INTRODUCTION
More than two billion people, mostly in tropical coun-tries are at risk from mosquito-borne diseases such as malaria, dengue, haemorrhagic fever and filariasis1. These
infectious diseases mainly impact the tropic’s poorest people. An estimated 50 million people are infected with dengue each year2. Malaria has a crippling effect on
Africa’s economic growth and perpetuates vicious cycles of poverty3. Approximately 300–500 million clinical cases
and >1 million deaths are recorded every year4.
The responsible pathogens are transmitted by bites of blood sucking mosquitoes which are considered to be harmful towards the populations in tropical and subtropi-cal regions5. The genera Culex, Aedes and Anopheles are
the most important vectors involved in diseases transmis-sion to humans.
Although there are proven strategies to control mos-quito-borne diseases, mosquitoes still cause a huge pub-lic health problem in Africa. Across African people are
Larvicidal potential of some plants from West Africa against
Culex
quinquefasciatus
(Say) and
Anopheles gambiae
Giles (Diptera: Culicidae)
Alain Azokou
1, 2, Mamidou W. Koné
1, 2,
Benjamin G. Koudou
1, 2, 3& Honora F. Tra Bi
11UFR Sciences de la Nature, Université Nangui Abrogoua, Abidjan, Côte d’Ivoire, 2Centre Suisse de Recherches Scientifiques en Côte
d’Ivoire, Abidjan, Côte d’Ivoire; 3Vector Group/Liverpool School of Tropical Medicine, Liverpool, UK
ABSTRACT
Background & objectives: Mosquitoes increased resistance to insecticides, and environmental concerns about the use of insecticides, pose a major challenge in the search for new molecules to deplete and incapacitate mosquito populations. Plants are the valuable source as practices consisting in exploiting plant materials as repellents, and are still in wide use throughout developing countries. The aim of the present study was to screen plants from Côte d’Ivoire for larvicidal activity against mosquitoes.
Methods: Resistant and sensitive larvae (III and IV instar) of Anophelesgambiae and Culex quinquefasciatus
were exposed to crude ethanol extracts (90%) of 45 plants and viability observed after 30 min, 6, 12 and 24 h post-incubation. After partition of active extracts, each fraction (hexane and chloroform washed with NaCl 1%, tannins and aqueous) was tested using the same protocol at various concentrations (1000–31.2 ppm).
Results: Of 49 extracts tested, 7 exhibited high potential (LC50 = 80 to 370 ppm) against resistant and sensitive III and IV instar larvae of An.gambiae and Cx. quinquefasciatus. These extracts were from Cissus populnea,
Cochlospermum planchonii, Heliotropium indicum, Phyllanthus amarus, Vitex grandifolia and Alchornea cordifolia. However, three most active plant species (LC50 = 80–180 ppm) were Cs. populnea, Cm. planchonii and P. amarus. Their hexane and chloroform fractions showed high larvicidal activity.
Conclusion: This study demonstrated that plants from Côte d’Ivoire have a real potential for malaria, yellow fever, filarial and dengue vector control. Those could be used as sources or provide lead compounds for the development of safe plant-based biocides.
Key words Anophelesgambiae;Culex quinquefasciatus; larvicidal activity; plants; phytochemistry; West Africa
exposed to mosquito bites because the larval habitats are widely distributed in humid areas such as flood areas and rice farms. These sites with larvae might be altered to decrease the mosquito population for the interruption of disease transmission. One of the strategies recommended by WHO is the use of organochlorines (DDT, endosul-fan), organophosphates (parathion, temephos) and car-bamates. However, these chemical interventions are se-verely compromised by the development of insecticide resistance in some mosquito vectors and environmental concerns6–8. Also in many African countries the most
widely tested interventions based on bednets treated with pyrethroid, have been difficult to implement correctly because of problems related to cost and acceptability8,
This situation highlights the need to search for new efficient products with fewer effects on environment9.
