Copyright2001 by the Genetics Society of America
Molecular Nature of 11 Spontaneous
de Novo
Mutations in
Drosophila melanogaster
Hsiao-Pei Yang,* Ana Y. Tanikawa* and Alexey S. Kondrashov
†*Department of Ecology and Evolutionary Biology, Cornell University, Ithaca, New York 14853 and†National Center for Biotechnology Information, National Institutes of Health, Bethesda, Maryland 20894
Manuscript received June 23, 2000 Accepted for publication November 27, 2000
ABSTRACT
To investigate the molecular nature and rate of spontaneous mutation in Drosophila melanogaster, we screened 887,000 individuals forde novorecessive loss-of-function mutations at eight loci that affect eye color. In total, 28 mutants were found in 16 independent events (13 singletons and three clusters). The molecular nature of the 13 events was analyzed. Coding exons of the locus were affected by insertions or deletions ⬎100 nucleotides long (6 events), short frameshift insertions or deletions (4 events), and replacement nucleotide substitutions (1 event). In the case of 2 mutant alleles, coding regions were not affected. Because ⵑ70% of spontaneous de novoloss-of-function mutations in Homo sapiens are due to nucleotide substitutions within coding regions, insertions and deletions appear to play a much larger role in spontaneous mutation inD. melanogasterthan inH. sapiens.If so, the per nucleotide mutation rate in D. melanogastermay be lower than inH. sapiens, even if their per locus mutation rates are similar.
S
PONTANEOUS mutation is the key genetic process Furthermore, since Drosophila populations are large, that supplies raw material for stabilizing (deleteri- even weak selection can be effective, leading to selection ous mutations) and directional (beneficial mutations) on silent sites causing substantial codon bias (Akashinatural selection. However, we know relatively little 1997). Thus, it is difficult to be sure that a particular about the molecular nature of this process,i.e., about DNA sequence within a Drosophila genome evolved how common different events (nucleotide substitutions, at a rate equal to the mutation rate (Pritchard and deletions, insertions, duplications, etc.) are among all Schaeffer1997). In contrast, there is little doubt that spontaneous mutations, or about the value of its basic mutations in hominoid pseudogenes were effectively quantitative parameter, the per nucleotide per genera- neutral.
tion spontaneous mutation rate (Drake et al. 1998; Second,can be estimated from the per locus
muta-Kondrashov1998). tion rate,m.If the mutational target at a locus,i.e., the
Two approaches can be used to estimate. First, one number of nucleotides whose changes will lead to a can measure the degree of divergence between homolo- phenotypically detectable mutation, ist, ⫽m/t. How-gous selectively neutral DNA sequences in related spe- ever, the size of the mutational target depends strongly cies, provided that the total number of generations from on the molecular nature of mutation. If we consider their last common ancestor is known with good preci- loss-of-function mutations,tfor insertions and deletions sion. The best data of this kind are available for the is close to the total numberNof protein-coding nucleo-human-chimpanzee pair, where sequence divergence, tides at a locus, since most of such events (except in-mostly due to nucleotide substitutions, between ortholo- frame insertions and deletions) lead to malfunction of gous pseudogenes isⵑ1.3%. This implies, assuming a the affected proteins. In contrast,tfor nucleotide substi-20-year generation time and 5 million years since the tutions may be closer toN/5, since onlyⵑ5% of substitu-last common ancestor, that≈2⫻10⫺8(Nachmanand
tions create an in-frame stop codon, and ⬍25% of
Crowell2000). Unfortunately, this approach cannot
missense substitutions (and very rare synonymous substi-currently be applied to Drosophila because the numbers
tutions) lead to total loss of function (Mohrenweiser
of generations that separate pairs of Drosophila species
1994;Kondrashov1998). Thus, studying the molecular are known, at best, only within a factor of 3–5, due to
nature of mutation is important in its own right and is large uncertainties regarding the absolute divergence
also essential for estimating. time and the number of generations per year in nature.
In humans, the majority of loss-of-function de novo spontaneous mutations are substitutions (Krawczaket al.2000). Thus,m⫽10⫺5for a locus with 2000 coding
Corresponding author:Hsiao-Pei Yang, Department of Ecology and
nucleotides implies ≈ 2 ⫻ 10⫺8, in agreement with
Evolutionary Biology, Corson Hall, Cornell University, Ithaca, NY
14853. E-mail: hy31@cornell.edu the rate of neutral evolution. Data of this kind, first
ent wild-caught females (G0) were crossed individually to unre-obtained for the hemophilia B locus (Sommer 1995),
lated G0 flies, and produced sibships of G1 flies (Figure 1). are now available for⬎20 human loci (Krawczaket al.
