metal-organic papers
m750
Akitsu and Einaga [Cu3Co2(CN)12(C4H12N2)6]4H2O doi:10.1107/S1600536806008105 Acta Cryst.(2006). E62, m750–m752 Acta Crystallographica Section E
Structure Reports Online
ISSN 1600-5368
Bis(
N
-ethylethylenediamine-
j
2N
,
N
000)copper(II)–
hexacyanocobaltate(III)–water (3/2/4): a
two-dimensional ladder structure of a
bimetallic assembly
Takashiro Akitsu* and Yasuaki Einaga
Department of Chemistry, Faculty of Science and Technology, Keio University, 3-14-1 Hiyoshi, Kohoku-ku, Yokohama 223-8522, Japan
Correspondence e-mail: akitsu@chem.keio.ac.jp
Key indicators
Single-crystal X-ray study
T= 298 K
Mean(C–C) = 0.006 A˚
Rfactor = 0.048
wRfactor = 0.150
Data-to-parameter ratio = 20.2
For details of how these key indicators were automatically derived from the article, see http://journals.iucr.org/e.
Received 23 January 2006 Accepted 6 March 2006
#2006 International Union of Crystallography
All rights reserved
The title compound, poly[[tetracyano-octa- -cyano-hexakis-(N-ethylethylenediamine)tricopper(II)dicobaltate(III)] tetra-hydrate], [Cu(C4H12N2)2]3[Co(CN)6]24H2O or {[Cu3Co2 -(CN)12(C4H12N2)6]4H2O}n, was crystallized from an aqueous
reaction mixture containing Cu(ClO4)2, N -ethylethylene-diamine and K3[Co(CN)6] in a self-assembling process. The polymeric chains are extended through –CoIII–CN–CuII– linkages involving four or two cyanide ligands of the centrosymmetric [Co(CN)6]
3
complex ions, forming a two-dimensional ladder structure. The CuIIatoms, one of which also lies on an inversion centre, have a distorted octahedral coordination geometry, the degree of tetragonal Jahn–Teller distortion beingT= 0.785 and 0.810 (Tis the ratio of mean in-plane Cu—N bond lengths to axial Cu—N bond lengths). This local Jahn–Teller distortion results from the anisotropic features of this two-dimensional cyano-bridged structure.
Comment
The design of photofunctional transition-metal complexes such as Fe–Co Prussian blue analogues is one of the challenges in materials science (Sato, 2003; Sato et al., 2003). Recently, Escaxet al.(2005) elucidated that photo-induced magnetiza-tion of Fe–Co Prussian blue analogues caused three-dimen-sional structural strain, which weakens the ligand field strength of the cyanide ligands. Magneto–structural correla-tions of cyano-bridged bimetallic assemblies (Ohba & Okawa, 2000; Okawa & Ohba 2002) and the photophysical properties of the [Co(CN)6]
3
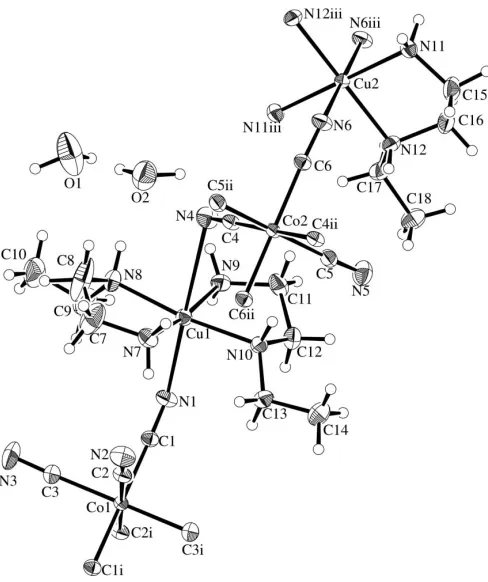
The asymmetric unit of (I) consists of one and a half [Cu(N -Eten)2]
2+
cations (N-Eten = N-ethylethylenediamine), two half [Co(CN)6]
3
anions, and two water molecules of crystal-lization (Fig. 1). Atoms Cu2, Co1 and Co2 are located at centre of symmetry. The [Cu(N-Eten)2]
2+
cations are linked to the [Co(CN)6]3anions through cyanide bridges to give a two-dimensional ladder-like structure, the vertical chains of which run along theaaxis (Fig. 2). The bond distance ranges include: Co—C 1.887 (3)–1.899 (3) A˚ , Cu—N (in-plane) 1.998 (3)– 2.070 (3) A˚ and C—N(cyanide) 1.133 (5)–1.157 (4) A˚ (Table 1). These values are comparable with the analogous cyano-bridged CuII–CoIII bimetallic assemblies (Ferbinteanu
et al., 1999; Mondalet al., 2001; Liet al., 2002; Marvaudet al., 2003; Sahaet al., 2004). The axial Cu—N bond distances are 2.366 (3)–2.828 (3) A˚ . There are intermolecular O—H O/N and N—H N hydrogen bonds (Table 2), where the acceptor N atoms are those of the cyanide groups.