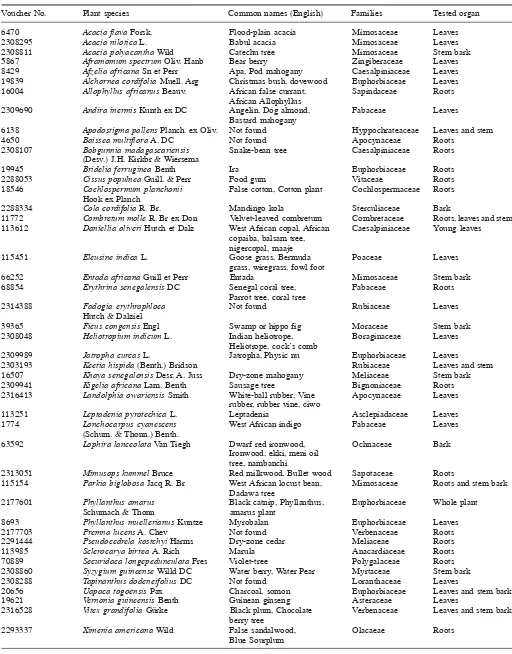
com-Table 1. Plant species selected for larvicidal screening
Voucher No. Plant species Common names (English) Families Tested organ
6470 Acacia flava Forsk. Flood-plain acacia Mimosaceae Leaves
2308295 Acacia nilotica L. Babul acacia Mimosaceae Leaves
2308811 Acacia polyacantha Wild Catechu tree Mimosaceae Stem bark
5867 Aframomum spectrum Oliv. Hanb Bear berry Zingiberaceae Leaves 8429 Afzelia africana Sn et Perr Apa, Pod mahogany Caesalpiniaceae Leaves 19839 Alchornea cordifolia Muell. Arg Christmas bush, dovewood Euphorbiaceae Leaves
16004 Allophyllus africanus Beauv. African false currant, Sapindaceae Roots
African Allophyllus
2309690 Andira inermis Kunth ex DC Angelin, Dog almond, Fabaceae Leaves
Bastard mahogany
6138 Apodostigma pallens Planch. ex Oliv. Not found Hyppochrateaceae Leaves and stem
4650 Baissea multiflora A. DC Not found Apocynaceae Roots
2308107 Bobgunnia madagascariensis Snake-bean tree Caesalpiniaceae Roots
(Desv.) J.H. Kirkbr & Wiersema
19945 Bridelia ferruginea Benth Ira Euphorbiaceae Roots
2288053 Cissus populnea Guill. & Perr Food gum Vitaceae Roots
18546 Cochlospermum planchonii False cotton, Cotton plant Cochlospermaceae Roots
Hook ex Planch
2288334 Cola cordifolia R. Br. Mandingo kola Sterculiaceae Bark
11772 Combretum molle R. Br ex Don Velvet-leaved combretum Combretaceae Roots, leaves and stem 113612 Daniellia oliveri Hutch et Dalz West African copal, African Caesalpiniaceae Young leaves
copaiba, balsam tree, nigercopal, maaje
115451 Eleusine indica L. Goose grass, Bermuda Poaceae Leaves
grass, wiregrass, fowl foot
66252 Entada africana Guill et Perr Entada Mimosaceae Stem bark
68854 Erythrina senegalensis DC Senegal coral tree, Fabaceae Roots
Parrot tree, coral tree
2314388 Fadogia erythrophloea Not found Rubiaceae Leaves
Hutch & Dalziel
39365 Ficus congensis Engl Swamp or hippo fig Moraceae Stem bark
2308048 Heliotropium indicum L. Indian heliotrope, Boraginaceae Leaves
Heliotrope, cock’s comb
2309989 Jatropha curcas L. Jatropha, Physic nu Euphorbiaceae Leaves
2303193 Keetia hispida (Benth.) Bridson Rubiaceae Leaves and stem
16507 Khaya senegalensis Desr. A. Juss Dry-zone mahogany Meliaceae Stem bark
2309941 Kigelia africana Lam. Benth Sausage tree Bignoniaceae Roots
2316413 Landolphia owariensis Smith White-ball rubber, Vine Apocynaceae Leaves
rubber, rubber vine, ciwo
113251 Leptadenia pyrotechica L. Leptadenia Asclepiadaceae Leaves
1774 Lonchocarpus cyanescens West African indigo Fabaceae Leaves (Schum. & Thonn.) Benth.
63592 Lophira lanceolata Van Tiegh Dwarf red ironwood, Ochnaceae Bark
Ironwood, ekki, meni oil tree, nambanchi
2313051 Mimusops kummel Bruce Red milkwood, Bullet wood Sapotaceae Roots
115154 Parkia biglobosa Jacq R. Br West African locust bean, Mimosaceae Roots and stem bark Dadawa tree
2177601 Phyllanthus amarus Black catnip, Phyllanthus, Euphorbiaceae Whole plant
Schumach & Thonn amarus plant
8693 Phyllanthus muellerianus Kuntze Myrobalan Euphorbiaceae Leaves
2177703 Premna lucens A. Chev Not found Verbenaceae Roots
2291444 Pseudocedrela kostchyi Harms Dry-zone cedar Meliaceae Roots
113985 Sclerocarya birrea A. Rich Marula Anacardiaceae Roots
70889 Securidaca longepedunculata Fres Violet-tree Polygalaceae Roots
2308860 Syzygium guineense Willd DC Water berry, Water Pear Myrtaceae Stem bark
2308288 Tapinanthus dodeneifolius DC Not found Loranthaceae Leaves
20656 Uapaca togoensis Pax Charcoal, somon Euphorbiaceae Leaves and stem bark
19621 Vernonia guineensis Benth Guinean ginseng Asteraceae Leaves
2316528 Vitex grandifolia Gürke Black plum, Chocolate Verbenaceae Leaves and stem bark
berry tree
2293337 Ximenia americana Wild False sandalwood, Olacaeae Roots
pounds6 and related commercial insecticides are
com-monly perceived as “safe” in comparison to synthetic re-pellents10. Traditionally plant based repellents have been
used for generations as protection measures against mos-quitoes. These are still extensively used throughout rural communities in Benin11, Tanzania12and Côte d’Ivoire.
