Review Article
The efficacy of metformin treatment for
myocardial infarction: a meta-analysis
of randomized controlled trials
Jialing Jiang1, Yong Luo2
1Department of Cardiology, The Second People’s Hospital of Liangjiang New District, Chongqing, China; 2
Depart-ment of Cardiology Medical, Jiangjin Central Hospital of Chongqing, Chongqing, China
Received January 5, 2019; Accepted July 12, 2019; Epub September 15, 2019; Published September 30, 2019
Abstract: Introduction: The efficacy of metformin treatment for myocardial infarction remains controversial. We conduct a systematic review and meta-analysis to explore the impact of metformin versus placebo on myocardial infarction. Methods: We searched PubMed, EMbase, Web of science, EBSCO, and Cochrane library databases until July 2018 for randomized controlled trials (RCTs) assessing the effect of metformin versus placebo on myocardial in-farction. This meta-analysis is performed using the random-effects model. Results: Six RCTs involving 1138 patients are included in this meta-analysis. Overall, compared with the control group of myocardial infarction, metformin treatment had no important impact on major adverse cardiac events (RR = 2.06; 95% CI = 0.90 to 4.74; P = 0.09), reinfarction (RR = 1.60; 95% CI = 0.67 to 3.85; P = 0.29), LVEF (MD = 1.25; 95% CI = -1.51 to 4.01; P = 0.38), NT-proBNP (MD = 0; 95% CI = -0.14 to 0.14; P = 1.0), glucose (MD = 0.05; 95% CI = -0.05 to 0.15; P = 0.31) and HbA1c (MD = 0; 95% CI = -0.02 to 0.02; P = 1.0), but results in the increase in LVEDV (MD = 0.50; 95% CI = 0.43 to 0.57; P < 0.00001) and LVESV were significant (MD = 3.80; 95% CI = 3.75 to 3.86; P < 0.00001). Conclusions: Metformin treatment may not provide significant benefits to patients with myocardial infarction.
Keywords: Metformin, myocardial infarction, major adverse cardiac events, randomized controlled trials, meta-analysis
Introduction
Myocardial infarction has spread wide through-out the world, with high mortality [1-3]. Patients with ST-segment elevation myocardial infarc-tion need immediate treatment with antithrom-botic agents and primary percutaneous
inter-vention to restore coronary blood flow [2, 4].
Myocardial damage and the risk of developing left ventricular dysfunction can be substantially reduced by timely reperfusion [5-8], but there are as many as 50% of patients presenting left ventricular dysfunction, and approximately 20% to 40% of patients having heart failure [9-11]. The dimethylbiguanide metformin is known as
a first-line treatment in patients with type II dia -betes mellitus, and shows some cardioprotec-tive effects in myocardial infarction irrespeccardioprotec-tive of glucose-lowering abilities [12, 13]. Metformin treatment prior to reperfusion is reported to
reduce infarct size in patients with diabetes mellitus [14]. Reduced N-terminal pro-brain
natriuretic peptide (NT pro-BNP) levels are
found after metformin treatment in patients with type II diabetes mellitus, which supports a
potential beneficial effect on the risk of heart
failure [15]. Metformin treatment during reper-fusion can result in the improvement of left ven-tricular ejection fraction (LVEF) in non-diabetic mice undergoing permanent coronary artery ligation [12].
However, the efficacy of metformin treatment
on myocardial infarction has not been well established. Recently, several studies on the topic have been published, and the results have
been conflicting [16-18]. With accumulating evi -dence, we therefore performed a systematic review and meta-analysis of RCTs to investigate
Materials and methods
Ethical approval and patient consent are not required because this is a systematic review and meta-analysis of previously published stud-ies. This systematic review and meta-analysis are conducted and reported in adherence to PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) [19]. Search strategy and study selection
Two investigators independently searched the following databases (from inception to July
2018): PubMed, EMbase, Web of science, EBSCO, and Cochrane library databases. The
electronic search strategy was conducted using the following keywords: metformin, and
myo-cardial infarction. We also checked the refer -ence lists of the screened full-text studies to identify other potentially eligible trials.
The inclusive selection criteria are as follows: (i) population: patients with myocardial infarction; (ii) intervention: metformin treatment; (iii) com-parison: placebo; (iv) study design: RCT. Patients with known diabetes, previous myo-cardial infarction, and severe renal dysfunction are excluded.
Data extraction and outcome measures
We have extracted the following information:
author, number of patients, age, gender, body
Quality assessment in individual studies
Methodological quality of the included studies
is independently evaluated using the modified
Jadad scale [20]. There are 3 items for Jadad scale: randomization (0-2 points), blinding (0-2 points), dropouts and withdrawals (0-1 points). The score of Jadad Scale varies from 0
to 5 points. An article with Jadad score ≤ 2 is
considered to be of low quality. If the Jadad
score ≥ 3, the study is thought to be of high
quality [21].