In the two-dimensional ladder structure, the horizontal bridge (related to Cu1) is more strained than the vertical bridge (related to Cu2, see Fig. 2). The degrees of tetragonal Jahn–Teller distortion of the Cu1 and Cu2 sites areT= 0.785 and 0.810, respectively (Tis the ratio of mean in-plane Cu—X
bond lengths to axial Cu—X bond lengths; Hathaway &
Billing, 1970). The T values for mononuclear
[Cu(en)2](ClO4)2, which shows considerable temperature-induced distortion, are 0.780, 0.785 and 0.791 at 297, 274 and 120 K, respectively (en = ethylenediamine; Akitsu & Einaga, 2004). Therefore, the structural dimensionality of the cyano-bridges in (I) may be ascribed to the anisotropic Jahn–Teller distortion of bimetallic assemblies in the solid state. On the other hand, no significant characteristic in-plane distortion (e.g. cis- ortrans-N—Cu—N bond angles) could be observed, notwithstanding the asymmetric introduction of N-ethyl groups.
Experimental
Compound (I) was obtained by slow diffusion of an aqueous solution (30 ml) of Cu(ClO4)2 (0.375 g, 1.00 mmol) and N -ethylethylene-diamine (0.176 g, 2.00 mmol) into an aqueous solution (30 ml) of K3[Co(CN)6] (0.165 g, 5.00 mmol) at 298 K. The mixed solution was left to stand in the dark for a few days. Blue plate-like single crystals of (I) were obtained (yield 0.224 g). Analysis calculated for C36H80N24Co2Cu3O4: C 35.39, H 6.60, N 27.52%; found: C 35.35, H 6.91, N 27.50%. IR (KBr): 2118 cm1(cyanide). Electronic spec-trum (diffuse reflectance): 17500 cm1(d–dtransition of CuIIion of 2Eground state).
Crystal data
[Cu3Co2(CN)12(C4H12N2)6]4H2O Mr= 1221.75
Triclinic,P1
a= 9.9783 (11) A˚
b= 11.976 (3) A˚
c= 12.060 (2) A˚
= 78.131 (15)
= 82.769 (11)
= 88.851 (12) V= 1399.1 (5) A˚3
Z= 1
Dx= 1.450 Mg m 3
MoKradiation Cell parameters from 25
reflections
= 10.1–14.6
= 1.76 mm1 T= 298 (2) K Plate, blue
0.600.300.10 mm
metal-organic papers
Acta Cryst.(2006). E62, m750–m752 Akitsu and Einaga [Cu
[image:2.610.48.292.68.359.2] [image:2.610.47.291.429.719.2]3Co2(CN)12(C4H12N2)6]4H2O
m751
Figure 1The asymmetric unit of (I), showing the atom-labelling scheme. Displacement ellipsoids are drawn at the 30% probability level. [Symmetry codes: (i) 2x, 1y, 1z; (ii) 1x,y, 2z; (iii)
x,y, 2z.]