From each G1sibship, at least 30 females were mated, in groups 2000). of 10, with strain V males (10–12 per group). Two or 3 days In contrast, we have little data on the molecular nature later, each group of females was allowed to lay eggs for 4 hr
ofde novospontaneous mutation inDrosophila melanogas- in a vial. During this time, 100–200 eggs were laid. After this,
the mothers were removed and stored at 16⬚. The G2offspring ter.Yamaguchiet al.(1994) found that, out of six
loss-that emerged in these vials were screened for salient eye-color of-function (null) mutations at theGpdhlocus obtained
phenotypes due to mutations at the four autosomal loci (kar in a mutation-accumulation experiment, five areP-ele- mutants could not be reliably detected) and at X-linked loci ment insertions and one is a long deletion. Loss-of- that affect eye color (only in males). Mosaic mutants were function alleles segregating in wild populations of D. ignored.
Since our design involves large G1sibships, we can
distin-melanogaster can be caused by both minor and
large-guishde novoclusters of mutations from preexisting heterozy-scale events (Nitasakaet al.1995;ten Haveet al.1995);
gosity. If an original G0fly carried a heterozygous loss-of func-unfortunately these authors did not discriminate
be-tion allele atpr,cn,bw,st, orkar, 25% the G2offspring of her tween substitutions and short indels. Data on molecular or his G1daughters should have a mutant phenotype. Seven evolution imply that indels (mostly deletions) constitute such families, each with⬎100 G2flies with abnormal eye color found in more than one vial, were identified and removed
ⵑ20% of all mutations in Drosophila,i.e., substantially
from the analysis. These families were clearly different from more than in mammals (Petrov et al. 1996;
Pritch-the threede novoclusters that we have found (see below). If
ard andSchaeffer 1997; Petrov andHartl 1998).
a single mutant was found in a vial, the G1females that laid
Petrov and Hartl (1998) found that deletions are eggs in it were allowed to lay eggs again to make sure that the much more common than insertions, whilePritchard singleton was not actually a small cluster. Thus, we effectively
andSchaeffer(1997) claim that they are about equally studied the mutation process in G1, while screening G2. Identifying and isolating mutant alleles:Mutants detected common.
in G2during screening were mated individually with flies of Thus, although extensive data onmare available for
the balancer strain, STV. Mendelian segregation within the many loci ofD. melanogaster(Schalet1960;Woodruff offspring from these crosses determined the chromosome
af-et al.1983), they cannot currently be used to estimate fected by a mutation and, for mutations at the four autosomal
. Obviously, we need more data on the molecular na- loci (pr,cn,bw, andst), identified the affected locus. Identity of the mutant locus was then confirmed by the appropriate ture of mutation in Drosophila. Here, we report the
complementation tests performed on offspring from G2 ⫻ results of specific locus tests (SLTs) of several loci that
STV cross. If a G2fly was not a mutant at one of our four affect eye color; four loci are autosomal [chromosome
autosomal loci, only male mutants were further analyzed by 2, purple (pr), cinnabar (cn), and brown (bw); chromo- the appropriate complementation tests with the following four some 3, scarlet (st)] and four are X-linked [prune (pn), X-linked loci:w,g,v, andpn.
To isolate an autosome that carried thede novo mutant vermilion (v), garnet (g), and white (w)]. Sixteen
inde-allele, we identified, using the appropriate crosses, male off-pendent de novo spontaneous mutational events were
spring from the G2⫻STV cross that carry bothSM1andTM3 detected in wild-type D. melanogaster, and molecular
balancers and an autosome carrying a mutant allele affecting analysis of 13 mutations was performed. eye color only at the locus where ade novomutation occurred. Only one such autosome was analyzed if the G2mutant was a male (because there is no crossing over in males). Five chromosomes were analyzed if the G2 mutant was a female MATERIALS AND METHODS
(in all such cases, the mutation occurred atbw, andbwis far Flies and cultural conditions:We sampledⵑ100 mated fe- away fromprandcnon chromosome 2), and the mutant allele males from a large wild population of D. melanogaster near different from the one present in strain V was regarded as Ithaca, NY. Flies were bred in 2.5 ⫻ 9.0-cm vials. Each vial the new one. All mutations discovered in fertile G2individuals contained 8 ml of food (1% agar, 0.1% propionic acid, 10% were homozygous viable and were, after being isolated, kept brewers yeast, and 10% glucose) seeded with a few grains of as pure strains, with the sole exception of the homozygous live baker’s yeast. At least 150 flies can develop simultaneously lethal loss-of-function mutation atpr, which was kept heterozy-in such a vial without a significant heterozy-increase of mortality. Flies gous with a second balancer strain (CyO).
