DOI: 10.4236/oalib.1105217 Feb. 27, 2019 1 Open Access Library Journal
BGC-823 and SGC-7901 Cells by Inducing Cell
Cycle Arrest and Apoptosis in Gastric Cancer
Hanni Zhang
1, Yunliang Guo
1, Keli Ge
1*, Yanan Wang
1,2*1The Center for Integrated Traditional Chinese and Western Medicine, Department of Medicine,
Qingdao University, Qingdao, China
2The Affiliated Hospital of Qingdao University, Qingdao, China
Abstract
Gastric cancer represents a major cause of cancer-related death worldwide. Although various tactics and anti-tumor drugs have been used to improve curative effects, five-year survival rate of lung cancer patients remains poor. Evodiamine, a sophora alkaloid, has been demonstrated to exert antitumor effects on many types of cancer. However, the molecular mechanism of evo-diamine against gastric cancer has not been clearly elucidated. In this study, we investigated the anti-tumor activity and the underlying mechanisms of EVO on gastric cancer cells, and found that it significantly inhibited the pro-liferation of BGC-823 and SGC-7901 cells by inducing cell cycle arrest at G2/M phase and cell apoptosis in a dose- and time-dependent manner. Its molecular mechanism may be that it reduces the expression of cell cycle- promoting protein Cdc25C and promotes the expression of cell cycle inhibi-tor p53, as well as prompts the activity of caspases pathways, such as the ex-pression level of cleaved caspase-3 and cleaved caspase-8; cleaved caspase-9 and cleaved PARP-1 are up-regulated, treated with EVO (10 μM) at different points in time (0, 3, 6, 9, 12, 24 h). Collectively, our data demonstrated that EVO was a potential anti-tumor agent against gastric cancer.
Subject Areas
Gastroenterology, Hepatology
Keywords
Evodiamine (EVO), BGC-823 Cells, SGC-7901 Cells, Proliferation, Cell Cycle, Cell Apoptosis
How to cite this paper: Zhang, H.N., Guo, Y.L., Ge, K.L. and Wang, Y.N. (2019) Evodiamine Inhibits the Proliferation of BGC-823 and SGC-7901 Cells by Inducing Cell Cycle Arrest and Apoptosis in Gastric Cancer. Open Access Library Journal, 6: e5217.
https://doi.org/10.4236/oalib.1105217
Received: January 30, 2019 Accepted: February 24, 2019 Published: February 27, 2019
Copyright © 2019 by author(s) and Open Access Library Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY 4.0).
DOI: 10.4236/oalib.1105217 2 Open Access Library Journal
1. Introduction
Gastric cancer is one of the most commonly digestive system carcinoma and remains the major cause of cancer-related death with characteristics of rapid progression, poor curative effect, easy metastasis, and unfavorable prognosis in the domestic and overseas. According to reports, the global incidence and the mortality rate of gastric cancer respectively rank fifth and third in clinical diag-nosed malignant tumors [1]. In China, it has been ranking from the second among all cancers with 15.8% annual incidence ratio and 17.6% mortality ratio [2]. At present, the major treatment methods for gastric cancer mainly remain surgical resection, chemotherapy and targeted therapy, even though new treat-ment approaches are emerging [3]. Therefore, to search for safer and more effec-tive therapy is an urgent problem in the treatment of gastric cancer.