Figure 2
Data collection
Rigaku AFC-7Rdiffractometer
!/2scans
Absorption correction: scan (Northet al., 1968)
Tmin= 0.398,Tmax= 0.839
7176 measured reflections 6418 independent reflections 5277 reflections withI> 2(I)
Rint= 0.059
max= 27.5
h=5!12
k=15!15
l=15!15 3 standard reflections
every 150 reflections intensity decay: 3.8%
Refinement
Refinement onF2 R[F2> 2(F2)] = 0.048 wR(F2) = 0.150 S= 1.16 6418 reflections 317 parameters
H-atom parameters constrained
w= 1/[2(F
o2) + (0.0966P)2
+ 0.7656P]
whereP= (Fo2+ 2Fc2)/3
(/)max< 0.001
max= 1.58 e A˚ 3
min=1.09 e A˚ 3
Extinction correction:SHELXL97
(Sheldrick, 1997)
[image:3.610.44.300.309.539.2]Extinction coefficient: 0.028 (3)
Table 1
Selected geometric parameters (A˚ ,).
Cu1—N9 1.998 (3) Cu1—N7 2.012 (3) Cu1—N8 2.058 (3) Cu1—N10 2.070 (3) Cu1—N1 2.366 (3) Cu1—N4 2.828 (3) Cu2—N11 2.027 (3) Cu2—N12 2.064 (2) Cu2—N6 2.522 (3) Co1—C1 1.887 (3) Co1—C2 1.895 (3)
Co1—C3 1.899 (3) Co2—C6 1.895 (3) Co2—C5 1.894 (3) Co2—C4 1.896 (3) N1—C1 1.157 (4) N2—C2 1.153 (4) N3—C3 1.133 (5) N4—C4 1.143 (4) N5—C5 1.153 (4) N6—C6 1.141 (4)
N9—Cu1—N7 168.90 (12) N9—Cu1—N8 92.95 (12) N7—Cu1—N8 84.29 (12) N9—Cu1—N10 84.96 (11) N7—Cu1—N10 96.43 (11) N8—Cu1—N10 172.71 (11) N9—Cu1—N1 95.31 (10) N7—Cu1—N1 95.71 (11) N8—Cu1—N1 97.37 (12) N10—Cu1—N1 89.79 (10) N11—Cu2—N12 84.54 (10) N11i—Cu2—N12 95.46 (10)
C1ii
—Co1—C2 90.36 (12) C1—Co1—C2 89.64 (12) C1—Co1—C3 88.58 (13) C2—Co1—C3 89.99 (15) C1—Co1—C3ii 91.42 (13) C2—Co1—C3ii
90.01 (15) C6—Co2—C5iii
92.56 (13) C6—Co2—C5 87.44 (12) C6—Co2—C4iii
91.05 (11) C5—Co2—C4iii
90.94 (13) C6—Co2—C4 88.95 (11) C5—Co2—C4 89.06 (13)
[image:3.610.44.296.598.711.2]Symmetry codes: (i) x;y;zþ2; (ii) xþ2;yþ1;zþ1; (iii) xþ1;y;zþ2.
Table 2
Hydrogen-bond geometry (A˚ ,).
D—H A D—H H A D A D—H A
O1—H1A N3iv
0.95 1.97 2.899 (8) 165 O1—H1B N5v
0.96 2.03 2.979 (6) 174 O2—H2A N2iv
0.95 2.15 3.086 (5) 170 O2—H2B O1 0.95 1.84 2.792 (6) 176 N7—H7C N2 0.90 2.36 3.206 (4) 156 N7—H7D N6iii
0.90 2.39 3.183 (4) 146 N8—H8C O2 0.91 2.29 3.146 (4) 157 N9—H9C N1vi
0.90 2.34 3.162 (4) 152 N9—H9D N4 0.90 2.92 3.204 (4) 100 N11—H11C N6i 0.90 2.99 3.280 (3) 101 N11—H11D N2iii
0.90 2.35 3.190 (4) 156
Symmetry codes: (i) x;y;zþ2; (iii) xþ1;y;zþ2; (iv) x1;y;z; (v)
x;y;z1; (vi)xþ1;yþ1;zþ1.
H atoms bonded to C and N atoms were placed in calculated positions, with C—H = 0.96–0.97 A˚ and N—H = 0.90–0.91 A˚, and included in the final cycles of refinement using riding constraints, with Uiso(H) = 1.2Ueq(C,N). The water H-atom positions were calculated based on some peaks observed in the difference Fourier map, with O—H = 0.95 A˚ and withUiso(H) = 1.2Ueq(O). The large displace-ments of atoms C7 and C8, the short C7—C8 distance and the smaller N7—C7—C8—N8 torsion angle than for the other chelate rings suggest positional disorder of these C atoms. The highest peak is located 0.94 A˚ from atom Cu1 and the deepest hole 0.80 A˚ also from Cu1.