were kept under a 12/12 light/dark cycle, at 25⬚ and 75% Molecular characterization of mutant alleles: Mutations humidity. Generation one (G1) parents (see below) were found in our screen can be due to molecular events at different stored at 16⬚, and remained fertile for at least 45 days. CO2 scales. Thus, we applied sequentially to each mutant three anesthesia was used. different techniques.
Tester strain and balancer strain:The tester strain, V, which
is homozygous for the allelespr1,cn1,bw1,st1, andkar1, and the 1. Cytogenetic analysis: Ectopic exchanges between transpos-able elements (TEs) situated in heterozygous positions balancer strain, STV, which has balancers on both autosomes
(SM1and TM3) and is heterozygous for alleles pr1, cn1, bw1, around a locus at which mutations are screened can lead to cytogenetically detectable events. Slides of squashed sali-st1, andkar1, were both created by introducing mutant alleles
paternally into the flies that originated from the same wild vary glands from five third instar larvae per mutant chromo-some were prepared, followingAshburner (1989), and population. Large population sizes, at least 1000 flies, were
maintained at all intermediate stages of the strain creation. scored for the presence of deletions and duplications. In all cases, no major chromosomal alterations were found. As a result, both strains are vigorous, despite carrying several
differ-Figure1.—Mating scheme for one family. R denotes meiosis, which forms the boundary between succes-sive generations. S denotes the occurrence of syngamy. P denotes “perigametic in-terval,” the time around mei-osis (from the last premei-otic DNA replication until the first DNA replication within the zygote). Fiand Mi
denote females and males of theith generation.
tively large events. Genomic DNA of ⵑ50 flies of each RESULTS mutant line was extracted with SDS lysis,
phenol-chloro-We screenedⵑ887,000D. melanogasterfor spontane-form extraction, and ethanol precipitation (Sambrooket
ousde novoloss-of-function mutations that occurred in
al. 1989). About 1g genomic DNA was restricted with
one or more restriction enzymes with 6-bp recognition the female germ line at four autosomal loci (pr,cn,bw, sequences, size-fractionated in 1% agarose gels, and blotted andst) and four X-linked loci (pn,v,g, andw). In total, onto S & S Nytran neutral charge membranes by capillary
28 mutants were found in 16 independent mutational transfer (Sambrook et al. 1989). The membranes were
events. Three events were clusters, of 10, 3, and 2 mu-probed with32P-random-primer-labeled DNA clones from
tants, respectively. No mutations were found atgorw. the corresponding gene at 58⬚overnight in hybridization
solution (0.01 g/ml BSA, 1 mm EDTA pH 7.2, 0.5 m Also, six X-linked eye color mutations at loci other than Na2HPO4 pH 7.2, and 7.5% SDS) and washed at 50⬚for pn, v, g, or w were found. These were not analyzed six to eight times in washing solution (40 mm Na2HPO4
because the loci involved are not yet known. Thirteen pH 7.2, 1 mmEDTA pH 7.2, and 1% SDS). The hybridized
of the 16 independent mutations were isolated into pure membranes were exposed to X-OMAT films at⫺80⬚ for
strains and analyzed molecularly. In 11 cases, the proba-3–14 days. Maps of restriction sites and probed regions of
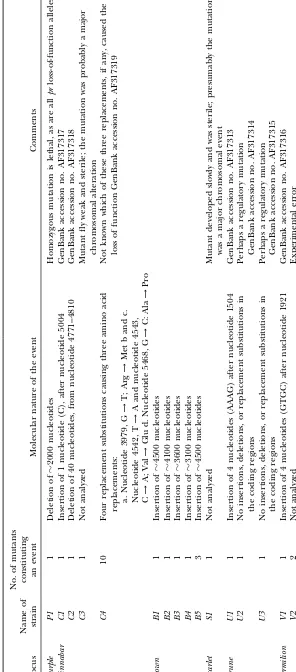
the genes analyzed are shown in Figure 2. ble cause of the mutant phenotype was found (Table 3. Sequencing:For those mutant alleles where no large differ- 1, Figure 3).
ences in the lengths of restriction fragments were detected, their coding regions were PCR amplified and sequenced. Primers for PCR reactions were designed using PRIMER3.
