DESIGN AND EVALUATION OF TORSEMIDE CONTROLLED RELEASE MATRIX TABLETS
Full text
Figure
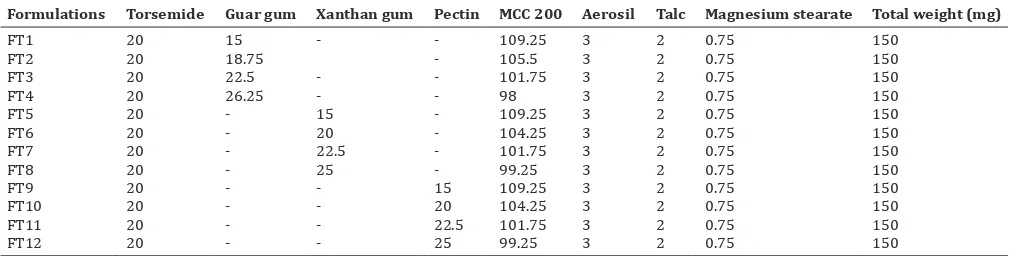
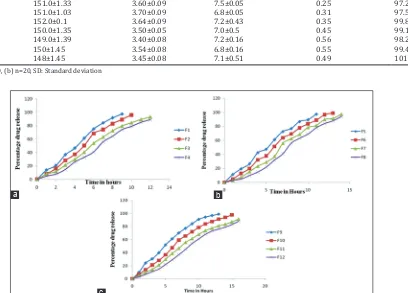

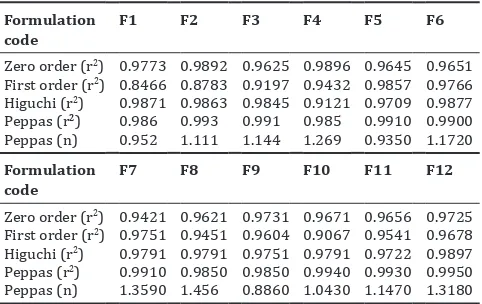
Related documents
AZFc region was highly deleted in infertile male patients compared to AZFa and AZFb region and this reports were congruous with previous research works
Finally temperature distribution in thermosyphon heat pipe (Fig. 9) as we see evaporator side gives the higher temperature and condenser side lower temperature is seen
In our study, patients with earlier stages of CKD have higher baseline sodium intake and they are able to reduce their sodium intake significantly following advice on
Axillary recurrence rate in breast cancer patients with negative sentinel lymph node (SLN) or SLN micrometastases: prospective analysis of 150 patients after SLN biopsy. Bilimoria
Social media enables the marketers to rethink about traditional one way communication flow of their marketing messages and how social media helps to incorporate a new
The proof of our scheme makes use of an algebraic method first used by Boneh and Boyen [1] and the security of our scheme reduces to the decisional Bilinear Diffie-Hellman
Grey scale US shows a well defined iso to hyperechoic lesion with echogenic foci No posterior acoustic enhancement or vascularity demonstrated.. The lesion was surgically
In conclusion, this study suggests that sevoflurane is a suitable inhalational induction agent for children in the Malaysian population and is comparable to