Data collection: WinAFC Diffractometer Control Software (Rigaku, 1999); cell refinement: WinAFC Diffractometer Control Software; data reduction:TEXSAN(Molecular Structure Corpora-tion, 1989); program(s) used to solve structure:SIR92(Altomareet al., 1994); program(s) used to refine structure: SHELXL97 (Shel-drick, 1997); molecular graphics:ORTEPII(Johnson, 1976); software used to prepare material for publication:TEXSAN.
This work was supported by a Grant-in-Aid for the 21st Century COE programme ‘KEIO Life Conjugate Chemistry’ from the Ministry of Education, Culture, Sports, Science and Technology, the Research Foundation for Opto-Science and Technology, and the Japan and Mizuho Foundation for the Promotion of Science.
References
Akitsu, T. & Einaga, Y. (2004).Bull. Chem. Soc. Jpn,77, 763–764. Alexander, J. J. & Gray, H. B. (1968).J. Am. Chem. Soc.90, 4260–4271. Altomare, A., Cascarano, G., Giacovazzo, C., Guagliardi, A., Burla, M. C.,
Polidori, G. & Camalli, M. (1994).J. Appl. Cryst.27, 435.
Azumi, T. & McGlynn, S. P. (2004).J. Phys. Chem. A,108, 6968–6974. Escax, V., Champion, G., Arrio, M.-A., Zacchigna, M., Cartier dit Moulin, C. &
Bleuzen, A. (2005).Angew. Chem. Int. Ed.44, 4798–4801. Falvello, L. R. (1997).J. Chem. Soc. Dalton Trans.pp. 4463–4475.
Ferbinteanu, M., Tanase, S., Andruh, M., Journaux, Y., Cimpoesu, F., Strenger, I. & Riviere, E. (1999).Polyhedron,18, 3019–3025.
Hathaway, B. J. & Billing, D. E. (1970).Coord. Chem. Rev.5, 143–207. Johnson, C. K. (1976).ORTEPII. Report ORNL-5138. Oak Ridge National
Laboratory, Tennessee, USA.
Li, B., Shen, X., Yu, K. & Xu, Z. (2002).J. Coord. Chem.55, 1191–1198. Marvaud, V., Decroix, C., Scuiller, A., Guyard-Duhayon, C., Vaissermann, J.,
Gonnet, F. & Verdaguer, M. (2003).Chem. Eur. J.8, 1678–1691. Molecular Structure Corporation (1989).TEXSAN. Version 1.11. MSC, 3200
Research Forest Drive, The Woodlands, TX 77381, USA.
Mondal, N., Dey, D. K., Mitra, S. & Gramlich, V. (2001).Polyhedron,20, 607– 613.
Murphy, B. & Hathaway, B. (2003).Coord. Chem. Rev.243, 237–262. North, A. C. T., Phillips, D. C. & Mathews, F. S. (1968).Acta Cryst.A24, 351–
359.
Ohba, M. & Okawa, H. (2000).Coord. Chem. Rev.198, 313–328. Okawa, H. & Ohba, M. (2002).Bull. Chem. Soc. Jpn,75, 1191–1203. Rigaku (1999).WinAFC Diffractometer Control Software. Rigaku
Corpora-tion, Tokyo, Japan.
Saha, M. K., Lloret, F. & Bernal, I. (2004).Inorg. Chem.43, 1969–1975. Sato, O. (2003).Acc. Chem. Res.36, 692–700.
Sato, O., Hayami, S., Einaga, Y. & Gu, Z.-Z. (2003).Bull. Chem. Soc. Jpn,76, 443–470.