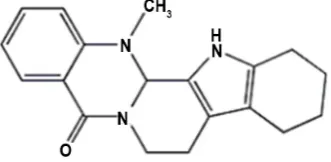
Evodiamine (EVO) (C19H17N3O) is one of the main active components in
dried roots and ripe fruits of Evodia rutaecarpa, which has a wide range of pharmacological effects and has few obviously side effects or toxicity [4] (Figure 1). Recently, it has been extensively studied for its chemopreventive potential against various cancers, for instance, hepatocellular carcinoma [5] [6], breast cancer [7], colon cancer [8], lung cancer [9], prostatic cancer [10] and osteosar-coma [11]. The data have certificated that EVO exerts its anticancer activities through inhibiting cancer cell proliferation, accelerating apoptosis, inducing cell cycle arrest, suppressing invasion and metastasis, and reducing chemothera-py-induced toxicity [12]. The related research has shown that evodiamine has inhibited the effective proliferation of gastric cancer in SGC-7901 cells [13], but its specific anti-tumor molecular mechanism is still unclear. In this study, we examined the mechanism of anti-tumor effects of EVO in BGC-823 and SGC-7901 cells, finding that it exerted its anti-proliferation effects by inducing cell cycle arrest at G2/M phase and cell apoptosis in gastric cancer cells, and tried to clarify its associated molecular mechanisms.
2. Materials & Methods
2.1. Cell lines and Culture
The Human gastric cancer cell lines (BGC-823, SGC-7901) were purchased from the National Cell Resource Center (Beijing, China). All cell lines were propagat-ed in DME/F-12 Mpropagat-edium (HyClone, USA), supplementpropagat-ed with 10% fetal bovine serum (FBS, Gibco, USA), 100 U/ml penicillin and 100 mg/ml streptomycin (HyClone, USA) in a humidified atmosphere with 5% CO2 at 37˚C. The cells
with 80% confluence were treated by EVO (National Vaccine and Serum Insti-tute, Beijing, China) of different concentrations.
2.2. Cell Viability Assay
DOI: 10.4236/oalib.1105217 3 Open Access Library Journal
Figure 1. Chemical structures of evodiamine
and its molecular formula (C19H17N3O).
respectively. Five parallel wells for each concentration. At each time point, Cell Counting Kit 8 (CCK-8) agent (Dojindo, Japan) was added to each well and in-cubated at 37˚C for 2 h. The numbers of viable cell were calculated by detecting the optical density (OD) at 450 nm using the microplate autoreader (Bio-Rad, CA, USA). IC50 was determined using the trimmed Spearman-Karber method.
Cell viability (%) = ODtreated/ODcontrol × 100.
2.3. Cell Morphology Observation
The Cells were seeded into 6-well plates at a density of 3 × 105 cells/well
over-night. Then, allowing their adhesion to the plate, the cells were treated with EVO (10 μM) for 48 h. The morphological changes of the cells were observed under a microscope.
2.4. Cell Colony Formation Assay
The cells were seeded at 500 cells/well in 6-well plates overnight, and then treated with EVO (the concentrations: 10 μM) for 5 days. Discarding the super-natant, in every well fixed with 4% paraformaldehyde for 20 minutes and stained with 0.1% Giemsa for 15 minutes at room temperature. The numbers of colony were scanned and counted with the microscope. The colony formation rate that colonies contained more than 50 cells was calculated according to the following equation “Colony formation rate (%) = (colony counts/number of seeded cells) × 100%”.
2.5. Cell Cycle Analysis
The cell cycle was detected by using flow cytometry (FCM) with propidium iodide (PI)/RNase staining solution (BD Biosciences, San Jose, CA, United States). Cells were seeded in 6-well plates at 3 × 105 cells per well and treated
with EVO (the concentrations: 10 μM) for 48 h. Following by collecting cells, fixed in ice-cold 70% ethanol at 4˚C overnight in darkness. Then washed with cold PBS for two times, and added with 100 μL RnaseA for 30 min at 37˚C, the cells were suspended in PI Staining Buffer at 4˚C for 20 min, finally analyzed on a flow cytometer (Becton Dickinson, Franklin Lakes, NJ, USA).