These plants are burned overnight in rooms to drive away nuisance mosquitoes. Some of these African plants have been shown to be larvicides13, 14.
The present study investigated 45 plants from West Africa for larvicidal activity against mosquitoes as safer natural alternatives to synthetic molecules. Most of the selected plants have been used for medicinal purposes for a long time, because these are not harmful to either humans or domestic animals.
MATERIAL & METHODS
Preparation of extracts
The plant species studied were selected on the basis of criteria (Table 1), such as lack of information on activity against Anopheles and Culex larvae, botanical families (Euphorbiaceae, Verbenaceae, and Meliaceae) from which number of larvicidal plant were reported and large distribution in West Africa. Voucher specimens are deposited at the herbarium (Base ivoire) of Centre Suisse de Recherches Scientifiques en Côte d’Ivoire, Adiopodoumé.
A quantity of the different plant parts were col-lected from April to October 2005 in the region of Ferkessedougou (northern Côte d’Ivoire), located in the Savanna area (9–11°N, 4– 7°W) of the Côte d’Ivoire. The roots, leaves and stem bark were dried in an air-condi-tioned room (22°C) and pounded by hand in a mortar. A quantity of 10 g of the powder was extracted with 100 ml of 90% ethanol under mechanical stiring (150 rpm) dur-ing 24 h and then filtered. The extracts were concentrated in a rotary evaporator (Rotavapor) at 40°C and lyophilized. In all, 49 extracts have been prepared for in vitro larvi-cidal screening.
Mosquito larvae tested
The larvae included wild resistant An. gambiae
strains, resistant Cx. quinquefasciatus strains, and sensi-tive Kisumu strain (from Kenya). The resistant strains were collected from breeding sites around the village of Adiopodoumé, located in the northern peri-urban part of Abidjan. These sites were selected because of their prox-imity to Centre Suisse de Recherches Scientifiques en Côte d’Ivoire (CSRS). Breeding sites located in this
vil-lage were around crop farms. After collection, the III and
IV instar larvae were transferred in plastic bottles and maintained at the laboratory.
The susceptible strain (Kisumu) was provided by the insectarium of CSRS. The eggs were put in distilled wa-ter maintained at 21–22°C and safe from contaminations. Eggs hatched after 24 h and larvae were fed with pow-dered cat kibble.
Larval bioassays
Larvicidal activity was assessed as per the protocol previously described by WHO with slight modification15.
The assays were performed in two steps: (i) Detection of susceptibility of larvae to extracts; and (ii) Determina-tion of larvicidal concentraDetermina-tion (LC95). The sensitivity of the larvae to the extracts was determined at single con-centration (1000 ppm). In 220 μl of distilled water or dimethylsulfoxide (DMSO) 220 mg of extract was dis-solved. Then 100 μl of extract solution was added to 5 ml of water from breeding site (wild strain) or distilled wa-ter (Kisumu strain). The final volume was adjusted to 10 ml and 20 larvae were added to each tube. A control tube containing only distilled water or 0.1% DMSO was pre-pared. Mortality is assessed by direct observation of lar-vae movements. An extract is active if 100% of larlar-vae died between 30 min and 24 h16. The tests were repeated
three times.
The extracts showing larvicidal activity at 1000 ppm were further diluted from 1000 to 31.2 ppm. The viability of larvae was observed after 30 min, 6, 12 and 24 h and scored according to larvae movements and physiological state: 0 = Dead larvae; 1 = Low or almost absence of movement; 2 = Activity; and 3 = Hyperactivity. The num-ber of dead larvae was counted to determine the mortal-ity rate and monitored for determination of KT50, the time required to kill 50% of the larvae.
Partition of active extracts
The three most active extracts were subjected to a liquid-liquid partition with different solvents of increas-ing polarity. In whole 10 g of plant powder was extracted with ethanol 90% using 10-fold solvent under mechani-cal stiring (150 rpm) during 14 h. The filtrate was succes-sively partitioned with hexane, chloroform and water. The chloroform fraction was washed with NaCl 1% (1 g/100 ml water) in order to remove tannins. All fractions were evaporated in a rotary evaporator to dryness at 40°C and lyophilized.
Larvicidal test with fractions prepared from active ex-tracts
against III and IV instar larvae of An. gambiae and Cx.
quinquefasciatus where 11 mg of each fraction was dissolved in 110 μl of DMSO. The test was performed as mentioned above. Mortality was assessed visually by direct observation of larvae movements. A fraction is active if 100% of larvae died between 30 min and 24 h of exposure.
