Copyright1998 by the Genetics Society of America
Genetic Characterization of the
Drosophila melanogaster Suppressor of deltex
Gene:
A Regulator of Notch Signaling
Maggy Fostier,* Dana A. P. Evans,* Spyros Artavanis-Tsakonas
†and Martin Baron*
*University of Manchester, School of Biological Sciences, Manchester M13 9PT, United Kingdom, and†Howard Hughes Medical Institute,
Boyer Center for Molecular Medicine, Yale Medical School, New Haven, Connecticut 06536
Manuscript received December 8, 1997 Accepted for publication August 24, 1998
ABSTRACT
The Notch receptor signaling pathway regulates cell differentiation during the development of multicel-lular organisms. A number of genes are known to be components of the pathway or regulators of the Notch signal. One candidate for a modifier of Notch function is the Drosophila Suppressor of deltex gene [Su(dx)]. We have isolated four new alleles of Su(dx) and mapped the gene between 22B4 and 22C2. Loss-of-function Su(dx) mutations were found to suppress phenotypes resulting from loss-Loss-of-function of Notch signaling and to enhance gain-of-function Notch mutations. Hairless, a mutation in a known negative regulator of the Notch pathway, was also enhanced by Su(dx). Phenotypes were identified for Su(dx) in wing vein development, and a role was demonstrated for the gene between 20 and 30 hr after puparium formation. This corresponds to the period when the Notch protein is involved in refining the vein competent territories. Taken together, our data indicate a role for Su(dx) as a negative regulator of Notch function.
D
URING the development of multicellular organ- andPosakony1992; Fortini and Artavanis-Tsako-isms, cell-to-cell signaling plays an important part nas1994;BaileyandPosakony1995;Lecourtoisand in specifying cell fates. One important signaling pathway Schweisguth 1995). The latter encodes seven basic is mediated by the Notch receptor, which is involved in helix-loop-helix nuclear proteins (Delidakisand Arta-many key cell fate decisions. Notch is a transmembrane vanis-Tsakonas1992;Knustet al. 1992) that functionprotein that is conserved during evolution. The extracel- as repressors of proneural genes (Oellerset al. 1994;
lular domain contains 36 epidermal growth factor Heitzleret al. 1996). Recent reports indicate that
dur-(EGF)-like repeats and three lin12/Notch cysteine-rich ing the signal transduction process, a proteolytic cleav-repeats. The intracellular domain includes six cdc10/ age releases the cytoplasmic domain of Notch, which ankyrin repeats and a proline, glutamate, serine, threo- is translocated to the nucleus (Schroeteret al. 1998; nine-rich (PEST) sequence (reviewed by Artavanis- StruhlandAdachi1998).
Tsakonaset al. 1995). Notch signaling is also involved in the development
In Drosophila melanogaster, Notch is involved in the of the margin and the veins of the Drosophila wing. development of the central and peripheral nervous sys- The wing anlage develops initially in the larval stages tems, oogenesis, and eye and wing differentiation (For- as an epithelial monolayer subdivided by anterior/pos-tini and Artavanis-Tsakonas 1993). During neuro- terior and dorsal/ventral axes, which have a major in-genesis, Notch activity is required for the epidermal vs. fluence on the growth and patterning of the imaginal neural cell fate decision. Cells receiving the Notch signal disc (Diaz-Benjumea and Cohen 1993; Basler and are inhibited from the neural fate in favor of the epider- Struhl1994).
mal fate by a process termed “lateral inhibition” (Arta- The dorsal/ventral boundary is defined during the vanis-Tsakonas and Simpson 1991; Heitzler and second larval instar stage, where dorsal selector factor Simpson1991). Notch signaling is activated by binding
apterous (Diaz-Benjumea andCohen 1993) activates of the transmembrane protein Delta to the Notch
recep-the expression of recep-the putative secreted protein fringe tor on an adjacent cell (HeitzlerandSimpson1991;
(Irvine and Wieschaus 1994). The juxtaposition of Rebay et al. 1991). In response to Notch activation,
dorsal fringe-expressing and ventral non-fringe-express-Suppressor of Hairless, a transcription factor, stimulates
ing cells initiates symmetrical activation of wing margin-the transcription of downstream genes, including those
specific genes, e.g., wingless, vestigial, and cut along the of the Enhancer of split [E(spl)] complex (Schweisguth
dorsal/ventral boundary (Kim et al. 1995, 1996; Doh-erty et al. 1996;Michelliet al. 1997). Fringe inhibits
Serrate-mediated Notch activation on the dorsal side of
Corresponding author: Martin Baron, School of Biological Sciences,
the boundary, but positively regulates Delta dependent University of Manchester, G38 Stopford Building, Oxford Rd.,
Man-chester M13 9PT, United Kingdom. E-mail: mbaron@man.ac.uk Notch activation. In contrast, on the ventral side of the
boundary, the absence of fringe allows Serrate-depen- 1995); however, the in vivo role of deltex and its mecha-dent signaling. This modulatory effect of fringe, to- nism of regulation are currently unknown.
gether with positive feedback loops of Serrate and Delta While much has been learned regarding the nature expression, restrict the activation of Notch to the dor- of Notch signal transduction, there remain many unre-sal/ventral boundary (Flemming et al. 1997; Panin et solved questions as to how this signal is regulated. The
al. 1997). analysis of genetic interactions has proven to be a
valu-The five longitudinal veins appear progressively from able tool for isolating components or regulators of sig-the distal to proximal side of sig-the wing, as lumen between naling pathways. By identifying and mapping mutations the two apposing surfaces (Garcia-Bellido1977). The that interact with Notch mutant phenotypes, we are aim-formation of the veins requires an interplay between the ing to identify novel regulatory genes. One candidate Notch and Drosophila EGF receptor (DER) pathways for a regulator of the Notch pathway is Suppressor of (Sturtevant and Bier 1995; de Celis et al. 1997). deltex [Su(dx)]. The mutations Su(dx)1and Su(dx)2were
Differentiation of the longitudinal veins is initiated dur- originally described as second-chromosome mutants ing third larval instar by the localized expression of the that dominantly suppressed the deltex phenotypes ( Mor-early vein marker veinlet (Sturtevantet al. 1993) and gan et al. 1931). Another Suppressor of deltex allele,
the Notch ligand Delta (Kooh et al. 1993). Veinlet is Su(dx)sp, has been identified more recently, having arisen
thought to amplify the activity of DER, a key component spontaneously in an AxE2, dxenu stock (Busseau et al.