2.6. Annexin V-FITC/PI Staining
[image:3.595.291.457.77.158.2]An-DOI: 10.4236/oalib.1105217 4 Open Access Library Journal nexin V-FITC/PI double staining solution (BD Biosciences, San Jose, CA, Unit-ed States) by FCM. Cells were plantUnit-ed into 6-well plates at 3 × 105 cells per well
and treated with EVO (10 μM) for 48 h. Then digested by trypsinization, washed with cold PBS for two times, and fixed cell suspension with 1 × Binding Buffer. The cells were then stained with Annexin V-FITC/PI according to the manufac-turer’s instruction. After incubation for 10 min at room temperature in dark-ness, the apoptotic cells were detected with flow cytometry.
2.7. Western Blot Analysis
The cells were planted into T25 flask at 5 × 105 cells per flask and treated with
EVO (10 μM) for 48 h, washed twice with PBS and then lysed with 300 μL of RIPA buffer for 30 min in ice. After centrifuged at 12,500 rpm for 20 min at 4˚C, the supernatants were transferred to clean microcentrifuge tubes. The total pro-tein concentration was determined using the bicinchoninic acid (BCA) (Beyo-time, China) method. Equal amount of protein (30 μg) from each sample was separated by 10% or 12% SDS-PAGE and transferred onto PVDF membranes. After being blocked in defatted milk (5% in Tris-buffered saline with Tween-20 buffer) at 37˚C for 1 h, the membrane was incubated with various primary anti-bodies overnight at 4˚C and then with appropriate secondary antianti-bodies for 1 h at room temperature. After each incubation period, the membrane was washed three times with TBST (Tris buffered saline with Tween-20). Signals were visua-lized by ECL detection reagents (Bio-Rad, CA, USA). The protein quantitative analysis was conducted by using the Image J software.
2.8. Statistical Analysis
Data are presented as the mean ± SD, every experiment was performed at least 3 times. The difference between the groups was assessed using a one-way analysis of variance (ANOVA) or student’s t-examination by the SPSS 22.0 software. A
P-value of less than 0.05 indicates a statistical significance.
3. Results and Discussion
3.1. EVO Can Significantly Inhibit the Proliferation of Human
Gastric Cancer Cells
DOI: 10.4236/oalib.1105217 5 Open Access Library Journal
Figure 2. The effects of EVO on cell proliferation in gastric cancer cell lines BGC-823 and SGC-7901 cells. (a) Exponentially
growing cells of BGC-823 and SGC-7901 cells were treated with EVO at the indicated concentrations (0 - 15 μM) for 24, 48, and 72 h; then, the percentages of viable cells were determined using CCK-8 assay. (b) The effect of EVO on the colony formation ability of BGC-823 and SGC-7901 cells. Data were shown as mean ± SD from at least three independent experiments. *P < 0.05 and **P < 0.01 vs. the Control group (culture medium only). (c) The IC50 value of EVO for BGC-823 and SGC-7901 cells. (d) The
[image:5.595.61.536.61.639.2]DOI: 10.4236/oalib.1105217 6 Open Access Library Journal respectively calculated by Graph-pad Prism7.0 software. The IC50 values of EVO
were respectively 10.01 μM and 9.73 μM in BGC-823 and SGC-7901 cells after intervenion for 24 h (Figure 2(c)), so the follow-up experiments were used 10 μM as the working concentration. The microscopic observation was shown that, the cell bodies were not reduced, rounded and shrunk, even separated from each other, but also there were a small amount of particulate matter appeared and more cell debris in the culture solution after 24 h, compared with the control group (Figure 2(d)). The results indicated that EVO has a better anti-gastric cancer activity.