DISCUSSION Standard PCR conditions (100 ng genomic DNA template,
0.5g of each primer, 200mdNTPs, 1.5 mmMgCl2, 10
Dealing with clusters of mutations: In multicellular
mmTris-HCl pH 8.4, 50 mmKCl, 1 unit ofTaqpolymerase;
organisms, a mutation can occur during any of many Promega, Madison, WI) were used with thermal cycles: 95⬚
cellular generations that constitute a single organismal for 3 min, followed by 35 cycles of 92⬚for 30 sec and 60⬚
for 1 min, and ending with 72⬚for 7 min. Length of DNA generation. As a result, a mutation can lead to a cluster amplified is within the range of 300–500 bp. The amplified of mutants (if it occurred well before gametogenesis DNA was purified with Ultrafree-MC centrifugal filters
within a parent of the individual screened), a singleton, (Millipore, Bedford, MA) for sequencing. Both DNA strains
or a mosaic (postzygotic) mutant (Figure 1; Russell
were directly sequenced by autosequencing. The sequences
and Russell 1996; Thompson et al. 1998; Russell
were compared to wild-type alleles deposited in GenBank
(accession nos. pn, Z12141; v, M34147; w, U64875; g, 1999).
U31351; pr, U36232; cn, U56245; bw, M20630; and st, Dealing with singletons is straightforward. Mosaics U39739) using basic BLAST search (version 2.0). Whenever are hard to use for quantitative studies because they a mismatch between the mutant sequence and the
wild-may be cryptic or difficult to detect (Schalet 1960, type sequence was found, the gene region where the
mis-1986). Eye-color mosaics, in particular, are manifested match was located was PCR amplified and sequenced again
ex-Figure2.—Molecular structures of genes at the eight loci. Boxes indicate exons, and the solid and open boxes indicate the coding and nontranslated regions, respectively. Sizes of these genes are relative to the scale on the top. For genes analyzed by Southern blots, includingcn,bw,pn, andv, locations of restriction sites analyzed and the probed regions are also shown in the bottom (shaded boxes) of each gene. More detailed sequence information can be found in references:pr,Kimet al.(1996);cn, Warrenet al.(1996);bw,Martin-Morriset al.(1993);st,Tearleet al.(1989);pn,Tenget al.(1991);v,Searleset al.(1990); g,Lloyd(1998);w,O’Hareet al.(1984).
pression (bw andwamong our eight loci) and only if fied as preexisting heterozygotes,m can be underesti-mated by as much as a factor of 5 (Selby 1998a,b). a sizeable fraction of the eye surface happens to be
affected. Thus, we ignored mosaics and concentrated Fortunately, even large clusters ofde novomutations can easily be distinguished from preexisting heterozygosity on mutations that occurred during G1(Woodruffand
Thompson1992;Drakeet al.1998). if sibships of 20–30 or more G1individuals are analyzed.
Thus, it is surprising that our SLT appears to be the Any SLT must be designed in such a way that a cluster
ofde novomutations can reliably be distinguished from first one to use this sibship-based design.
Molecular nature of mutations:Among the 13 events
preexisting heterozygosity. This is easy if a cluster is
small, provided that enough offspring from each G1 analyzed, 5 mutations, all at bw, were insertions of lengths from 3100 to 4500 nucleotides, 1 was a deletion individual are analyzed. However, mutations occurring
just before the perigametic interval, which is the time of 2000 nucleotides, 4 were short frameshift insertions or deletions in coding exons, and only 1 event, a cluster between the last premeiotic DNA replication and the
first DNA replication within the zygote (P in Figure 1), of 10 cnmutants, was probably due to a replacement nucleotide substitution (Table 1). Five long insertions which are quite common (RussellandRussell1996;
Russell1999), produce large clusters, such that up to were probably caused by transposable elements, al-though this is not certain.
50% of gametes produced by the affected G1individual
also common (Nitasaka et al. 1995; ten Have et al. 1995). However, data on autosomal recessive loss-of-function alleles, both in Drosophila and in Homo, may not accurately reflect the properties ofde novomutation because many generations elapse between the origin of a mutation and its detection, which allows selection to act on heterozygotes.