in determining the fate of vein cells (Sturtevant et 1994). We have determined the map location of the al. 1993). Notch activity is present in the presumptive Su(dx) gene and have described four new Su(dx) alleles.
intervein cells to limit vein differentiation to a “vein Wing phenotypes for Su(dx) loss-of-function alleles and competent” region (Fehonet al. 1991). During the pu- genetic interactions with Notch pathway mutants are
pal stage, Notch expression becomes restricted to the presented. The results show that the wild-type function boundaries of the vein precursor territories. At the vein of Su(dx) is as a negative regulator of the Notch boundaries, Notch signaling activates expression of pathway.
E(spl)mb, which subsequently represses veinlet expres-sion and refines the vein-competent territories to a
nar-rower region (de Celiset al. 1997). The role of Notch MATERIALS AND METHODS
signaling in wing development is reflected by the
pheno-Drosophila stocks and culture conditions: The following types of Notch mutants. Notch loss-of-function mutations
mutations were as described previously: Su(dx)sp(Busseauet
result in flies displaying wing margin notches and
al. 1994), dxenuand dxsm(XuandArtavanis-Tsakonas1990),
broader vein territories, while those that increase the
nd (Hinget al. 1994), and AxE2(XuandArtavanis-Tsakonas
Notch signal cause wing vein gaps (Schellenbarger 1990). The deficiency stock df(2L)yanJ2 was a gift from Zhi
and Mohler 1978; de Celis and Garcia-Bellido Chun Lai. The UAS-E(spl) line was provided by Jose de Celis,
1994). and the MS1096 line was as described previously (Capdevila
andGuerrero1994). All other mutants, balancer and com-The precise regulation of the Notch signal is crucial
pound X chromosomes, used are described inLindsleyand to its biological role, and a number of proteins that
Zimm(1992). Flies were cultured on standard cornmeal-yeast regulate this signal have been identified. The Hairless medium in temperature-controlled incubators at 258
unless (H) protein negatively regulates the Notch pathway by otherwise stated.
direct inhibition of Suppressor of Hairless (Brouet al. Mutagenesis screen:Virgin female wand dxenusn3; Su(dx)sp
and male1/Y; cn flies were collected and aged 3 days at 258. 1994; Bang et al. 1995). Other regulatory molecules
The male flies were exposed to 3000 rads of X rays (filtered bind to the intracellular domain of Notch itself. These
to remove the soft X-ray component). The males were then include dishevelled, a possible negative regulator (
Axel-allowed to mate for 48 hr before being removed from the rodet al. 1996), and deltex (dx), a positive regulator vials. Rescued male progeny were collected and crossed to
(Matsuno et al. 1995). The deltex protein contains virgin females of the genotype C1(A)y/Y; CyO/Sco. Only the
three domains separated by stretches of glutamine-rich female progeny from this cross were viable. CyO females
C1(A)y/Y; CyO/* cn were collected and mated to1/Y; CyO/
sequence (Busseau et al. 1994; Matsuno et al. 1995)
Sco males. Female C1(A)y/Y; CyO/* cn and male1/Y; CyO/
The N-terminal domain is responsible for binding to
* cn flies were collected and used to establish a stock balanced
the intracellular domain of Notch. The middle section
for the mutated second chromosome.
contains a proline-rich sequence that has been pro- After the recovery of balanced stocks, males1/Y; CyO/* posed to be an SH3 domain-binding site, and the C cn were crossed to wand dxenusn3; Su(dx)sp virgin females to
terminus contains a ring zinc-finger motif. Mutations confirm that the recovered second chromosome again failed to complement Su(dx)spin the lethal rescue assay. This ruled
in deltex resemble loss-of-function mutations of Notch
out the possibility that the original recovered male was a back-pathway genes, displaying wing margin loss and vein
ground escaper from lethality. thickening phenotypes (XuandArtavanis-Tsakonas
For genetic interaction analysis and complementation test-1990;GormanandGirton1992). Coexpression of del- ing, the C1(A)y chromosome was removed from the stock, tex and Notch in tissue culture regulates the nuclear except for Su(dx)4, which was kept as a C1(A)y stock because
TABLE 1 and rescue the mutant alleles are described in materi-als and methods.After isolation and balancing of the
Cytological mapping ofSu(dx)by testing failure
mutated second chromosome, each line was checked
to complement theSu(dx)spallele in the
for rescue of the nd-dx interaction. Four mutations were
rescue ofnd dxmale flies
obtained that failed to complement Su(dx)spin this
res-Rescue of nd dx cue assay and that had visible cytological chromosome Allele Cytology males over Su(dx)sp
aberrations at the tip of 2L (listed in Table 1). Su(dx)7
and Su(dx)52 were independently derived deficiencies
Df(2L)Su(dx)7 22B4-E1 Yes
that were identical at the cytological level (n.b., the
Df(2L)Su(dx)52 22B4-E1 Yes
Su(dx)52stock has subsequently been lost). Su(dx)56was
Df(2L)dp-79b 22A2-3;22D5-E1 Yes
Df(2L)S2 21C6-D1;22A6-B1 No an inversion with a breakpoint within the range of the
Df(2L)ast 22D1-2;22B2-3 No two deficiencies. In the case of Su(dx)4, a modification
Df(2L)Janj2 22C2-E1 No
of the crosses used to establish a stable line was
neces-In(2L)Su(dx)56 22B-C;22C-D Yes
sary. The cross of C1(A)y/Y; CyO/Su(dx)4
cn with 1/Y;
T(2:Y)Su(dx)4 22B-C Yes
CyO/Sco resulted in no viable female C1(A)y/Y; CyO/ Su(dx)4 cn progeny. However, viable male and female
progeny were recovered when both male and female phenotypes, control crosses were performed by crossing
mu-parents carried the second and Y chromosomes from tant flies to OregonR wild-type flies.