3.2. EVO Induces Gastric Cancer Cell Cycle Arrest at the G2/M
Checkpoint
We have verified that uncontrolled cell mitosis represents one of the hallmarks of cancer. Thus, we used the PI staining to inspect the effects of EVO on the cell cycle distrution upon BGC-823 and SGC-7901 cells by FCM. Luckly, cell cycle analysis revealed that the proportion of gastric cancer in G2/M phase was signif-icantly increased after treatment for 24 h. Specifically, after all cells were treated with 10 μM EVO for 24 h, the G0/G1 checkpoint ratio of BGC-823 and SGC-7901 cells were respectively decreased to 8.57% ± 2.83% (t = 10.681, P < 0.001) and 23.11% ± 4.84% (t = 5.376, P < 0.01); the S-phase ratio were respec-tively rose up to 19.31% ± 4.34% (t = 2.121, P < 0.05) and 16.24% ± 10.23% (t = −0.196, P > 0.05); the cell cycle ratio in G2/M checkpoint were increased to 54.13% ± 6.81% (t = −11.552, P < 0.001) and 47.93% ± 9.18% (t = −10.776, P < 0.001), compared with the control group (Figure 3(a) and Figure 3(b)). Thus, EVO mainly induces gastric cancer cell cycle impeded at the G2/M checkpoint.
3.3. EVO Reinforces the Apoptosis of Gastric Cancer Cells
To determine if EVO could synergistically aggravate the apoptosis of gastric cancer cells, Annexin V-FITC/PI staining and FCM method were applied to detect the apoptotic events. After treatment with EVO (10 μM) for 24 h, the total apoptotic percentages were respectively 18.93% ± 5.78% (t = −12.728, P < 0.001) and 17.24% ± 5.07% (t = −12.956, P < 0.001), much higher than the 4.88% ± 1.96% and 3.74% ± 2.49% of the control group. Among them, EVO obviously induced the late apoptosis of cells, the apoptotic percentages were 16.13% ± 4.53% (t = −10.844, P < 0.001) and 10.87% ± 5.67% (t = −8.854, P < 0.01), re-spectively, in EVO-treated BGC-823 and SGC-7901 cells (Figure 4(a) and Fig-ure 4(b)). Together, the findings indicated that EVO inhibits the malignant pro-liferation of gastric cancer cells by inducing apoptosis.
3.4. EVO Mediates the Activity of Apoptosis-Related Proteins and
Cell Cycle-Related Proteins in Gastric Cancer Cells
DOI: 10.4236/oalib.1105217 7 Open Access Library Journal
Figure 3. EVO induced gastric cancer cell cycle arrest at the G2/M phase. The length of
each cell cycle phase was calculated and compared from three independent experiments. *P < 0.05, **P < 0.01, ***P < 0.001 vs. the Control group.
examined by western-blot analysis. After treatment with EVO (10 μM) at different points in time (0, 3, 6, 9, 12, 24 h), the results showed that the expression levels of p53, cleaved-caspase-3, cleaved-caspase-8, cleaved-caspase-9 and cleaved-PARP-1 were significantly up-regulated, but the expression level of cdc25c was marketa-bly reduced, in the EVO-treated BGC-823 and SGC-7901 cells in a time-de- pendent manner compared with their control groups (Figure 5(a) and Figure 5(b)). Therefore, we concluded that EVO can induce gastric cancer cells apopto-sis by regulating the activity caspases pathways, and accelerate cell cycle arrested at the G2/M checkpoint by changing the expression levels of p53 and cdc25c.
4. Conclusions
DOI: 10.4236/oalib.1105217 8 Open Access Library Journal
Figure 4. Apoptosis-induced effect of EVO was evaluated by FCM in BGC-823 and
SGC-7901 cells, with Annexin V-FITC/PI staining. The apoptotic percentages from three independent experiments were analyzed and compared.
that it significantly inhibited the proliferation of BGC-823 and SGC-7901 cells by inducing cell cycle arrest at G2/M phase and cell apoptosis in a dose- and time-dependent manner.