TLC phytochemical analysis
Plant extracts (hexane, and chloroform) showing lar-vicidal activity were investigated by thin layer chroma-tography (TLC). TLC plates were prepared from 10 μl of extract solution (10 mg/ml in methanol) on silicagel 60 F254 plates (aluminum), developed in hexane-ethyl acetate (1:1) as mobile phase. After drying, the chromatograms were analyzed at 254 and 366 nm, pre- and post-spraying with specific reagents according to the nature of
chemi-cals17–19.The retention factor (Rf) values were calculated,
using the following formula:
Distance moved by the compound Distance moved by the solvent front
RESULTS
In this study, we investigated the larvicidal activities of 45 plants, traditionally used in Côte d’Ivoire. Of the 49 ethanol crude extracts 7 (14.29%) showed high activ-ity against III and IV instar larvae of Anopheles and Culex
at 1000 ppm 24 h post-exposure. These seven extracts were obtained from six plant species: A. cordifolia, P.
amarus, H. indicum, C. populnea, V. grandifolia and Cm.
planchonii. Six of the extracts had effect on viability of susceptible and resistant larvae of Anopheles, resulting
Rf =
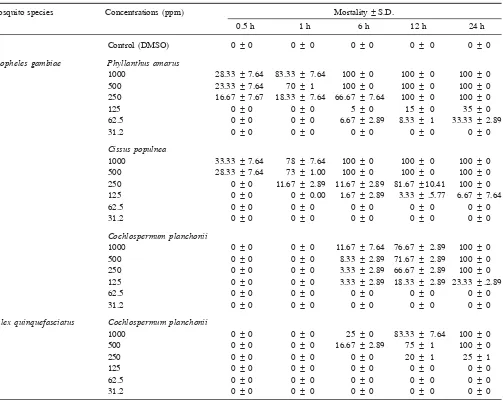
Table 2. Mortality rates of resistant larvae of Anopheles gambiae and Culexquinquefasciatus in the presence of active plant species
Mosquito species Concentrations (ppm) Mortality ± S.D.
0.5 h 1 h 6 h 12 h 24 h
Control (DMSO) 0 ± 0 0 ± 0 0 ± 0 0 ± 0 0 ± 0
Anopheles gambiae Phyllanthus amarus
1000 28.33 ± 7.64 83.33 ± 7.64 100 ± 0 100 ± 0 100 ± 0
500 23.33 ± 7.64 70 ± 1 100 ± 0 100 ± 0 100 ± 0
250 16.67 ± 7.67 18.33 ± 7.64 66.67 ± 7.64 100 ± 0 100 ± 0
125 0 ± 0 0 ± 0 5 ± 0 15 ± 0 35 ± 0
62.5 0 ± 0 0 ± 0 6.67 ± 2.89 8.33 ± 1 33.33 ± 2.89
31.2 0 ± 0 0 ± 0 0 ± 0 0 ± 0 0 ± 0
Cissus populnea
1000 33.33 ± 7.64 78 ± 7.64 100 ± 0 100 ± 0 100 ± 0
500 28.33 ± 7.64 73 ± 1.00 100 ± 0 100 ± 0 100 ± 0
250 0 ± 0 11.67 ± 2.89 11.67 ± 2.89 81.67 ± 10.41 100 ± 0
125 0 ± 0 0 ± 0.00 1.67 ± 2.89 3.33 ± .5.77 6.67 ± 7.64
62.5 0 ± 0 0 ± 0 0 ± 0 0 ± 0 0 ± 0
31.2 0 ± 0 0 ± 0 0 ± 0 0 ± 0 0 ± 0
Cochlospermum planchonii
1000 0 ± 0 0 ± 0 11.67 ± 7.64 76.67 ± 2.89 100 ± 0
500 0 ± 0 0 ± 0 8.33 ± 2.89 71.67 ± 2.89 100 ± 0
250 0 ± 0 0 ± 0 3.33 ± 2.89 66.67 ± 2.89 100 ± 0
125 0 ± 0 0 ± 0 3.33 ± 2.89 18.33 ± 2.89 23.33 ± .2.89
62.5 0 ± 0 0 ± 0 0 ± 0 0 ± 0 0 ± 0
31.2 0 ± 0 0 ± 0 0 ± 0 0 ± 0 0 ± 0
Culex quinquefasciatus Cochlospermum planchonii
1000 0 ± 0 0 ± 0 25 ± 0 83.33 ± 7.64 100 ± 0
500 0 ± 0 0 ± 0 16.67 ± 2.89 75 ± 1 100 ± 0
250 0 ± 0 0 ± 0 0 ± 0 20 ± 1 25 ± 1
125 0 ± 0 0 ± 0 0 ± 0 0 ± 0 0 ± 0
62.5 0 ± 0 0 ± 0 0 ± 0 0 ± 0 0 ± 0
31.2 0 ± 0 0 ± 0 0 ± 0 0 ± 0 0 ± 0
in death of larvae.