the original mutated male. Subsequent polytene
chro-Polytene chromosome analysis:Male flies1/Y; CyO/* cn
were crossed to virgin females of the genotype Bc/CyO. Non- mosome analysis revealed that Su(dx)4 was a reciprocal
CyO male progeny1/Y; * cn/Bc were crossed to wild-type 2:Y translocation. The translocation breakpoint lay females, and the culture was grown at 188. Third-instar larvae within the range of the Su(dx)7and Su(dx)52deficiencies.
were collected; those not displaying the dominant Bc larval
Higher-resolution mapping of Su(dx) was achieved by marker were dissected, and the salivary glands were used to
testing deficiency stocks available in the region. The prepare spreads of polytene chromosomes. Standard methods
of preparation and polytene chromosome staining were used results, summarized in Table 1, place the Su(dx) locus (Ashburner 1989a), and the chromosomes were inspected between 22B4 and 22C2.
by phase contrast microscopy with a3100 oil immersion lens. Characterization ofSu(dx)alleles:No phenotypes for
Electron microscopy:The flies were dehydrated in 100%
Su(dx) alleles have been reported previously, other than
ethanol and subsequently gold coated. Electron microscopy
their interactions with other mutations such as deltex was performed on a SEM 360 Cambridge instrument.
(Busseauet al. 1994). Here we describe phenotypes for Su(dx) in the wing veins and identify the developmental
RESULTS stage at which Su(dx) activity is required. We also
de-scribe genetic interactions with Notch pathway mutants.
Mapping of Su(dx): A recessive viable mutation in
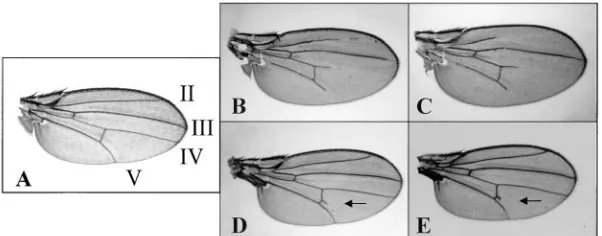
Wing vein phenotypes: Homozygous Su(dx)sp flies have
Notch, notchoid (nd), has a strong genetic interaction
a wild-type vein pattern at 258. However, when they with deltex, and flies homozygous for both nd and dx are
were kept at 298, a recessive wing vein gap phenotype not viable (Xu and Artavanis-Tsakonas 1990). We
appeared (Figure 1B). The phenotype was manifested have found that this lethal interaction can be rescued
most often in veins L.IV and L.V, distal to the posterior dominantly by Su(dx)spat 188, but at 258rescue required
cross-vein. Gaps were found frequently in L.II as well, two copies of Su(dx)sp(data not shown). Rescue was also
but never in L.III. Su(dx)4
, Su(dx)7
, and Su(dx)56
displayed achieved by a heteroallelic combination of Su(dx)spand
wing vein gaps at 298when placed over Su(dx)sp(Figure
Su(dx)2 (data not shown). Thus, it was expected that
1C). At 258, the same combinations of alleles had intact a deficiency of Su(dx) over Su(dx)sp would also rescue
longitudinal veins, but forked or incomplete cross-veins lethality. This was the basis of a deficiency screen to
(Figure 1, D and E). map the Su(dx) gene and generate new alleles of Su(dx).
Full details of the crosses used to perform the screen Developmental staging of Su(dx) function in the wing veins:
Figure1.—Phenotypes of Su(dx). (A) Wild-type wing; the longitudinal veins are numbered II, III, IV, and V. (B) Flies homozygous for Su(dx)spcn at
298display wing vein gaps in L.II, L.IV, and L.V. (C) At 298, Su(dx)7cn/Su(dx)56cn flies display wing
vein gaps like the other heteroallelic combina-tions of Su(dx). (D) At 258, Su(dx)7cn/Su(dx)56cn
flies have normal longitudinal veins, but they have a forked posterior cross-vein (arrow). (E) At 258,
Su(dx)7cn/Su(dx)spcn flies show the same forked
TABLE 2 perature-sensitive period between 16 and 22 hr APF at 298and 24–28 hr APF at 258, during which Su(dx)spaffects Temperature shift analysis of theSu(dx)
wing vein formation. Because pupal development is
wing vein gap phenotype
z80% slower at 258 than at 298 (Ashburner 1989b), the temperature-sensitive period of Su(dx)spcan be
ex-Wing vein gap phenotype
trapolated toz20–28 hr APF at 258. L2 and/or Small L4
Interaction with deltex: deltex mutations result in
thick-L4 and L5 and/or L5 None
ened veins and wing margin loss phenotypes that are
Age (%) (%) (%)
expected from a reduction in Notch signaling. The new A. Temperature shift from 258to 298 alleles Su(dx)4, Su(dx)7, and Su(dx)56were compared with
hAPF, 258
Su(dx)spfor their ability to suppress dxenuand dxsm
pheno-24 (n592) 75 22 3
types. The new alleles of Su(dx) suppressed the
pheno-26 (n523) 9 48 43
types of both dxenu(data not shown) and dxsm(see Figure
30 (n5103) 0 1 99
2); however, the Su(dx)spallele was the strongest
suppres-B. Temperature shift from 298to 258
sor even though Su(dx)7is a deficiency.
hAPF, 298
Interaction with Notch: Null mutations of Notch, such
12 (n528) 0 0 100
as Notch54l9, show a dominant phenotype of loss of wing
14–16 (n547) 0 25 75
18–20 (n5122) 54 29 17 margin at the distal tip of the wing (LindsleyandZimm 22–24 (n592) 96 2 2 1992). Su(dx)sp, Su(dx)7, and Su(dx)56mutants dominantly
suppressed the strength and penetrance of the wing hAPF, hours APF.
