud-mundsson KO. Somatic SETBP1 mutations in myeloid malignancies. Nat Genet 2013; 45: 942-6.
[6] Patnaik MM, Parikh SA, Hanson CA, Tefferi A. Chronic myelomonocytic leukaemia: a concise clinical and pathophysiological review. Br J Haematol 2014; 165: 273-86.
[7] Laborde RR, Patnaik MM, Lasho TL, Finke CM, Hanson CA, Knudson RA, Ketterling RP, Parda-nani A. SETBP1 mutations in 415 patients with primary myelofibrosis or chronic myelomono -cytic leukemia: independent prognostic impact in CMML. Leukemia 2013; 27: 2100-2. [8] Such E, Germing U, Malcovati L, Cervera J,
Kuendgen A, Della Porta MG, Nomdedeu B, Ar-enillas L. Development and validation of a prognostic scoring system for patients with chronic myelomonocytic leukemia. Blood 2013; 121: 3005-15.
[9] Germing U, Strupp C, Knipp S, Kuendgen A, Giagounidis A, Hildebrandt B, Aul C, Haas R. Chronic myelomonocytic leukemia in the light of the WHO proposals. Haematologica 2007; 92: 974-7.
[10] Lee SH, Erber WN, Porwit A, Tomonaga M, Pe-terson LC. ICSH guidelines for the standardiza-tion of bone marrow specimens and reports. Int J Lab Hematol 2008; 30: 349-64.
[11] Cibull TL, Thomas AB, O’Malley DP, Billings SD. Myeloid leukemia cutis: a histologic and immu-nohistochemical review. J Cutan Pathol 2008; 35: 180-5.
[12] Qubaja M, Marmey B, Le Tourneau A, Haiat S, Cazals-Hatem D, Fabiani B, Diebold J, Marie JP. The detection of CD14 and CD16 in paraffin-embedded bone marrow biopsies is useful for the diagnosis of chronic myelomonocytic leu-kemia. Virchows Arch 2009; 454: 411-9. [13] Orazi A, Chiu R, O’Malley DP, Czader M, Allen
SL, An C, Vance GH. Chronic myelomonocytic leukemia: The role of bone marrow biopsy im-munohistology. Mod Pathol 2006; 19: 1536-45.
[14] Bacher U, Haferlach T, Schnittger S, Kreipe H, Kröger N. Recent advances in diagnosis, mo-lecular pathology and therapy of chronic my-elomonocytic leukaemia. Br J Haematol 2011; 153: 149-67.
[15] Villeneuve P, Kim DT, Xu W, Brandwein J, Chang H. The morphological subcategories of acute monocytic leukemia (M5a and M5b) share similar immunophenotypic and cytogenetic features and clinical outcomes. Leuk Res 2008; 32: 269-73.
[16] Dunphy CH, Orton SO, Mantell J. Relative con-tributions of enzyme cytochemistry and flow cytometric immunophenotyping to the evalua-tion of acute myeloid leukemias with a mono-cytic component and of flow cytometric immu -nophenotyping to the evaluation of absolute monocytoses. Am J Clin Pathol 2004; 122: 865-74.
[17] Xu Y, McKenna RW, Karandikar NJ, Pildain AJ, Kroft SH. Flow cytometric analysis of mono-cytes as a tool for distinguishing chronic myelo-monocytic leukemia from reactive monocyto-sis. Am J Clin Pathol 2005; 124: 799-806. [18] Raspadori D, Damiani D, Lenoci M, Rondelli D,
Testoni N, Nardi G, Sestigiani C, Mariotti C. CD56 antigenic expression in acute myeloid leukemia identifies patients with poor clinical prognosis. Leukemia 2001; 15: 1161-4. [19] Kern W, Bacher U, Haferlach C, Schnittger S,
Haferlach T. Acute monoblastic/monocytic leu-kemia and chronic myelomonocytic leuleu-kemia share common immunophenotypic features but differ in the extent of aberrantly expressed antigens and amount of granulocytic cells. Leuk Lymphoma 2011; 52: 92-100.
[20] Xu Y, McKenna RW, Wilson KS, Karandikar NJ, Schultz RA, Kroft SH. Immunophenotypic iden-tification of acute myeloid leukemia with mono -cytic differentiation. Leukemia 2006; 20: 1321-4.
[21] Ngo NT, Lampert IA, Naresh KN. Bone marrow trephine morphology and immunohistochemi-cal findings in chronic myelomonocytic leukae -mia. Br J Haematol 2008; 141: 771-81. [22] Rollins-Raval MA, Roth CG. The value of
immu-nohistochemistry for CD14, CD123, CD33, my-eloperoxidase and CD68R in the diagnosis of acute and chronic myelomonocytic leukae-mias. Histopathology 2012; 60: 933-42. [23] Naresh KN. Morphological evaluation of
mono-cytes and monocyte precursors in bone mar-row trephine biopsies - need for establishing diagnostic criteria. Haematologica 2009; 94: 1623-4.
[24] Lau SK, Chu PG, Weiss LM. CD163: a specific marker of macrophages in paraffin-embedded tissue samples. Am J Clin Pathol 2004; 122: 794-801.
[25] Meggendorfer M, Bacher U, Alpermann T, ferlach C, Kern W, Gambacorti-Passerini C, Ha-ferlach T, Schnittger S. SETBP1 mutations oc-cur in 9% of MDS/MPN and in 4% of MPN cases and are strongly associated with atypical CML, monosomy 7, isochromosome i(17) (q10), ASXL1 and CBL mutations. Leukemia 2013; 27: 1852-60.
specific mutation in chronic neutrophilic leuke -mia. Leukemia 2013; 27: 1870-3.
[27] Cristóbal I, Blanco FJ, Garcia-Orti L, Marcote-gui N, Vicente C, Rifon J, Novo FJ, Bandres E. SETBP1 overexpression is a novel leukemo-genic mechanism that predicts adverse out-come in elderly patients with acute myeloid leukemia. Blood 2010; 115: 615-25.
[28] Hou HA, Kuo YY, Tang JL, Chou WC, Yao M, Lai YJ, Lin CC, Chen CY. Clinical implications of the SETBP1 mutation in patients with primary my-elodysplastic syndrome and its stability during disease progression. Am J Hematol 2014; 89: 181-6.
[29] Piazza R, Valletta S, Winkelmann N, Redaelli S, Spinelli R, Pirola A, Antolini L, Mologni L. Recur-rent SETBP1 mutations in atypical chronic my-eloid leukemia. Nat Genet 2013; 45: 18-24. [30] Fernandez-Mercado M, Pellagatti A, Di Genua