Alchornea cordifolia extract exhibited activity only against Kisumu strain. The extract of Cm. planchonii was the only active against larvae of Culex. Mere weak or no effect on larvae was observed following exposure to the remaining 42 extracts.
The decrease in viability is more pronounced at high concentrations from 1000 to 250 ppm at all examination points. At the lowest concentrations, no effect on larvae was observed with any of the extracts tested. These re-sults show a dose response activity.
Phyllanthus amarus and Cs. populnea caused 100% mortality of resistant larvae of Anopheles after 6 h con-tact (Table 2). The mortality rates range between 6.67 and 100% for Anopheles and 25–100% for Culex.
The most active extracts causing 100% mortality of larvae were Cm. planchonii, P. amarus and Cs. populnea
24 h post-incubation. For these extracts, the LC50 were 80–180 ppm against Anopheles and 370 ppm against
Culex (Table 3).Phyllanthus amarus and Cs. populnea
killed resistant An. gambiae, with KT50 ranged between 41 and 42 min. Cochlospermum planchonii caused death of Anopheles and Culex with KT50 values of 125 and 145 min respectively.
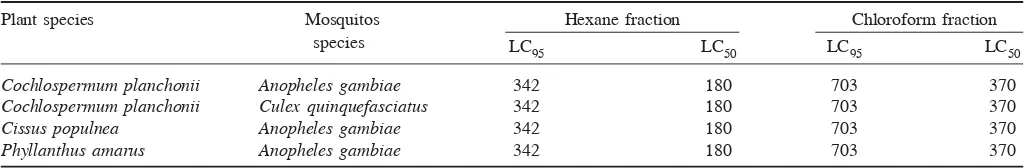
Incubation with extract of Cm. planchonii (Table 3) and related fractions (hexane, and chloroform) resulted in death of larvae of both Anopheles and Culex, at LC50 values ranging between 80 and 370 ppm (Table 4).
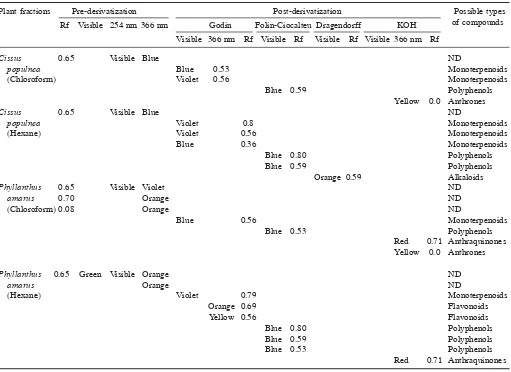
Following exposure to Cs. populnea extract, sensi-tive and resistant larvae of An. gambiae diedatLC50 val-ues of 80 and 180 ppm respectively. Its hexane fraction was more active (LC50 = 180 ppm) than the chloroform fraction, LC50 =370 ppm (Table 4). The TLC phytochemi-cal analysis revealed at least trace amount of monoter penoids, polyphenols and alkaloids (Table 5).
In this study P. amarus exhibited high larvicidal po-tential against An. gambiae (LC50 =80–180 ppm). Incu-bation with derivatives (hexane and chloroform) caused death of larvae at LC50 =180–370 ppm between 12–24 h (Table 4). No effect was observed with aqueous and tannin fractions. Preliminary phytochemical studies have shown presence of monoterpenoids, flavonoids, anthrones and anthraquinones (Table 5).
DISCUSSION
Plant phytochemicals have more specific effects and could be usefully integrated with other control measures to design comprehensive, appropriate and effective man-agement protocols with less collateral harm to the envi-ronment and non-target species20.
Exposures to studied plants resulted in death of sus-ceptible and resistant larvae of An. gambiae. For the ac-tive plant species, the mortality rates range between 6.67 and 100% for Anopheles and 25–100% for Culex after 24
Table 3. LC50 and LC95 (ppm) of ethanol extracts of active plant species on III and IV instar larvae of Anopheles gambiae and
Culexquinquefasciatus
Plant species Plant parts Anopheles gambiae Culex quinquefasciatus
Sensitive strain Kissumu Resistant strain Resistant strain
LC50 LC95 LC50 LC95 LC50 LC95
Cochlospermum planchonii Roots 80 22.22 180 342 370 703
Phyllanthusamarus Whole plant 80 22.22 180 342 ND ND
Heliotropium indicum Leaves 180 342 370 703 ND ND
Cissus populnea Roots 80 22.22 180 342 ND ND
Vitex grandifolia Leaves 180 22.22 370 703 ND ND
Vitex grandifolia Stem bark 180 342 370 703 ND ND
ND = Not determined.