margin phenotype of Notch. The strength of the interac-tions varied among the different alleles, with Su(dx)sp
showing the strongest suppression (Figure 3). The temperature-sensitive wing vein gap phenotype of
Interaction with nd: Su(dx) mutations were tested for
the Su(dx)spmutant allowed us to determine the
develop-interaction with nd, a recessive Notch allele with a wing mental stage in which Su(dx) functions during wing
margin loss phenotype (Figure 4A) that is similar to the vein formation. Temperature shift analysis of Su(dx)sp
loss of one copy of Notch (Hing et al. 1994). Su(dx)4,
was performed using white prepupae that were collected
Su(dx)7, and Su(dx)56alleles dominantly suppressed the
from stocks kept at 258, aged, and shifted to 298 at
wing margin phenotype of nd (Figure 4B). Su(dx)sp,
how-specific time points. The results have revealed a critical
ever, in addition to suppressing the margin phenotype, stage between 24 and 28 hr after puparium formation
interacted dominantly to produce wing vein gaps (Fig-(APF) at 258(Table 2A). Pupae that were shifted to the
ure 4C). Additional phenotypes were observed in flies restrictive temperature before this stage resulted in most
that were homozygous for both nd and Su(dx)sp
. This
adults displaying a wing vein gap phenotype. A
tempera-combination was near lethal, but escapers were further ture upshift after 28 hr APF allowed normal
develop-enhanced for the vein gap phenotype and displayed ment of the veins.
horizontally held out and downward arching wings (Fig-Shift-down experiments were used to define the start
ure 4D). of the temperature-sensitive period of Su(dx)sp. White
Homozygous Su(dx)spalso interacts with heterozygous
prepupae were collected from stocks bred at 298 and
nd to produce wing vein gaps (Figure 4F). Su(dx)7and
transferred to 258. Veins developed normally in most
Su(dx)56failed to complement Su(dx)spfor the wing vein
flies when the temperature downshift occurred before
gap phenotype in combination with nd/1(Figure 4G). 14–16 hr APF (Table 2B). Increasing penetrance and
Su(dx)4could not be tested for complementation in this
severity of phenotype were evident when pupae were
assay because of the 2:Y reciprocal translocation. We kept for longer times APF at 298. A period of 22–24 hr
have also tested the original Su(dx)1and Su(dx)2alleles
APF was the minimum time at 298 required for most
in this assay and have shown that they fail to complement pupae to develop a phenotype indistinguishable from
Su(dx)sp. It is interesting to note that the interaction of
flies cultured permanently at 298.
Thus, temperature-shift analysis has revealed a tem- Su(dx) with nd is not a straightforward suppression of
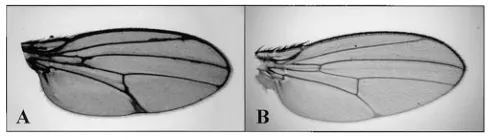
Figure 2.—Genetic interactions between
Su(dx) and deltex. (A) deltex males, dxsmt2v/Y; cn/
1, display deltas at the tip of the longitudinal veins and small notches in the distal wing margin. (B) dxsm t2 v/Y; Su(dx)7 cn/1 flies. Su(dx)7, like
the other Su(dx) alleles, dominantly suppresses the deltex phenotype. (C) dxsmt2v/Y; Su(dx)sp,cn
flies. Only the Su(dx)spallele shows complete
combination. Heteroallelic combinations of Su(dx)4,
Su(dx)7, and Su(dx)56 with Su(dx)sp showed a similarly
strong enhancement of AxE2. Females heterozygous for
AxE2did not have wing vein gaps in a wild-type genetic
background (Figure 5C). However, in combination with homozygous Su(dx)spor in heteroallelic combinations of
Su(dx)sp, Su(dx)1, Su(dx)2, Su(dx)7, and Su(dx)56, AxE2/1
females displayed a strong loss-of-vein phenotype (Fig-ure 5D).
Interaction with other Notch pathway genes: We have
ex-amined the genetic interactions of Su(dx) with H, Dl,
Ser, and E(spl)mb. H/1 displayed a small LV vein gap Figure3.—Genetic interactions between Su(dx) and N54l9.
(A) y waN54l9/1; cn/1flies display notches at the distal wing phenotype at a low penetrance in a wild-type
back-margin. (B) The dominant interaction with Su(dx)sp,cn
sup-ground. The penetrance of the vein gap phenotype was presses the Notch wing margin phenotype. (C) y waN54l9/1;
increased by a dominant interaction of Su(dx)sp with
Su(dx)7cn/1flies also show suppression of the Notch
pheno-H/1(data not shown), and this phenotype was strongly type, as y waN54l9/1; Su(dx)56cn/1flies (D) do.
enhanced when homozygous for Su(dx)sp(Figure 6B).
The bristle loss phenotype of Hairless was also examined. Counting of microchaetae and macrochaetae revealed the wing margin phenotype. The new wing vein gap
an enhanced loss-of-microchaetae phenotype in a ho-phenotype introduced implies a hyperactivation of
mozygous Su(dx) background (see Table 3); however, a Notch signaling in this combination of mutants.
significant reduction of the average number of
macro-Interaction with a gain-of-function Notch allele (AxE2
): The
chaetae present was not observed.