Sheldrick, G. M. (1997).SHELXL97. University of Go¨ttingen, Germany. Simmons, C. J. (1993).New J. Chem.17, 77–95.
metal-organic papers
m752
Akitsu and Einaga [Cusupporting information
sup-1
Acta Cryst. (2006). E62, m750–m752
supporting information
Acta Cryst. (2006). E62, m750–m752 [https://doi.org/10.1107/S1600536806008105]
Bis(
N
-ethylethylenediamine-
κ
2N
,
N
′
)copper(II)
–
hexacyanocobaltate(III)
–
water
(3/2/4): a two-dimensional ladder structure of a bimetallic assembly
Takashiro Akitsu and Yasuaki Einaga
poly[[tetracyano-octa-µ-cyano-hexakis(N– ethylethylenediamine)tricopper(II)dicobalt(II)] tetrahydrate]
Crystal data
[Cu3Co2(CN)12(C4H12N2)6]·4H2O Mr = 1221.75
Triclinic, P1 Hall symbol: -P 1
a = 9.9783 (11) Å
b = 11.976 (3) Å
c = 12.060 (2) Å
α = 78.131 (15)°
β = 82.769 (11)°
γ = 88.851 (12)°
V = 1399.1 (5) Å3
Z = 1
F(000) = 637
Dx = 1.450 Mg m−3
Mo Kα radiation, λ = 0.71073 Å Cell parameters from 25 reflections
θ = 10.1–14.6°
µ = 1.76 mm−1 T = 298 K Plate, blue
0.60 × 0.30 × 0.10 mm
Data collection
Rigaku AFC-7R diffractometer
Radiation source: Rigaku rotating anode generator
Graphite monochromator
ω/2θ scans
Absorption correction: ψ scan (North et al., 1968)
Tmin = 0.398, Tmax = 0.839 7176 measured reflections
6418 independent reflections 5277 reflections with I > 2σ(I)
Rint = 0.059
θmax = 27.5°, θmin = 2.5°
h = −5→12
k = −15→15
l = −15→15
3 standard reflections every 150 reflections intensity decay: 3.8%
Refinement
Refinement on F2
Least-squares matrix: full
R[F2 > 2σ(F2)] = 0.048 wR(F2) = 0.150 S = 1.16 6418 reflections 317 parameters 0 restraints
Primary atom site location: structure-invariant direct methods
Secondary atom site location: inferred from neighbouring sites
Hydrogen site location: constr H-atom parameters constrained
w = 1/[σ2(Fo2) + (0.0966P)2 + 0.7656P]
where P = (Fo2 + 2Fc2)/3
(Δ/σ)max < 0.001
Δρmax = 1.58 e Å−3
Δρmin = −1.09 e Å−3
Extinction correction: SHELXL97 (Sheldrick, 1997), Fc*=kFc[1+0.001xFc2λ3/sin(2θ)]-1/4
supporting information
sup-2
Acta Cryst. (2006). E62, m750–m752
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
x y z Uiso*/Ueq
supporting information
sup-3
Acta Cryst. (2006). E62, m750–m752