DOI: 10.4236/oalib.1105217 9 Open Access Library Journal
Figure 5. EVO suppressed the activity of p 53 signaling and caspases pathways in gastric
cancer cells. BGC-823 and SGC-7901 cells were cultured in T25 flask and treated with EVO (10 μM) at different points in time (0, 3, 6, 9, 12, 24 h), then the expression of the indicated factors was examined by Western blot analysis. β-actin was used as the loading control. The densitometry analysis of every factor was performed, normalized with the corresponding β-actin content.
activation of CDC2, which can inhibit the activation of the CDC2/CyclinB1 complexes, inducing cancer cell cycle arrest at the G2/M checkpoint [16] [17]. At the same time, the expression of cdc25c is regulated by cell cycle inhibition pro-tein p53. It can combine with cdc25c promoter to inhibit its transcription and maintain the smooth operation of cell cycle [18] [19]. Similarly, the results have shown that EVO can increase the expression of p 53 and reduce the expression of cdc25c in BGC-823 and SGC-7901 cells after treatment for 24 h.
DOI: 10.4236/oalib.1105217 10 Open Access Library Journal caspase-3, cleaved caspase-8, cleaved caspase-9 and cleaved PARP-1 in BGC-823 and SGC-7901 cells, treated with EVO at different time, thus having prompted the activity of caspases pathways, which induces gastric cancer cells apoptosis.
Above all, EVO may induce gastric cancer cell arrest at G2/M checkpoint by promoting the expression of cell cycle inhibitor p53 and raising p53 expression and reducing the expression of cell cycle-promoting protein Cdc25C, as well as prompting the activity of caspases pathways to induce the apoptosis of gastric cancer cells. It provides new thoughts for our future research in which EVO is a new potential anticarcinogen for treatment of gastric cancer.
Conflicts of Interest
The authors declare no conflicts of interest regarding the publication of this pa-per.
References
[1] Ferlay, J., Soerjomataram, I., Dikshit, R., et al. (2015) Cancer Incidence and Mortal-ity Worldwide: Sources, Methods and Major Patterns in GLOBOCAN 2012. Inter-national Journal of Cancer, 136, E359-E386. https://doi.org/10.1002/ijc.29210
[2] Chen, W.Q., Zheng, R.S., Peter, D., et al. (2016) Cancer Statistics in China, 2015.
CA: A Cancer Journal for Clinicians, 2, 115-132. https://doi.org/10.3322/caac.21338
[3] Mihmanli, M., Ilhan, E., Idiz, U.O., et al. (2016) Recent Developments and Innova-tions in Gastric Cancer. World Journal of Gastroenterology, 22, 4307-4320.
https://doi.org/10.3748/wjg.v22.i17.4307
[4] Feng, Y., Wu, C.Y. and Li, J. (2017) Research Progress on the Advantages and Poss-ible Mechanisms of Traditional Chinese Medicine in the Treatment of Gastric Can-cer. Liaoning Journal of Traditional Chinese Medicine, 44, 200-203.
[5] Zhang, Q.R., Zhou, Z.Y., Pan, Z.H., et al. (2018) Evodiamine Inhibits the Growth of Huh7 Cells and Enhances the Sensitivity of Cells to TRAIL. Chinese Journal of Pa-thophysiology, 34, 212-217.
[6] Yang, F., Shi, L., Liang, T., et al. (2017) Anti-Tumor Effect of Evodiamine by In-ducing Akt-Mediated Apoptosis in Hepatocellular Carcinoma. Biochemical and Biophysical Research Communications, 485, 54-61.
https://doi.org/10.1016/j.bbrc.2017.02.017
[7] Han, S., Woo, J.K., Jung, Y., et al. (2016) Evodiamine Selectively Targets Cancer Stem-Like Cells through the p53-p21-Rb Pathway. Biochemical and Biophysical Research Communications, 469, 1153-1158.
https://doi.org/10.1016/j.bbrc.2015.12.066
[8] Huang, J., Chen, Z.H., Ren, C.M., et al. (2015) Antiproliferation Effect of Evodia-mine in Human Colon Cancer Cells Is Associated with IGF-1/HIF-1α Downregula-tion. Oncology Reports, 34, 3203-3211. https://doi.org/10.3892/or.2015.4309
[9] Mohan, V., Agarwal, R. and Singh, R.P. (2016) A Novel Alkaloid, Evodiamine Causes Nuclear Localization of Cytochrome-c and Induces Apoptosis Independent of p53 in Human Lung Cancer cells. Biochemical and Biophysical Research Com-munications, 477, 1065-1071. https://doi.org/10.1016/j.bbrc.2016.07.037
DOI: 10.4236/oalib.1105217 11 Open Access Library Journal of Traditional Chinese Medicine, 34, 115-118.