Table 4. LC95 and LC50 (ppm) of chloroform and hexane fractions
Plant species Mosquitos Hexane fraction Chloroform fraction
species LC
95 LC50 LC95 LC50
Cochlospermum planchonii Anopheles gambiae 342 180 703 370
Cochlospermum planchonii Culex quinquefasciatus 342 180 703 370
Cissus populnea Anopheles gambiae 342 180 703 370
h exposure. In a previous study, Schinus terebinthifolia
essential oil displayed activity after 72 h, the mean mor-tality percentage ranged from 0.5 to 96.75% for Cx. quinquefasciatus and 13.75 to 97.91% for An. gambiae12. Cochlospermum planchonii, Cs. populnea and P.
amarus extracts caused cent percent mortality of larvae 24 h post-incubation, with LC50 of 80–180 ppm and LC95 values of 22.22–342 ppm. Other active species such as A.
cordifolia, H. indicum and V. grandifolia exhibited activity with LC50 and LC95 values ranging between 180–370 and 342–703 ppm respectively. Following par-tition of crude extracts and larvicidal assays, only hexane and chloroform fractions exhibited activity against lar-vae, with LC50 and LC95 values of 180 and 342 ppm re-spectively for hexane. Chloroform fraction showed LC50 and LC95 values of 370 and 703 ppm respectively. The results revealed that increased larval mortality was ob-served with increased concentration of the extracts tested against An. gambiae and Cx. quinquefasciatus. Similar
finding was obtained against An. stephensi with the leaf of Adansonia digitata4. Chloroform extract of the plant
showed LC50 and LC90 values of 88.55 and 168.14 ppm respectively, while its hexane extract showed LC50 and LC90 of 111.32 and 178.63 ppm respectively in 24 h. However, in the present study, hexane fractions displayed stronger potential than chloroform fractions against An. gambiae and Cx.quinquefasciatus.
The KT50 values ranged between 41–125 and 145 min against resistant An. gambiae and Cx. quinquefasciatus
respectively. Previous study demonstrated that the time required to knock down 50% of the wild adult An. gambiae
in Tanzania was 11.29 min for S. terebinthifolia essential oil12.
The larvicidal activity of some studied plants such as
Cm. planchonii, H. indicum and A. cordifolia was reported against Ae. aegypti14. The ethanolic extracts of these
spe-cies caused death of larvae 30 min and 24 h post-in-cubation respectively at single concentration tested of
Table 5. Possible compounds present in fractions of the most active plants
Plant fractions Pre-derivatization Post-derivatization Possible types
Rf Visible 254 nm 366 nm Godin Folin-Ciocalteu Dragendorff KOH of compounds
Visible 366 nm Rf Visible Rf Visible Rf Visible 366 nm Rf
Cissus 0.65 Visible Blue ND
populnea Blue 0.53 Monoterpenoids
(Chloroform) Violet 0.56 Monoterpenoids
Blue 0.59 Polyphenols
Yellow 0.0 Anthrones
Cissus 0.65 Visible Blue ND
populnea Violet 0.8 Monoterpenoids
(Hexane) Violet 0.56 Monoterpenoids
Blue 0.36 Monoterpenoids
Blue 0.80 Polyphenols
Blue 0.59 Polyphenols
Orange 0.59 Alkaloids
Phyllanthus 0.65 Visible Violet ND
amarus 0.70 Orange ND
(Chloroform) 0.08 Orange ND
Blue 0.56 Monoterpenoids
Blue 0.53 Polyphenols
Red 0.71 Anthraquinones Yellow 0.0 Anthrones
Phyllanthus 0.65 Green Visible Orange ND
amarus Orange ND
(Hexane) Violet 0.79 Monoterpenoids
Orange 0.69 Flavonoids
Yellow 0.56 Flavonoids
Blue 0.80 Polyphenols
Blue 0.59 Polyphenols
Blue 0.53 Polyphenols
Red 0.71 Anthraquinones
500 μg/ml. The present study gave further data on the potential use of these plants against malaria, yellow fe-ver, filarial and dengue vector control.
The active plants contain phytochemicals such as monoterpenoids and flavonoids. The fresh rhizomes of
Cm. planchonii yield essential oils, with a high rate of oxygenated compounds (86.4% of ketones and esters)21. Cissuspopulnea and P. amarus also contained essential oils22, 23.Several authors have demonstrated strong
re-sponses of mosquito odour receptors to volatiles produced by plants. Essential oils were found to be larvicidal against
Anopheles and Culex17, 24, 25. The finding of the present
study is in line with the high potential of non-polar (dichloromethane, chloroform and hexane) extracts4, 19
demonstrated against mosquito larvae.