Our data also agree with early results byMukaiand
Cockerham(1977),Voelkeret al.(1980), andHarada
et al.(1993), who found that the mutation rate toward
loss-of-function (null) alleles of several proteins isⵑ10 times higher than toward active alleles with changed electrophoretic mobility. This is to be expected if inser-tions and deleinser-tions are common. Indeed, all six null mutants found at the Gpdh locus during a mutation-accumulation experiment were major insertions (five) or deletions (one;Yamaguchiet al.1994).
The molecular nature of spontaneous mutation in humans is rather different. Replacement and nonsense nucleotide substitutions in coding regions causeⵑ70% of X-linked recessive (Sommer 1995; Giannelli et al. 1998;Lemahieuet al.1999;Tuffery-Giraudet al.1999) and autosomal dominant (haplo-insufficient) de novo loss-of-function mutations (Krantzet al.1998;Prosser
and van Heyningen1998; Clough et al. 1999; Loh-mann1999;Mayeret al.1999;Niidaet al.1999; West-ermanet al.1999;Krawczaket al.2000). For individual loci, however, the fraction of substitutions varies from
⬎90% (hemophilia B, Sommer1995; Giannelliet al. 1998) to⬍50% (Alagille syndrome,Krantzet al.1998). Thus, insertions and deletions should account for only
ⵑ5% of all spontaneous human mutations, which is confirmed by data on DNA sequence evolution (
Nach-manandCrowell2000).
This difference may be at least partially due to methyl-ation of mammalian DNA at CpG dinucleotides, which drastically increases substitution rates at many such nu-cleotides. As a result,⬎30% of human nucleotide substi-tutions occur at CpG hotspots (Shianget al.1994;
Som-Figure3.—Southern blot analysis ofbwmutant alleles.
Sam-mer 1995). In contrast, DNA in Drosophila is not ples of genomic DNA from each strain were digested with
methylated (seeLykoet al.1999), and CpG dinucleo-EcoRI (a), and withEcoRI andHindIII (b). Probed fragments
and their expected sizes in wild-type (wt) alleles are indicated. tides apparently mutate at a normal rate. (Lanes 1–7 in a correspond to wt, B1, B2, B3, B4, B5, andbw1
Per locus mutation ratem:Because mutations at the
and lanes 1–6 in b correspond to wt B5, B4, B1, B2, B3, and four X-linked loci could be detected only in male off-bw1respectively. Thin lines on the left are marker sizes.)
spring, our estimate of the per locus rate for indepen-dent mutational events isk⫽16/B⫽3.0⫻10⫺6, where B is the total number of loci screened. Since B⫽ 4⫻ among all de novomutations, because almost 100% of
insertions and deletions, but onlyⵑ10–20% of substitu- 887,000⫹4⫻443,500⫽5,322,000, and the total num-ber of mutants found was 28 (Table 1), our estimate of tions, within the coding regions inactivate a protein
(Kondrashov1998). Thus, our data imply that inser- the per locus mutation rate is m⫽ 28/B⫽5.3 ⫻ 10⫺6. The number of rare independent events has a Poisson tions and deletions, both major and short, constitute at
leastⵑ20% of all spontaneous mutations in Drosophila, distribution, so that the 95% confidence interval for k is 1.7–4.9 ⫻10⫺6. The confidence limits formcannot which agrees well with estimates byPetrovandHartl
(1998; 1999) based on patterns of DNA sequence evolu- be calculated precisely, due to insufficient data on the fraction of clusters among all mutational events and on tion.
Among loss-of-function mutations segregating in wild the distribution of cluster size. Assuming that between 50 and 75% of all spontaneous mutations occur in
molecular part of the work; R. MacIntyre and C. Webb for useful
ters (Thompsonet al.1998), we can therefore tentatively
suggestions, the anonymous reviewer who suggested that CpG
methyl-conclude that 2.0⫻10⫺6⬍m⬍15.0⫻10⫺6.
ation in mammals may be responsible for differences between
Dro-This estimate is in agreement with those obtained sophila and humans; and the following people for providing gene previously for bothD. melanogaster(3–5⫻10⫺6;Mukai
clones: W. Warren (cn), L. Searles (v), P. Kim (bw), and K. O’Hare
andCockerham1977;Voelkeret al.1980;Woodruff (w). This work was supported by a Fellowship for Study Abroad from the Republic of China Government to H.-P. Yang and a National
et al.1983;Haradaet al.1993) and mammals (Drake
Science Foundation grant DEB-9815621 to Sergey V. Nuzhdin.
et al.1998).