H9B 0.5177 0.3388 0.3983 0.088* C10 0.5700 (6) 0.2273 (5) 0.2936 (4) 0.0784 (14) H10A 0.4828 0.1915 0.3042 0.096* H10B 0.6391 0.1713 0.2869 0.096* H10C 0.5783 0.2857 0.2253 0.096* C11 0.3459 (3) 0.3751 (4) 0.7417 (3) 0.0504 (8) H11A 0.3074 0.3083 0.7952 0.061* H11B 0.2757 0.4321 0.7304 0.061* C12 0.4621 (4) 0.4219 (3) 0.7885 (3) 0.0460 (7) H12A 0.4933 0.4939 0.7398 0.056* H12B 0.4327 0.4352 0.8645 0.056* C13 0.6988 (3) 0.3831 (3) 0.8212 (3) 0.0446 (7) H13A 0.7278 0.4500 0.7633 0.054* H13B 0.7688 0.3261 0.8184 0.054* C14 0.6861 (5) 0.4151 (5) 0.9366 (4) 0.0685 (12) H14A 0.7716 0.4427 0.9489 0.082* H14B 0.6591 0.3492 0.9949 0.082* H14C 0.6194 0.4737 0.9396 0.082* C15 −0.0717 (4) 0.0411 (3) 1.2262 (3) 0.0534 (9) H15A 0.0125 0.0256 1.2587 0.064* H15B −0.1442 0.0399 1.2881 0.064* C16 −0.0646 (4) 0.1558 (3) 1.1471 (3) 0.0518 (9) H16A −0.1518 0.1751 1.1209 0.061* H16B −0.0401 0.2139 1.1864 0.061* C17 0.0388 (4) 0.2557 (3) 0.9589 (3) 0.0476 (8) H17A −0.0507 0.2653 0.9350 0.057* H17B 0.1010 0.2452 0.8933 0.057* C18 0.0772 (5) 0.3630 (3) 0.9930 (4) 0.0592 (10) H18A 0.1666 0.3555 1.0151 0.071* H18B 0.0144 0.3761 1.0561 0.071* H18C 0.0751 0.4262 0.9297 0.071*
Atomic displacement parameters (Å2)
U11 U22 U33 U12 U13 U23
supporting information
sup-4
Acta Cryst. (2006). E62, m750–m752
N8 0.0535 (16) 0.0476 (15) 0.0335 (14) 0.0040 (12) −0.0075 (12) −0.0146 (11) N9 0.0325 (12) 0.0414 (14) 0.0420 (14) −0.0030 (10) −0.0073 (10) −0.0071 (11) N10 0.0383 (12) 0.0321 (12) 0.0270 (11) −0.0058 (9) −0.0013 (9) −0.0039 (9) N11 0.0350 (12) 0.0343 (12) 0.0311 (12) 0.0008 (9) −0.0003 (9) −0.0038 (9) N12 0.0375 (12) 0.0303 (12) 0.0420 (14) −0.0041 (9) −0.0054 (10) −0.0129 (10) C1 0.0317 (13) 0.0291 (13) 0.0291 (13) −0.0028 (10) −0.0029 (10) −0.0012 (10) C2 0.0299 (13) 0.0330 (14) 0.0378 (15) −0.0052 (10) −0.0002 (11) −0.0024 (12) C3 0.0438 (17) 0.0497 (18) 0.0369 (16) −0.0061 (14) 0.0005 (13) −0.0169 (14) C4 0.0273 (12) 0.0341 (14) 0.0329 (15) −0.0062 (10) 0.0008 (10) −0.0020 (11) C5 0.0319 (13) 0.0394 (15) 0.0313 (14) −0.0019 (11) 0.0005 (11) −0.0056 (11) C6 0.0286 (13) 0.0323 (13) 0.0260 (13) −0.0019 (10) −0.0034 (10) −0.0004 (10) C7 0.075 (3) 0.096 (4) 0.123 (5) 0.041 (3) −0.038 (3) −0.071 (4) C8 0.154 (6) 0.121 (5) 0.089 (4) 0.093 (5) −0.069 (4) −0.073 (4) C9 0.117 (4) 0.061 (3) 0.038 (2) −0.014 (3) −0.013 (2) −0.0079 (18) C10 0.092 (3) 0.109 (4) 0.040 (2) 0.004 (3) −0.014 (2) −0.025 (2) C11 0.0333 (15) 0.067 (2) 0.049 (2) 0.0072 (15) 0.0015 (14) −0.0114 (17) C12 0.0482 (18) 0.0498 (19) 0.0425 (18) 0.0079 (14) −0.0040 (14) −0.0169 (14) C13 0.0434 (17) 0.0490 (18) 0.0446 (18) −0.0093 (14) −0.0079 (14) −0.0148 (14) C14 0.073 (3) 0.089 (3) 0.055 (2) −0.014 (2) −0.015 (2) −0.035 (2) C15 0.074 (2) 0.057 (2) 0.0296 (16) −0.0025 (18) 0.0019 (16) −0.0137 (14) C16 0.071 (2) 0.0425 (18) 0.0438 (19) 0.0024 (16) 0.0038 (17) −0.0195 (15) C17 0.057 (2) 0.0324 (15) 0.053 (2) −0.0086 (14) −0.0045 (16) −0.0100 (14) C18 0.074 (3) 0.0295 (16) 0.075 (3) −0.0045 (16) −0.007 (2) −0.0143 (16)