Abruptex class of mutations in Notch is defined by a wing
The phenotype of the Dl171
allele in a wild-type back-vein gap phenotype (LindsleyandZimm1992) thought
ground is illustrated in Figure 7A. Thickening of the to result from an increase in Notch signaling (Heitzler
veins can be observed, particularly at the distal ends and andSimpson1993;de Celiset al. 1996). Flies
homozy-around the anterior and posterior cross-veins. Genetic gous for AxE2 display a loss of vein at the distal tip of
interaction with heterozygous Su(dx)spresulted in
sup-L.V (Figure 5A). This phenotype was weakly enhanced
pression of the vein-thickening phenotypes, particularly by heterozygous Su(dx)sp, Su(dx)4, Su(dx)7, and Su(dx)56
at the distal tips of the veins (Figure 7B). We did not mutations (data not shown), and it was strongly
en-find a significant suppression of the wing margin pheno-hanced by homozygous Su(dx)sp at 258. This
double-type of Ser1, a dominant mutation in the second
Dro-homozygous combination of AxE2; Su(dx)spwas found to
sophila Notch ligand. be poorly viable, but escapers that had strong wing vein
To test for genetic interactions with E(spl)mb, we have gaps were observed (Figure 5B). These flies also had
used a transgenic line that ectopically expresses the held-out and downward arching wings, a phenotype that
was indistinguishable from the homozygous nd; Su(dx) E(spl)mbprotein. During the refinement of the wing
Figure4.—Genetic interactions between Su(dx) and nd. (A) wand/Y flies display distal margin notches. (B) wand/Y; Su(dx)7
cn/1 flies. Su(dx)7 dominantly suppresses the nd phenotype. (C) wand/Y; Su(dx)sp/1 flies show suppression of the nd wing
margin phenotype, but this interaction also results in a wing vein gap phenotype. (D) wand/Y; Su(dx)sp /Su(dx)spflies show a
strong enhancement of the wing vein gap phenotype and also have horizontally held out and downwardly arching wings. This phenotype is also observed in AxE2/Y; Su(dx)sp/Su(dx)spflies (data not shown). (E) Female wand/1flies appear wild type. (F)
wand/1; Su(dx)spcn/Su(dx)spcn flies display vein gaps. (G) The same phenotype is apparent in wand/1; Su(dx)spcn/Su(dx)56cn
TABLE 3
Enhancement ofHairlessmicrochaetae loss phenotype bySu(dx)sp
No. flies Average no.
Genotype counted microchaetae t P
1; 1
1 1
H
1 55 69.3 11.9 ,0.001
1; Y
Su(dx)sp;
Su(dx)sp
H
1 47 57.6
Figure5.—Genetic interactions between Su(dx) and AxE2.
(A) Male y AxE2/Y flies display a L.V distal wing vein gap. (B)
A severe enhancement of the wing vein gap at 258is seen in 1; 1
1 1
H
1 55 79.2
the near-lethal combination y AxE2/Y; Su(dx)spcn/Su(dx)spcn.
9.7 ,0.001 (C) Female y AxE2/1 flies appear wild type. (D) Female y
AxE2/FM7c; Su(dx)sp cn/Su(dx)spcn flies have wing vein gaps
at 258. 1;
1
Su(dx)sp;
Su(dx)sp
H
1 54 62.9
Average number of microchaetae contained in the middle vein territories, Notch signaling results in accumulation eight longitudinal rows were calculated for each genotype, of E(spl)mbprotein in the vein boundary cells, which and the data were subjected to a statistical t-test.
represses vein differentiation. Thus, Notch activation can be mimicked by ectopic expression of E(spl)mb,
has been mapped between 22B4 and 22C2 on the sec-which causes a vein gap phenotype when expressed in
ond chromosome. We have demonstrated that Su(dx) the wing (de Celiset al. 1997). We have used the
Gal4-mutants have phenotypes that are independent of muta-expressing line MS1096 to drive the expression of
tions in other genes. A forked cross-vein phenotype is E(spl)mbin the developing wing disc. These flies have
observed at 258, and a longitudinal vein gap phenotype wing vein gap and bristle loss phenotypes. MS1096;
UAS-is apparent at 298.
E(spl)mb/CyO flies were crossed to either wild-type or
The new alleles of Su(dx) described in this paper
domi-Su(dx)spmales. Su(dx)spwas found to strongly enhance the
nantly suppressed the deltex phenotype and failed to wing vein gap phenotype of the E(spl)mb-expressing
complement Su(dx)spin genetic interactions with
differ-progeny (Figure 8B). We also observed a strong
en-ent Notch alleles and with Hairless. By comparison of hancement of the loss-of-macro- and -microchaetae
phe-Su(dx) alleles with the deficiency phe-Su(dx)7, we conclude
notype (Figure 8B). In a wild-type background, ectopic
that the phenotypes observed result from a loss-of-func-expression of E(spl)mbresulted in an average loss of
tion of the Su(dx) gene product. In complementation 2.3 macrochaetae compared with an average loss of 13.4
tests over the Su(dx)spallele, the phenotype of the
defi-macrochaetae in a Su(dx)sp/1
background (P,0.001).
ciency Su(dx)7 is not significantly different from those
The strength of the enhanced wing vein and bristle loss
of Su(dx)4 and Su(dx)56. This suggests that the latter
al-phenotypes was comparable to the expression of two
leles may be near to null mutations. Su(dx)4
is lethal copies of the UAS-E(spl)mbtransgene in a wild-type
back-over Su(dx)7; however, it is premature to speculate on
ground (Figure 8C).
the null phenotype because it is possible that additional genes are removed by the combination of these
muta-DISCUSSION tions.
Su(dx)spis an antimorphic allele: Su(dx)spconsistently
Four new Su(dx) mutations with visible cytological abnormalities have been obtained, and the Su(dx) gene
Figure7.—Genetic interaction of Su(dx)sp with Delta. (A)
Dl171/1flies display vein thickening at the distal tips of the
Figure6.—Genetic interactions between Su(dx) and H1. (A)
H1/1flies display a narrower or missing distal tip of vein L.V. longitudinal veins and around the anterior and posterior
cross-veins. (B) Wings from Dl171/1; Su(dx)sp/1flies showing
sup-(B) The vein gap phenotype is strongly enhanced in a Su(dx)sp/
displayed a stronger phenotype in its genetic interac- wing vein precursors in the imaginal disc (Fehon et al. 1991). Second, during pupal development, Notch
tions compared to the other loss-of-function alleles of
Su(dx), including the deficiency Su(dx)7. In a dominant signaling is essential in determining the correct
thick-ness of the veins and maintaining the vein territories interaction, Su(dx)sp more completely suppressed the
Notch and deltex phenotypes. At 188, one copy of Su(dx)sp (Shellenbarger and Mohler 1978; de Celis et al.