Per nucleotide mutation rate:Despite similar values
of mfor loss-of-function mutations in Drosophila and
in humans (DrostandLee1995),in Drosophila may LITERATURE CITED be substantially smaller than in humans because the
Akashi, H.,1997 Codon bias evolution inDrosophila: population
mutational target size for indels, which are more com- genetics of mutation-selection drift. Gene205:269–278.
Ashburner, M.,1989 Drosophila: A Laboratory Manual.Cold Spring
mon in Drosophila, is 5–10 times larger than for
substi-Harbor Laboratory Press, Cold Spring substi-Harbor NY.
tutions.
Bauer, V. L.,andC. F. Aquadro,1997 Rates of DNA sequence
Because the average length of the coding regions of evolution are not sex-biased in Drosophila melanogaster andD. the eight loci used in our screening is 1620 nucleotides, simulans.Mol. Biol. Evol.14:1252–1257.
Clough, M. V., J. D. HamlingtonandI. McIntosh,1999 Restricted
our estimate for the component ofdue to insertions
distribution of loss-of-function mutations within the LMX1B
and deletions is indel ⫽ (1.2 ⵑ 10.0) ⫻ 10⫺9. Not a genes of nail-patella syndrome patients. Hum. Mutat.14:459–465. single nonsense substitution was found in 887,000 flies Drake, J. W., B. Charlesworth, D. CharlesworthandJ. F. Crow,
1998 Rates of spontaneous mutation. Genetics148:1667–1686.
screened at the four autosomal loci in which we
calcu-Drost, J. B.,andW. R. Lee,1995 Biological basis of germline
muta-late that there are 526 possible substitutions that would tion: comparisons of spontaneous germline mutation rates create an in-frame stop codon, nor in 443,500 flies among drosophila, mouse, and human. Environ. Mol. Mutagen.
25:48–64.
screened at the four X-linked loci in which we calculate
Eyre-Walker, A.,andP. D. Keightley,1999 High genomic
delete-775 possible substitutions that could create an in-frame rious mutation rates in hominids. Nature397:344–347. stop codon. Thus, assuming that all nucleotide substitu- Giannelli, F., P. M. Green, S. S. Sommer, M. C. Poon, M. Ludwig
et al., 1998 Haemophilia B: database of point mutations and
tions occur with the same rate, no nonsense
substitu-short additions and deletions—eighth edition. Nucleic Acids Res.
tions happened within the target equivalent of (526⫻ 26:265–268. 887,000⫹ 775⫻ 443,500)/3 ⫽2.7 ⫻ 108 nucleotides
Harada, K., S. Kusakabe, T. YamazakiandT. Mukai,1993 Sponta-neous mutation rates in null and band-morph mutations of
en-(division over 3 is because a nucleotide can be
substi-zyme loci inDrosophila melanogaster.Jpn. J. Genet.68:605–616.
tuted in three ways). If we ignore clustering, this implies
Kim, N., J. Kim, D. Park, C. Rosen, D. DorsettandJ. Yim,1996
thatsub⬍ 1.2⫻10⫺8 with 95% confidence. Structure and expression of wild-type and suppressible alleles of
theDrosophila purplegene. Genetics142:1157–1168.
In contrast to mammals, there is no evidence that the
Kondrashov, A. S.,1988 Deleterious mutations and the evolution
spontaneous mutation in Drosophila is male biased, and
of sexual reproduction. Nature336:435–440.
there are approximately equal numbers of cell divisions Kondrashov, A. S.,1998 Measuring spontaneous deleterious
muta-tion process. Genetica103:183–197.
in the female and male germ lines (Bauerand
Aqua-Krantz, I. D., R. P. Colliton, A. Genin, E. B. Rand, L. Liet al.,
dro1997). Screening ofⵑ100,000 flies for mutations
1998 Spectrum and frequency of Jagged1 ( JAG1) mutations in
in the male germ line did not provide any evidence for Alagille syndrome patients and their families. Am. J. Hum. Genet. elevated mutation rate (data not reported). Thus, we 62:1361–1369.
Krawczak, M., E. V. Ball, I. Fenton, P. D. Stenson, S. Abeysinghe
probably did not underestimatekandmby our
proce-et al., 2000 Human gene mutation database—a biomedical
in-dure. formation and research resource. Hum. Mut.15:45–51.