Geometric parameters (Å, º)
Cu1—N9 1.998 (3) N10—C12 1.475 (4)
Cu1—N7 2.012 (3) N10—C13 1.492 (4)
Cu1—N8 2.058 (3) N10—H10D 0.9100
Cu1—N10 2.070 (3) N11—C15 1.479 (4)
Cu1—N1 2.366 (3) N11—H11C 0.9000
Cu1—N4 2.828 (3) N11—H11D 0.9000
Cu2—N11 2.027 (3) N12—C17 1.484 (4) Cu2—N11i 2.027 (3) N12—C16 1.480 (5)
Cu2—N12 2.064 (2) N12—H12C 0.9100
Cu2—N12i 2.064 (2) C7—C8 1.354 (7)
Cu2—N6 2.522 (3) C7—H7A 0.9700
Co1—C1ii 1.887 (3) C7—H7B 0.9700
Co1—C1 1.887 (3) C8—H8A 0.9700
Co1—C2ii 1.895 (3) C8—H8B 0.9700
Co1—C2 1.895 (3) C9—C10 1.512 (6)
Co1—C3 1.899 (3) C9—H9A 0.9700
Co1—C3ii 1.899 (3) C9—H9B 0.9700
Co2—C6iii 1.895 (3) C10—H10A 0.9600
Co2—C6 1.895 (3) C10—H10B 0.9600
Co2—C5iii 1.894 (3) C10—H10C 0.9600
Co2—C5 1.894 (3) C11—C12 1.517 (5)
supporting information
sup-5
Acta Cryst. (2006). E62, m750–m752
Co2—C4 1.896 (3) C11—H11B 0.9700
O1—H1A 0.9500 C12—H12A 0.9700
O1—H1B 0.9500 C12—H12B 0.9700
O2—H2A 0.9500 C13—C14 1.508 (5)
O2—H2B 0.9500 C13—H13A 0.9700
N1—C1 1.157 (4) C13—H13B 0.9700
N2—C2 1.153 (4) C14—H14A 0.9600
N3—C3 1.133 (5) C14—H14B 0.9600
N4—C4 1.143 (4) C14—H14C 0.9600
N5—C5 1.153 (4) C15—C16 1.501 (5)
N6—C6 1.141 (4) C15—H15A 0.9700
N7—C7 1.475 (5) C15—H15B 0.9700
N7—H7C 0.9000 C16—H16A 0.9700
N7—H7D 0.9000 C16—H16B 0.9700
N8—C8 1.444 (5) C17—C18 1.499 (5)
N8—C9 1.458 (5) C17—H17A 0.9700
N8—H8C 0.9100 C17—H17B 0.9700
N9—C11 1.468 (5) C18—H18A 0.9600
N9—H9C 0.9000 C18—H18B 0.9600
N9—H9D 0.9000 C18—H18C 0.9600
N9—Cu1—N7 168.90 (12) N2—C2—Co1 178.9 (3) N9—Cu1—N8 92.95 (12) N3—C3—Co1 177.6 (4) N7—Cu1—N8 84.29 (12) N4—C4—Co2 177.5 (3) N9—Cu1—N10 84.96 (11) N5—C5—Co2 176.8 (3) N7—Cu1—N10 96.43 (11) N6—C6—Co2 176.3 (3) N8—Cu1—N10 172.71 (11) C8—C7—N7 115.6 (4) N9—Cu1—N1 95.31 (10) C8—C7—H7A 108.4 N7—Cu1—N1 95.71 (11) N7—C7—H7A 108.4 N8—Cu1—N1 97.37 (12) C8—C7—H7B 108.4 N10—Cu1—N1 89.79 (10) N7—C7—H7B 108.4 N11—Cu2—N12 84.54 (10) H7A—C7—H7B 107.4 N11i—Cu2—N12 95.46 (10) C7—C8—N8 116.2 (5)
N11—Cu2—N12i 95.46 (10) C7—C8—H8A 108.2
N11i—Cu2—N12i 84.54 (10) N8—C8—H8A 108.2
C1ii—Co1—C2ii 89.64 (12) C7—C8—H8B 108.2
C1—Co1—C2ii 90.36 (12) N8—C8—H8B 108.2
