MR Imaging, Single photon Emission CT, and Neurocognitive Performance after Mild Traumatic Brain Injury
Full text
Figure
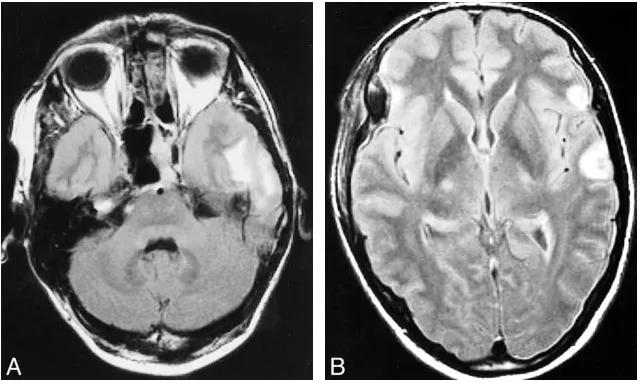
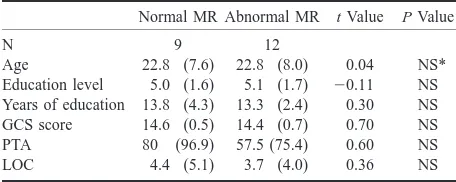
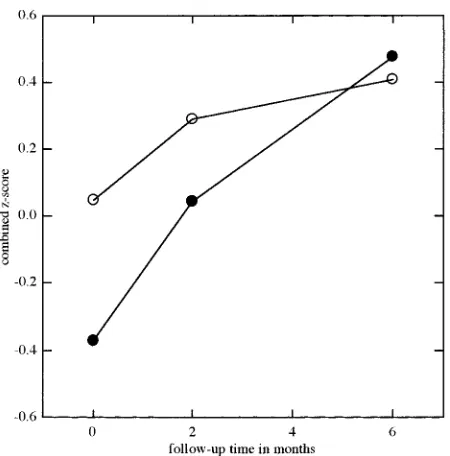
Related documents
There are currently many different assessment formats used around Australia for assessing speech pathology students’ competency in their clinical placements, none of which have
According to these results as indicated in Table 1, one could find that among the most causes of accident is the unlicensed drivers who spread widely in the city without
The overall results of the study indicate that drivers embedded in the environment in which the pension funds function, the characteristics of the pension funds and the
• In terms of building a viable value creation plan, focus on long-term value drivers is the most critical factor, followed by mutual contribution, clear responsibility, and
Effect of stocking rate and supplementation on performance of dairy cows grazing native grassland in small-scale systems in the highlands of central Mexico... Shakhane LM, Mulcahy
To support the argument, this paper provides a prelimi- nary study on the transaction costs borne by firms in the industrial manufacturing sector based on two industrial surveys
The objectives of the research project are to gain more in-depth practical knowledge regarding the factors that affect the success and failure of new product
In the second experiment, it was found that there was interaction between feed types and feeding frequencies which significantly (P<0.05) affected feed consumption,