Out ofⵑ100 spontaneousde novomutations that are Lemahieu, V., J. M. GastierandU. Francke,1999 Novel mutations in the Wiskott-Aldrich syndrome protein gene and their effects
estimated to occur in a human genome every
genera-on transcriptigenera-onal, translatigenera-onal, and clinical phenotypes. Hum.
tion, at least 2–3 are probably deleterious (Eyre- Mut.14:54–66.
WalkerandKeightley1999). In contrast, the genomic Lloyd, V. K.,1998 Drosophila melanogasterdelta-adaptin subunit of AP-3 (garnet) gene, complete cds. GenBank/EMBL/DDBJ:
deleterious mutation rate U in Drosophila is poorly
AF044287.
known, although it is probably below that in humans Lohmann, D. R.,1999 RB1 gene mutations in retinoblastoma. Hum. (Kondrashov1998). Drosophila, being sexual, may fal- Mutat.14:283–288.
Lyko, F., B. H. Ramsahoye, H. Kashevsky, M. Tudor, M. A.
Mas-sify the mutational deterministic hypothesis for the
trangeloet al., 1999 Mammalian (cytosine-5)
methyltransfer-maintenance of sexual reproduction if it hasU ⬍0.5– ases cause genomic DNA methylation and lethality inDrosophila.
ⵑ1.0 (Kondrashov1988). Assuming that, as inCaeno- Nat. Genet.23:363–366.
Martin-Morris, L. E., K. Loughney, E. O. Kershisnik, G.
Poor-rhabditis elegans (Shabalina and Kondrashov 1999),
tinga andS. Henikoff,1993 Characterization of sequences
ⵑ30% of 3 ⫻ 108 nucleotides in the D. melanogaster
responsible for trans-inactivation of theDrosophila browngene.
diploid genome are controlled by selection, we can con- Cold Spring Harbor Symp. Quant. Biol.58:577–584.
Mayer, K., W. BallhausenandH.-D. Rott,1999 Mutation
screen-clude thatU⬍ 0.5–1.0 and, therefore, this hypothesis
ing of the entire coding regions of the TSC1 and the TSC2 gene
must be rejected for this species, if ⬍0.5–1.0⫻10⫺8.
with the protein truncation test (PTT) identifies frequent splicing We thank S. A. Shabalina, F. A. Kondrashov and V. A. Kondrashov defects. Hum. Mutat.14:401–411.
Mutat. Res.304:119–137. 1990 Structure and transcription of theDrosophila melanogaster
Mukai, T.,andC. C. Cockerham,1977 Spontaneous mutation rates vermiliongene and several mutant alleles. Mol. Cell. Biol.10:
at enzyme loci inDrosophila melanogaster.Proc. Natl. Acad. Sci. 1423–1431.
USA74:2514–2517. Shabalina, S. A.,andA. S. Kondrashov,1999 Pattern of selective
Nachman, M. W.,andS. L. Crowell,2000 Estimate of the mutation constraint inC. elegansandC. briggsaegenomes. Genet. Res.74:
rates per nucleotide in humans. Genetics156:297–304. 23–30.
Niida, Y., N. Lawrence-Smith, A. Banwell, E. Hammer, J. Lewis Shiang, R., L. M. Thompson, Y. Z. Zhu, D. M. Church, T. J. Fielder
et al., 1999 Analysis of both TSC1 and TSC2 for germline muta- et al., 1994 Mutations in the transmembrane domain ofFGFR3
tions in 126 unrelated patients with tuberous sclerosis. Hum. cause the most common genetic form of dwarfism, Achondropla-Mutat.14:412–422. sia. Cell78:335–342.
Nitasaka, E., T. YamazakiandM. M. Green,1995 The molecular Sommer, S. S.,1995 Recent human germ-line mutation: inferences analysis of brown eye color mutations isolated from geographi- from patients with hemophilia B. Trends Genet.11:141–147. cally discrete populations ofDrosophila melanogaster.Mol. Gen. Tearle, R. G., J. M. Belote, M. McKeown, B. S. BakerandA. J. Genet.247:164–168. Howells,1989 Cloning and characterization of thescarletgene
O’Hare, K., C. Murphy, R. LevisandG. M. Rubin, 1984 DNA ofDrosophila melanogaster.Genetics122:595–606.
sequence of the white locus of Drosophila melanogaster.J. Mol. ten Have, J. F. M., M. M. GreenandA. J. Howells,1995 Molecular Biol.180:437–455. characterization of spontaneous mutations at thescarletlocus of
Petrov, D. A.,andD. L. Hartl,1998 High rate of DNA loss in the Drosophila melanogaster.Mol. Gen. Genet.249:673–683.