C1ii—Co1—C2 90.36 (12) H8A—C8—H8B 107.4
C1—Co1—C2 89.64 (12) N8—C9—C10 115.7 (4) C1ii—Co1—C3 91.42 (13) N8—C9—H9A 108.4
C1—Co1—C3 88.58 (13) C10—C9—H9A 108.4 C2ii—Co1—C3 90.01 (15) N8—C9—H9B 108.4
C2—Co1—C3 89.99 (15) C10—C9—H9B 108.4 C1ii—Co1—C3ii 88.58 (13) H9A—C9—H9B 107.4
C1—Co1—C3ii 91.42 (13) C9—C10—H10A 109.5
C2ii—Co1—C3ii 89.99 (15) C9—C10—H10B 109.5
C2—Co1—C3ii 90.01 (15) H10A—C10—H10B 109.5
supporting information
sup-6
Acta Cryst. (2006). E62, m750–m752
C6—Co2—C5iii 92.56 (13) H10A—C10—H10C 109.5
C6iii—Co2—C5 92.56 (13) H10B—C10—H10C 109.5
C6—Co2—C5 87.44 (12) N9—C11—C12 108.5 (3) C6iii—Co2—C4iii 88.95 (11) N9—C11—H11A 110.0
C6—Co2—C4iii 91.05 (11) C12—C11—H11A 110.0
C5iii—Co2—C4iii 89.06 (13) N9—C11—H11B 110.0
C5—Co2—C4iii 90.94 (13) C12—C11—H11B 110.0
C6iii—Co2—C4 91.05 (11) H11A—C11—H11B 108.4
C6—Co2—C4 88.95 (11) N10—C12—C11 108.2 (3) C5iii—Co2—C4 90.94 (13) N10—C12—H12A 110.1
supporting information
sup-7
Acta Cryst. (2006). E62, m750–m752
C16—N12—H12C 108.0 H18A—C18—H18C 109.5 Cu2—N12—H12C 108.0 H18B—C18—H18C 109.5 N1—C1—Co1 177.7 (3)
N9—Cu1—N1—C1 172.2 (3) C9—N8—C8—C7 102.0 (7) N7—Cu1—N1—C1 −9.2 (4) Cu1—N8—C8—C7 −28.0 (8) N8—Cu1—N1—C1 −94.1 (3) C8—N8—C9—C10 57.9 (6) N10—Cu1—N1—C1 87.3 (3) Cu1—N8—C9—C10 −175.8 (3) N9—Cu1—N7—C7 78.5 (7) Cu1—N9—C11—C12 38.1 (3) N8—Cu1—N7—C7 2.5 (4) C13—N10—C12—C11 171.5 (3) N10—Cu1—N7—C7 175.2 (3) Cu1—N10—C12—C11 41.2 (3) N1—Cu1—N10—C12 78.8 (2) N9—C11—C12—N10 −53.6 (4) N9—Cu1—N10—C13 −143.5 (2) C12—N10—C13—C14 61.6 (4) N7—Cu1—N10—C13 47.6 (2) Cu1—N10—C13—C14 −174.5 (3) N1—Cu1—N10—C13 −48.1 (2) Cu2—N11—C15—C16 34.7 (4) N12—Cu2—N11—C15 −7.3 (2) C17—N12—C16—C15 172.3 (3) N12i—Cu2—N11—C15 172.7 (2) Cu2—N12—C16—C15 45.8 (3)
N11i—Cu2—N12—C16 158.8 (2) N11—C15—C16—N12 −54.5 (4)
Cu1—N7—C7—C8 −19.3 (7) C16—N12—C17—C18 64.0 (4) N7—C7—C8—N8 32.7 (10) Cu2—N12—C17—C18 −175.6 (3)
Symmetry codes: (i) −x, −y, −z+2; (ii) −x+2, −y+1, −z+1; (iii) −x+1, −y, −z+2.
Hydrogen-bond geometry (Å, º)
D—H···A D—H H···A D···A D—H···A
O1—H1A···N3iv 0.95 1.97 2.899 (8) 165
O1—H1B···N5v 0.96 2.03 2.979 (6) 174
O2—H2A···N2iv 0.95 2.15 3.086 (5) 170
O2—H2B···O1 0.95 1.84 2.792 (6) 176 N7—H7C···N2 0.90 2.36 3.206 (4) 156 N7—H7D···N6iii 0.90 2.39 3.183 (4) 146
N8—H8C···O2 0.91 2.29 3.146 (4) 157 N9—H9C···N1vi 0.90 2.34 3.162 (4) 152
N9—H9D···N4 0.90 2.92 3.204 (4) 100 N11—H11C···N6i 0.90 2.99 3.280 (3) 101
N11—H11D···N2iii 0.90 2.35 3.190 (4) 156