1997;Huppertet al. 1997). The latter role is reflected by
can rescue the nd-dx lethal interaction, whereas the
de-ficiency Su(dx)7is unable to do this on its own (data not the gradual shift in Notch expression from the intervein
regions to a sharp border of cells along the veins by 24 shown). Furthermore, Su(dx)spdominantly interacts with
nd to produce wing vein gaps, while for the other Su(dx) hr APF. The expression of Notch and one of its target genes, E(spl)mb, is maintained in these cells#35 hr APF mutants, two alleles are required to produce this
pheno-type. These results indicate that Su(dx)spis an antimor- (de Celis et al. 1997). In addition, the Notch ligand
Delta was shown to be required for vein cell specification phic allele.
It is intriguing that an antimorphic allele that displays between 20 and 30 hr APF at 258(Huppertet al. 1997).
Thus, the developmental stage requiring Su(dx) activity strong genetic interactions with Notch pathway mutants
has a relatively weak phenotype on its own. This raises is consistent with a role in regulating the Notch signal during wing vein formation.
the possibility that there is functional overlap with other
genes. This is not without precedent in Notch signaling. Su(dx) is a negative regulator of the Notch pathway:
A number of observations indicate that the wild-type For example, the E(spl) family of DNA-binding genes
has been shown to have a partially redundant function function of Su(dx) is as a negative regulator of the Notch pathway. The temperature-sensitive wing vein gap in mediating the consequences of Notch activation
(DelidakisandArtavanis-Tsakonas1992). phenotype described in this paper is similar to that observed for gain-of-function Abruptex alleles of Notch.
Developmental stages of Su(dx) function:Analysis of
the phenotypes of Su(dx) and its interactions with Notch Complementation tests over the deficiency have shown that the Su(dx) mutants described result in a loss of pathway mutants has shown that Su(dx) functions at
different stages of development: the developing wing function of Su(dx). This is an important prerequisite for interpreting the wild-type function of Su(dx). margin, wing vein differentiation, and macro- and
mi-crochaetae development. In addition, the held-out wing The haplo-insufficient phenotype of Notch is sup-pressed by Su(dx) mutations, as is the mutation of Delta, phenotype seen in interactions with AxE2and nd
muta-tions is suggestive of a role for Su(dx) in muscle forma- the Notch ligand. In contrast, the gain-of-function AxE2
mutation of Notch is enhanced by Su(dx). This is similar tion. Notch signaling has previously been implicated
in muscle development (Baker andSchubiger 1996; to the known genetic interactions of Hairless with these
Notch mutants (Banget al. 1995). Hairless is a negative
Anantet al. 1998).
The temperature-sensitive wing vein gap phenotype regulator of the Notch pathway, and it functions by binding to and inhibiting Suppressor of Hairless, a of Su(dx)sp was used to determine the developmental
stage in which Su(dx) functions in vein formation. Tem- Notch-responsive transcription factor (Brouet al. 1994).
The fact that Su(dx) enhanced the Hairless phenotype perature shift analysis has revealed a
temperature-sensi-tive period between 20 and 28 hr APF at 258. This pupal indicates that the two genes are regulating the Notch signal in the same direction. Similarly, the observed stage coincides with the apposition of dorsal and ventral
wing surfaces, during which the veins are established suppression of deltex is as expected. Because deltex is a positive regulator of Notch function, its mutation (Fristrom et al. 1993). An important role for Notch
signaling in the process of vein formation is evident. should be compensated by a mutant that leads to a hyperactivation of the Notch signal.
Notch activity is first required in the specification of
Figure 8.—Genetic interactions between
Su(dx) and ectopic expression of E(spl)mb. MS1096; UAS-E(spl)mb/CyO flies were crossed to
either wild-type or Su(dx)spmales, and the
non-CyO progeny were examined for wing vein gaps
and bristle loss phenotypes. (A) MS1096/Y;
UAS-E(spl)mb/1flies have wing vein and microchaetae and macrochaetae loss phenotypes. (B) MS1096/
Y; UAS-E(spl)mb/Su(dx)spflies have a strongly
en-hanced wing vein phenotype, as well as micro-chaetae and macromicro-chaetae loss phenotypes. (C) Phenotypes of MS1096; UAS-E(spl)m b/UAS-E(spl)mbflies. Increasing the dosage of E(spl)mb
Activation of the Notch pathway can be mimicked by LITERATURE CITED ectopic E(spl)mbexpression in the wing, which results
Anant, S., R. SudiptoandK. V. Raghavan,1998 Twist and Notch in gaps in the veins. The strength of this phenotype is negatively regulate adult muscle differentiation in Drosophila.
Development 125: 1361–1369. dependent on the dosage of the expressed E(spl)mb,
Artavanis-Tsakonas, S.,andP. Simpson,1991 Choosing a cell fate: and the phenotype is enhanced in a Su(dx) mutant
back-a view from the Notch locus. Trends Genet. 7: 403–408. ground. We hypothesize that the Su(dx) mutation leads Artavanis-Tsakonas, S., K. Matsuno and M. E. Fortini, 1995
Notch signaling. Science 268: 225–232. to an elevation of Notch signaling and increased
expres-Ashburner, M.,1989a Drosophila: A Laboratory Manual. Cold Spring
sion of endogenous E(spl)mb, which augments the ecto- Harbor Laboratory Press, Cold Spring Harbor, NY.
pically expressed protein levels. However, we cannot rule Ashburner, M., 1989b Drosophila: A Laboratory Handbook. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY. out the alternative possibility that the enhanced
pheno-Axelrod, J. D., K. Matsuno, S. Artavanis-TsakonasandN.
Perri-type may be caused by an upregulation of the
down-mon,1996 Interaction between Wingless and Notch signaling stream response to the activity of expressed E(spl)mb. pathways mediated by dishevelled. Science 271: 1826–1832.