Drosophila melanogasterandDrosophila virilisspecies groups. Mol. Teng, D. H. F., L. B. Bender, C. M. Engele, S. TsubotaandT.
Biol. Evol.15:293–302. Venkatesh,1991 Isolation and characterization of theprune
Petrov, D. A.,andD. L. Hartl,1999 Patterns of nucleotide
substitu-locus ofDrosophila melanogaster.Genetics128:373–380. tion inDrosophilaand mammalian genomes. Proc. Natl. Acad. Thompson, J. N., R. C. WoodruffandH. Y. Huai,1998 Mutation Sci. USA96:1475–1479.
rate: a simple concept has become complex. Environ. Mol.
Muta-Petrov, D. A., E. R. Lozovskaya and D. L. Hartl,1996 High
gen.32:292–300. intrinsic rate of DNA loss inDrosophila.Nature384:346–349.
Tuffery-Giraud, S., S. Chambert, J. DemailleandM. Claustres, Pritchard, J. K.,andS. W. Schaeffer,1997 Polymorphism and
1999 Point mutations in the dystrophin gene: evidence for fre-divergence at aDrosophilapseudogene locus. Genetics147:199–
quent use of cryptic splice sites as a result of splicing defects. 208.
Hum. Mutat.14:359–368.
Prosser, J.,andV. van Heyningen,1998 PAX6 mutations reviewed.
Voelker, R. A., H. E. SchafferandT. Mukai,1980 Spontaneous Hum. Mutat.11:93–108.
allozyme mutations inDrosophila melanogaster: rate of occurrence
Russell, L. B.,1999 Significance of the perigametic interval as a
and nature of the mutants. Genetics94:961–968. major source of spontaneous mutations that result in mosaics.
Warren, W. D., S. Palmer andA. J. Howells, 1996 Molecular Environ. Mol. Mutagen.34:16–23.
characterization of thecinnabarregion ofDrosophila melanogaster:
Russell, L. B.,andW. L. Russell,1996 Spontaneous mutations
identification of the cinnabar transcription unit. Genetica 98:
recovered as mosaics in the mouse specific-locus test. Proc. Natl.
249–262. Acad. Sci. USA93:13072–13077.
Westerman, A. M., M. M. Entius, P. P. C. Boor, R. Koole, E. de Sambrook, J., E. F. FritschandT. Maniatis,1989 Molecular
Clon-Baaret al., 1999 Novel mutations in the LKB1/STK11 gene in
ing: A Laboratory Manual, Ed. 2. Cold Spring Harbor Laboratory
Dutch Peutz-Jeghers families. Hum. Mutat.13:476–481. Press, Cold Spring Harbor, NY.
Woodruff, R. C.,andJ. N. Thompson, Jr.,1992 Have premeiotic
Selby, P. B.,1998a Major impacts of gonadal mosaicism on
heredi-clusters of mutation been overlooked in evolutionary theory? J. tary risk estimation, origin of hereditary diseases, and evolution.
Evol. Biol.5:457–464. Genetica102/103:445–462.
Woodruff, R. C., B. SlatkoandJ. N. Thompson, Jr.,1983 Factors
Selby, P. B.,1998b Discovery of numerous clusters of spontaneous
affecting mutation rates in natural populations, pp. 37–124 in mutations in the specific-locus test in mice necessitates major
The Genetics and Biology of Drosophila, Vol. 3C, edited byM.
Ash-increases in estimates of doubling doses. Genetica102/103:463–
487. burner, H. L. CarsonandJ. N. Thompson, Jr.Academic Press,
Schalet, A. P.,1960 A study of spontaneous visible mutations in NY.
Drosophila melanogaster. Ph.D. Thesis, Indiana University, Yamaguchi, Y., T. S. Takano, T. Yamazaki andK. Harada,1994
Bloomington, IN. Molecular analysis ofGpdhnull mutations that arose in mutation
Schalet, A. P.,1986 The distribution of and complementation rela- accumulation experiments inDrosophila melanogaster.Heredity73:
tionships between spontaneous x-linked recessive lethal muta- 397–404. tions recovered from crossing long-term laboratory stocks of