[14] Evan, G.I. and Vousden, K.H. (2001) Proliferation, Cell Cycle and Apoptosis in Cancer. Nature, 411, 342-348. https://doi.org/10.1038/35077213
[15] Sanchez, I. and Dynlacht, B.D. (2005) New Insights into Cyclins, CDKs, and Cell Cycle Control. Seminars in Cell and Developmental Biology, 16, 311-321.
https://doi.org/10.1016/j.semcdb.2005.02.007
[16] Peter, M., Le Peuch, C., Labbé, J.C., et al. (2002) Initial Activation of Cyclin-B1-cdc2 Kinase Requires Phosphorylation of Cyclin B1.EMBO Reports, 3, 551-556.
https://doi.org/10.1093/embo-reports/kvf111
[17] Chien, C.C., Wu, M.S., Shen, S.C., et al. (2014) Activation of JNK Contributes to Evodiamine-Induced Apoptosis and G2/M Arrest in Human Colorectal Carcinoma Cells: A Structure-Activity Study of Evodiamine. PLoS ONE, 9, e99729.
https://doi.org/10.1371/journal.pone.0099729
[18] Lu, X., Liu, D.A. And Xu, Y. (2013) The Gain of Function of p53 Cancer Mutant in Promoting Mammary Tumorigenesis. Oncogene, 32, 2900-2906.
https://doi.org/10.1038/onc.2012.299
[19] Yin, X., Zhang, R., Feng, C., et al. (2014) Diallyl Disulfide Induces G2/M Arrest and Promotes Apoptosis through the p53/p21 and MEK-ERK Pathways in Human Esophageal Squamous Cell Carcinoma. Oncology Reports, 32, 1748-1756.
https://doi.org/10.3892/or.2014.3361
[20] Godefroy, N., Lemaire, C., Renaud, F., et al. (2004) P53 Can Promote Mitochondria- and Caspase-Independent Apoptosis. Cell Death & Differentiation, 11, 785-787.
https://doi.org/10.1038/sj.cdd.4401398
[21] Wawryk-Gawda, E., Chylińska-Wrzos, P., Lis-Sochocka, M., et al. (2014) P53 Pro-tein in Proliferation, Repair and Apoptosis of Cells. Protoplasma, 251, 525-533.
https://doi.org/10.1007/s00709-013-0548-1
[22] St. Clair, S., Giono, L., Varmeh-Ziaie, S., et al. (2004) DNA Damage-Induced Downregulation of Cdc25C Is Mediated by p53 via Two Independent Mechanisms: One Involves Direct Binding to the cdc25C Promoter. Molecular Cell, 16, 725-736.
https://doi.org/10.1016/j.molcel.2004.11.002
[23] Fu, Z., Han, X., Du, J., et al. (2018) Euphorbialunulata Extract Acts on Multidrug Resistant Gastric Cancer Cells to Inhibit Cell Proliferation, Migration and Invasion, Arrest Cell Cycle Progression, and Induce Apoptosis. Journal of Ethnopharmacolo-gy, 212, 8-17.https://doi.org/10.1016/j.jep.2017.08.014
[24] Zhang, X. and Song, T. (2002) Study on Caspase-3 and Apoptosis. Medical Review, 8, 621-623.
[25] Xu, Y., Gao, C.C., Pan, Z.G., et al. (2018) Irigenin Sensitizes TRAIL-Induced Apop-tosis via Enhancing Pro-Apoptotic Molecules in Gastric Cancer Cells. Biochemical and Biophysical Research Communications, 496, 998-1005.