Support for a negative regulatory function for Su(dx) Bailey, A. M.,andJ. W. Posakony,1995 Suppressor of Hairless directly activates transcription of Enhancer of split complex genes also comes from comparison of Su(dx) phenotypes with
in response to Notch receptor activity. Genes Dev. 9: 2609–2622. those resulting from ectopic expression of activated Baker, R.,andG. Schubiger,1996 Autonomous and nonautono-Notch and wild-type deltex proteins. It is possible to mous Notch functions for embryonic muscle and epidermis
devel-opment in Drosophila. Develdevel-opment 122: 617–626. make a constitutively activated Notch receptor by
ex-Bang, A. G., A. M. BaileyandJ. W. Posakony,1995 Hairless pro-pressing a truncated form that lacks the extracellular motes stable commitment to the sensory organ precursor cell domain (Rebayet al. 1993). The Notch pathway can also fate by negatively regulating the activity of the Notch signaling
pathway. Dev. Biol. 172: 479–494. be upregulated by overexpression of wild-type deltex
Basler, K.,andG. Struhl,1994 Compartment boundaries and the (Matsunoet al. 1995). When activated Notch or wild- control of Drosophila limb pattern by hedgehog protein. Nature
type deltex are expressed under control of a heat shock 368:208–214.
Brou, C., F. Logeat, M. Lecourtois, J. Vandekerckhove, P.
Kouril-promoter 0–24 hr APF, a wing vein gap phenotype
ap-skyet al., 1994 Inhibition of the DNA-binding activity of Dro-pears. In both cases, this phenotype is strongly enhanced sophila Suppressor of Hairless and of its human homolog, KBF2/ in a heterozygous nd background, similar to the interac- RBP-J kappa, by direct protein-protein interaction with
Drosoph-ila Hairless. Genes Dev. 8: 2491–2503. tion between Su(dx) mutants and nd. Thus, the Su(dx)
Busseau, I., R. J. Diederich, T. XuandS. Artavanis-Tsakonas,
mutation mimics an elevation of the Notch signal. 1994 A member of the Notch group of interacting loci, deltex Taken together, our data indicate a role for Su(dx) encodes a cytoplasmic basic protein. Genetics 136: 585–596.
Capdevila, J.,andI. Guerrero,1994 Targetted expression of the as a negative regulator of the Notch pathway. The
exis-signaling molecule Decapentaplegic induces pattern duplications tence of feedback regulatory loops in the control of
and growth alterations in Drosophila wings. EMBO J. 13: 4459– Notch signaling makes the position of Su(dx) protein 4468.
de Celis, J. F.,andA. Garcia-Bellido,1994 Roles of the Notch in the Notch pathway difficult to define through genetic
gene in Drosophila wing morphogenesis. Mech. Dev. 46: 109–122. analysis. Su(dx) mutants were first identified through
de Celis, J. F., A. Garcia-BellidoandS. J. Bray,1996 Activation their interaction with deltex. It cannot be concluded that and function of Notch at the dorsal-ventral boundary of the wing the corresponding proteins interact directly, however, imaginal disc. Development 122: 359–369.
de Celis, J. F., S. J. Bray and A. Garcia-Bellido, 1997 Notch especially as we have shown significant genetic
interac-signaling regulates veinlet expression and establishes boundaries tions of Su(dx) with a number of Notch pathway genes. between veins and interveins in the Drosophila wing. Develop-The precise function of Su(dx) will only be resolved ment 124: 1919–1928.
Delidakis, C.,andS. Artavanis-Tsakonas,1992 The Enhancer of through cloning of the gene and analysis of its function
split [E(spl)] locus of Drosophila encodes seven independent at the molecular level, which is in progress. In a recent helix-loop-helix proteins. Proc. Natl. Acad. Sci. USA 89: 8731– mutagenesis screen, we have detected a number of en- 8735.
Diaz-Benjumea, F., andS. M. Cohen,1993 Interaction between hancers of Su(dx) that may be alleles of functionally
dorsal and ventral cells in the imaginal disc directs wing develop-related genes. It is likely, therefore, that the further
ment in Drosophila. Cell 75: 741–752.
characterization of Su(dx) and its interacting mutations Doherty, D., G. Feger, S. Younger-Shepherd, L. Y. JanandY. N.
Jan,1996 Delta is a ventral to dorsal signal complementary to will be fruitful for the understanding of Notch pathway
Serrate, another Notch ligand, in Drosophila wing formation. regulation.
Genes Dev. 10: 421–434.
We thank Iain Dawson for advice and help with polytene chromo- Fehon, R. G., K. Johansen, I. RebayandS. Artavanis-Tsakonas,
1991 Complex cellular and subcellular regulation of Notch ex-some analysis, and Bob Diederich, Mark Fortini, Tian Xu, Mike
Cor-pression during embryonic and imaginal development of Dro-nell, Luke Alphey, Jenny Gleason, and Roger Wood for valuable
dis-sophila: implications for Notch function. J. Cell. Biol. 113: 657– cussion. Samantha Newby gave us valuable assistance with the electron
669. microscopy. We thank Zhi Chun Lai and Gerry Rubin for supplying
Flemming, R. J., Y. GuandN. A. Hukriede,1997 Serrate-mediated the yanJ2deficiency stock, Jose de Celis for supplying the UAS-E(spl)mb
activation of Notch is specifically blocked by the product of the line, and Matthew Freeman for the MS1096 Gal4 line. M.B. is
sup-gene fringe in the dorsal compartment of the Drosophila wing ported by a Zeneca Senior Fellowship, M.F. by the Biotechnology and imaginal disc. Development 124: 2973–2981.