The selection of plants based on their botanical fam-ily or genus can be valuable criteria for identifying high larvicidals. Of the 7 active species, 2 were Euphorbiaceae. Several species of this family were reported to be larvi-cidal against mosquitos. Ricinus communis26, Acalypha indica27, and Acalypha alnifolia1 have shown activity
against resistant and susceptible Anopheles and Culex
larvae. Alchornea cordifolia, Bridelia aubrevillei and
B. grandis caused death of Ae. aegypti18. Thus,
Euphorbiaceae is a promising family for vector control.
Vitex grandifolia displayed activity on resistant and susceptible larvae of Anopheles; disappointingly in this study, the species lacked activity against Cx. quinquefasciatus. Other species of the same genus, V. trifolia, V. peduncularis and V. altissima exhibited activ-ity on IV instar larvae of Cx. quinquefasciatus28. The
ex-tract of V. negundo was repellent against adult mosqui-toes29. Therefore, there is no doubt that Vitex spp are of
great interest in control ofmosquitoes.
This is the first hand report of the larvicidal activity of studied plants discussing whether some are well-known for treating malaria. Phytochemical investigations, repel-lent study and field evaluation are ongoing.
CONCLUSION
In the present study, the larvicidal potential of 45 plants from West Africa was evaluated against sensitive and resistant An. gambiae and Cx. quinquefasciatus. Some of these plants exhibited high larvicidal activity. The re-sults show that some of plants traditionally used in West Africa could gain place in control of African malaria vectors. The efficacy exhibited by these plants has given an opportunity for further investigation on eggs and adult mosquitoes and to evaluate them in small-scale field trials.
ACKNOWLEDGEMENTS
The authors would like to sincerely address their deep-est acknowledgements to the Centre Suisse de Recherches Scientifiques en Côte d’Ivoire (CSRS) for financially sup-porting this study. They would also like to thank the Nangui Abrogoua University for the scientific and aca-demic support.
REFERENCES
1. Murugan K, Kovendan K, Vincent S, Barnard DR. Biolarvicidal and pupicidal activity of Acalypha alnifolia Klein ex Willd. (Fam-ily: Euphorbiaceae) leaf extract and microbial insecticide,
Metarhizium anisopliae (Metsch.) against malaria fever mosquito,
Anopheles stephensi Liston. (Diptera: Culicidae). Parasitol Res
2012; 110: 2263–70.
2. Report on global surveillance of epidemic-prone infectious dis-eases: Dengue and dengue hemorrhagic fever (chapter 6) 2008. Available from: http://www.who.int/csr/resources/publications/ surveillance/ (Accessed on May 2011).
3. Malaria: A major cause of child death and poverty in Africa 2004. Available from: http://rbm.who.int/docs/rps publications/ unicef_malaria_fr.pdf (Accessed on July 2012).
4. Krishnappa K, Elumalai K, Dhanasekaran S, Gokulakrishnan J. Larvicidal and repellent properties of Adansonia digitata against medically important human malarial vector mosquito
Anopheles stephensi (Diptera: Culicidae). J Vector Borne Dis
2012; 49: 86–90.
5. Rahuman AA, Bagavan A, Kamaraj C, Vadivelu M, Zahir AA, Elango G, et al. Evaluation of indigenous plant extracts against larvae of Culex quinquefasciatus Say (Diptera : Culicidae).
Parasitol Res 2009; 104: 637–43.
6. Ruikar AD, Pawar PV, Sen A, Phalgune UD, Puranik VG, Deshpande N R. Larvicidal potential of Mimusops elengi against
Aedes aegypti (L) and Culex quinquefasciatus (Say). J Vector Borne Dis 2012; 49: 111–13.
7. Chouaibou MS, Chabi J, Bingham GV, Knox TB, N’dri L, Kesse NB, et al. Increase in susceptibility to insecticides with aging of wild Anopheles gambiae mosquitoes from Côte d’Ivoire. BMC Infect Dis 2012; 12: 214.
8. Promsiri S, Naksathit A, Kruatrachue M, Thavara U. Evalua-tions of larvicidal activity of medicinal plant extracts to Aedes aegypti (Diptera : Culicidae) and other effects on a non-target fish. Insect Sci 2006; 13: 179–88.
9. Oliveira PV, Ferreira JC, Moura FS, Lima GS, de Oliveira FM, Oliveira PE, et al. Larvicidal activity of 94 extracts from ten plant species of northeastern of Brazil against Aedes aegypti L. (Diptera : Culicidae). Parasitol Res 2010; 107: 403–7. 10. Maia MF, Moore SJ. Plant-based insect repellents: A review of
their efficacy, development and testing. Malar J 2011; 10: S11. 11. Abagli AZ, Alavo TBC. Essential oil from bush mint, Hyptis suaveolens, is as effective as DEET for personal protection against mosquito bites. Open Entomol J 2011; 5: 45–8.
12. Kweka EJ, Nyindo M, Mosha F, Silva AG. Insecticidal activity of the essential oil from fruits and seeds of Schinus terebinthifolia
Raddi against African malaria vectors. Parasit Vectors 2011; 4:
129.
gambiae Giles et composition chimique des huiles essentielles extraites de quatre plantes cultivées au Cameroun. Biotechnol Agron Soc Environ 2009; 13: 77–84.
14. Kamanzi KA, Koné M, Traoré D. Activité larvicide et mollusci-cide des plantes médicinales de Côte d’Ivoire Bioterre. Rev inter Sci vie et terre 2002; spécial: 22–9.
15. Guidelines for laboratory and field testing of mosquito larvi-cides. WHO/CDS/WHOPES/GCDPP/ 2005.13.
16. Cepleanu F. Validation and application of three beech-top bioassays for screening of crude plant extracts and subsequent activity – Guided isolation. PhD Thesis. Lausanne: University of Lausanne 1993; p. 259.
17. Mallikharjuna PB, Rajanna LN, Seetharam YN, Sharanabasappa GK. Phytochemical studies of Stychnos potatorum Lf:A medi-cal plant. E-J Chem 2007; 4: 510–8.
18. Wagner H, Bladt S, Zgainski EM. Plant drug analysis: A thin layer chromatography atlas. Berlin: Springer-Verlag 1984; p. 368.
19. Chaaib KF. Investigation phytochimique d’une brosse à dents africaine Zanthoxylum zanthoxyloides (Lam.) Zepernick et Timler syn. Fagara zantrhoxyloides L. (Rutaceae)- Thèse de doctorat.
Lausanne : Université de Lausanne 2004; p. 211.
20. Silva AG, Almeida DL, Ronchi SN, Bento AC, Scherer R, Ramos AC, et al. The essential oil of Brazilian pepper, Schinus terebinthifolia Raddi in larval control of Stegomyia aegypti
(Linnaeus, 1762). Parasit Vectors 2010; 3: 79.
21. Ouattara L, Kondou J, Obame LC, Karou DS, Traoré A, Bessière JM. Chemical composition and antibacterial activity of
Cochlospermum planchonii Hook. f. ex Planch essential oil from Burkina Faso. Pak J Biol Sci 2007; 10: 4177–9.
22. Pandey SK, Upadhyay S, Tripathi AK. Insecticidal and repellent activities of thymol from the essential oil of Trachyspermum ammi
(Linn) Sprague seeds against Anopheles stephensi. Parasitol Res
2009; 105: 507–12.
23. Koliopoulos G, Pitarokili D, Kioulos E, Michaelakis A, Tzakou O. Chemical composition and larvicidal evaluation of Mentha,
Salvia and Melissa essential oils against the West Nile virus mos-quito Culex pipiens. Parasitol Res 2010; 107: 327–35. 24. Osibote EAS, Ogunlesi M, Okiei W, Asekun T, Familoni OB.
Assessment of antimicrobial activity of the essential oil from the stem powder of Cissus populnea and the leaves of Sesamum radiatum, herbal medications for male infertility factor. Res J Med Plant 2010; 4: 14–20.
25. Koffi NG, Tiébré MS, Aké AE, Zirihi GN. Etude ethnobotanique des plantes utilisées pour traiter l’hypertension artérielle en médecine traditionnelle par les populations Abbey et Krobou d’Agboville (Côte d’Ivoire). J Eur Sci Rech 2009; 35: 85–98. 26. Govindarajan M, Jebaneson A, Pushpanathan T, Samiduraï K.
Studies on effect of Acalypha indica (Euphorbiaceae) leaf ex-tracts on the malaria vector, Anopheles stephensi. Parasitol Res
2008; 103: 691–5.
27. Aouinty B, Oufara S, Mellouki F, Mahari S. Evaluation préliminaire de l’activité larvicide des extraits aqueux des feuilles du ricin (Ricinus communis L.) et du bois de thuya (Tetraclinis articulata Vahl.) sur les larves de quatre moustiques culicudés :
Culex pipiens (Linn.), Aedes caspius (Pallas), Culiseta longiareolata (Aitken) et Anopheles maculipennis (Meigen).
Biotechnol Agron Soc Environ 2006; 10: 67–71.
28. Kannaathsan K, Senthilkumar A, Chandrasekaran M, Venkatesalu V. Differential larvicidal efficacy of four species of
Vitex against Culex quinquefasciatus larvae. Parasitol Res 2007;
101: 1721–3.
29. Karunamoorthi K, Ramanujam S, Rathinasamy R. Evaluation of leaf extracts of Vitex negundo L. (Family: Verbenaceae) against larvae of Culex tritaeniorhynchus and repellent activity on adult vector mosquitoes. Parasitol Res 2008; 103: 545–50.
Correspondence to: Prof. M.W. Koné, Centre Suisse de Recherches Scientifiques en Côte d’Ivoire, 01 BP 1303, Abidjan 01, Côte d’Ivoire. E-mail: mamidou.kone@csrs.ci