Biological Sciences Research Council Cell Commitment and Determi- Fortini, M. E.,andS. Artavanis-Tsakonas,1993 Notch: neuro-nation (BBSRC CAD) initiative, and D.E. by a Medical Research Coun- genesis is only part of the picture. Cell 75: 1245–1247. cil Realising Our Potential Award (MRC ROPA). S.A.T. is supported Fortini, M. E.,andS. Artavanis-Tsakonas,1994 The Suppressor by Howard Hughes Medical Institute. We also acknowledge the sup- of Hairless protein participates in Notch receptor signaling. Cell
Fristrom, D., M. WilcoxandJ. Fristrom,1993 The distribution Matsuno, K., R. J. Diederich, M. J. Go, C. M. BlaumuellerandS.
of PS integrins, laminin A and F-actin during key stages in Dro- Artavanis-Tsakonas,1995 Deltex acts as a positive regulator sophila wing development. Development 117: 509–523. of Notch signaling through interactions with the Notch ankyrin
Garcia-Bellido, A.,1977 Inductive mechanism in the process of repeats. Development 121: 2633–2644.
wing vein formation in Drosophila. Wilhelm Roux’s Arch. Dev. Micchelli, C. A., E. J. RulifsonandS. S. Blair,1997 The function Biol. 182: 93–106. and regulation of cut expression on the wing margin of
Drosoph-Gorman, M. J.,andJ. R. Girton,1992 A genetic analysis of deltex ila: Notch, wingless and a dominant negative role for Delta and and its interaction with the Notch locus in Drosophila melanogaster. Serrate. Development 124: 1485–1495.
Genetics 131: 99–112. Morgan, T. H., A. H. Bridges, J. SchultzandJ. Schultz, 1931 The
Heitzler, P.,andP. Simpson,1991 The choice of cell fate in the constitution of the germinal material in relation to heredity. Year epidermis of Drosophila. Cell 64: 1083–1092. Book Carnegie Inst. 30: 408–415.
Heitzler, P.,and P. Simpson,1993 Altered EGF-like sequences Oellers, N., M. DehioandE. Knust,1994 bHLH proteins encoded provide evidence for a role of Notch as a receptor in cell fate by the Enhancer of split complex of Drosophila negatively inter-decisions. Development 117: 1113–1123. fere with transcriptional activation mediated by proneural genes.
Heitzler, P., M. Bourouis, L. Ruel, C. CarteretandP. Simpson, Mol. Gen. Genet. 244: 465–473.
1996 Genes of the Enhancer of split and achaete-scute com- Panin, V. M., V. Papayannopoulos, R. WilsonandK. D. Irvine, plexes are required for a regulatory loop between Notch and 1997 Fringe modulates Notch-ligand interactions. Nature 387: Delta during lateral signaling in Drosophila. Development 122: 908–912.
161–171. Rebay, I., R. J. Fleming, R. G. Fehon, L. Cherbas, P. Cherbaset
Hing, H. K., X. SunandS. Artavanis-Tsakonas,1994 Modulation al., 1991 Specific EGF repeats of Notch mediate interactions of wingless signaling by Notch in Drosophila. Mech. Dev. 47: with Delta and Serrate: implications for Notch as a multifunc-261–268.
tional receptor. Cell 67: 687–699.
Huppert, S. S., L. J. JacobsenandM. A. T. Muskavitch,1997 Feed- Rebay, I., R. G. FehonandS. Artavanis-Tsakonas,1993 Specific back regulation is central to Delta-Notch signaling required for
truncations of Drosophila Notch define dominant activated and Drosophila wing vein morphogenesis. Development 124: 3283–
dominant negative forms of the receptor. Cell 74: 319–329. 3291.
Schellenbarger, D. L.,andJ. D. Mohler,1978
Temperature-sensi-Irvine, K. D.,andE. Wieschaus, 1994 Fringe, a boundary-specific
tive periods and autonomy of pleiotropic effects of l1 Nts, a
condi-signaling molecule, mediates interactions between dorsal and
tional Notch lethal in Drosophila. Dev. Biol. 62: 432–446. ventral cells during Drosophila wing development. Cell 79: 595–
Schroeter, E. H., J. A. KisslingerandR. Kopan,1998 Notch-1 606.
signaling requires ligand induced proteolytic release of
intracellu-Kim, J., K. D. IrvineandS. B. Carroll, 1995 Cell recognition,
lar domain. Nature 393: 382–386. signal induction and symmetrical gene activation at the dorso
Schweisguth, F.,andJ. W. Posakony,1992 Suppressor of Hairless, ventral boundary of the developing Drosophila wing. Cell 82:
the Drosophila homolog of the mouse recombination signal-795–802.
binding protein gene, controls sensory organ cell fates. Cell 69:
Kim, J., A. Sebring, J. J. Esch, M. E. Kraus, K. Vorwerket al., 1996
1199–1212. Integration of positional signals and regulation of wing formation
Struhl, G.,andA. Adachi,1998 Nuclear access and action of notch and identity by Drosophila vestigial gene. Nature 382: 133–138.
in vivo. Cell 93: 649–660.
Knust, E., H. Schrons, F. GraweandJ. A. Campos-Ortega,1992
Sturtevant, M. A.,andE. Bier,1995 Analysis of the genetic hierar-Seven genes of the Enhancer of split complex of Drosophila
melano-chy guiding wing vein development in Drosophila. Development
gaster encode helix loop helix proteins. Genetics 132: 505–518.
121:785–801.
Kooh, P. J., R. G. FehonandM. A. Muskavitch,1993 Implications
Sturtevant, M. A., M. RoarkandE. Bier,1993 The Drosophila of dynamic patterns of Delta and Notch expression for cellular
interactions during Drosophila development. Development 117: rhomboid gene mediates the localized formation of wing veins 493–507. and interacts genetically with components of the EGF-R signaling
Lecourtois, M.,andF. Schweisguth,1995 The neurogenic Sup- pathway. Genes Dev. 7: 961–973.
pressor of Hairless DNA-binding protein mediates the transcrip- Xu, T.,andS. Artavanis-Tsakonas,1990 deltex, a locus interacting tional activation of the Enhancer of split complex genes triggered with the neurogenic genes, Notch, Delta and mastermind in by Notch signaling. Genes Dev. 9: 2598–2608. Drosophila melanogaster. Genetics 126: 665–677.
Lindsley, D. L.,andG. G. Zimm, 1992 The Genome of Drosophila