Statistical analysis
We estimate the mean difference (MD) with 95% confidence interval (CI) for continuous out
-comes (LVEF, LVEDV, LVESV, NT-proBNP, glu
-cose, and HbA1c) and risk ratio (RR) with 95%
CIs for dichotomous outcomes (major adverse cardiac events and reinfarction). A random-effects model is used regardless of
heteroge-neity. Heterogeneity is reported using the I2 statistic, and I2 > 50% indicates significant
heterogeneity [22]. Whenever significant het -erogeneity is present, we search for potential sources of heterogeneity via omitting one stu- dy in turn for the meta-analysis or performing subgroup analysis. All statistical analyses are performed using Review Manager Version 5.3 (The Cochrane Collaboration, Software Update,
Oxford, UK).
Figure 1. Flow diagram of study searching and selection process.
mass index, diabetes, and de- tailed methods in each group etc. Data have been extracted independently by two investi-gators, and discrepancies are
resolved by consensus. We
also contacted the corre-sponding authors to obtain any data when necessary. The primary outcomes are major adverse cardiac events and reinfarction. Secondary outcomes include left ventric-ular ejection fraction (LVEF), left ventricular end-diastolic volume (LVEDV), left ventricu-lar end-systolic volume (LVE-
[image:2.612.88.370.73.296.2]Results
Literature search, study characteristics and quality assessment
A detailed flowchart of the search and selection
results is shown in Figure 1. Four hundred thir-ty-four potentially relevant articles are
identi-fied initially. Finally, 5 RCTs that meet our inclu -sion criteria are included in the meta-analysis [16-18, 23, 24].
The baseline characteristics of the 5 eligible RCTs in the meta-analysis are summarized in
Table 1. The 5 studies were published between 2014 and 2017, and sample sizes range from 152 to 379 with a total of 1138. Two RCTs report the patient sample with different follow up times [16, 24], and the follow-up time in the included RCTs range from 24 h to 2 years. Four RCTs involve metformin given at 500 mg, twice daily [16-18, 24], while the remaining one RCT involve 250 mg of metformin given 3 times a day [23].
Among the 5 studies included here, 2 studies report major adverse cardiac events [16, 24], 3 studies report reinfarction [16, 23, 24], 3 stud-ies report LVEF [17, 18, 24], 2 studstud-ies report LVEDV and LVESV [17, 18], 2 studies report
NT-proBNP, glucose and HbA1c [17, 24]. Jadad
scores of the 5 included studies vary from 3 to 5, and all 5 studies are considered to be high-quality according to high-quality assessment. Primary outcomes: major adverse cardiac events and reinfarction
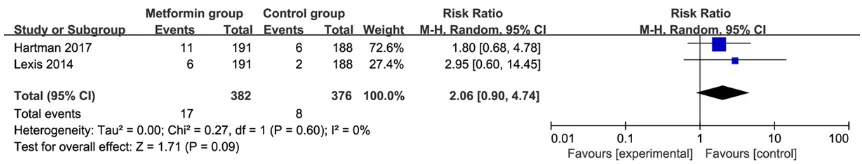
These outcome data are analyzed with the ran-dom-effects model, and the pooled estimate of the 2 included RCTs suggested that compared to the control group with myocardial infarction,
metformin treatment shows no significant
impact on major adverse cardiac events (RR = 2.06; 95% CI = 0.90 to 4.74; P = 0.09) with no heterogeneity among the studies (I2 = 0%, het-erogeneity P = 0.60) (Figure 2), and reinfarction (RR = 1.60; 95% CI = 0.67 to 3.85; P = 0.29) with no heterogeneity among the studies (I2 = 5%, heterogeneity P = 0.31) (Figure 3).
Sensitivity analysis
No heterogeneity is observed among the includ-ed studies for the primary outcomes. Thus we
ting one study in turn or a subgroup analysis to detect the heterogeneity.
Secondary outcomes
Metformin treatment shows no substantial
influence on LVEF (MD = 1.25; 95% CI = -1.51
to 4.01; P = 0.38; Figure 4) compared to pla-cebo in patients with myocardial infarction. Although LVEDV (MD = 0.50; 95% CI = 0.43 to 0.57; P < 0.00001; Figure 5) and LVESV (MD = 3.80; 95% CI = 3.75 to 3.86; P < 0.00001;
Figure 6) in the metformin group are found to be higher than placebo intervention. In
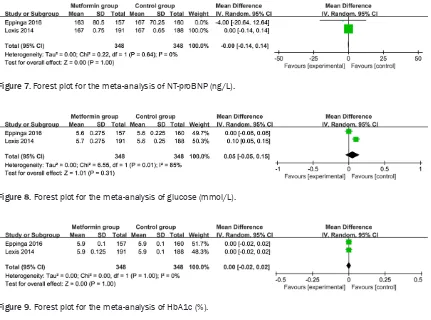
addi-tion, there is no significant difference of NT-proBNP (MD = 0; 95% CI = -0.14 to 0.14; P =
1.0; Figure 7), glucose (MD = 0.05; 95% CI = -0.05 to 0.15; P = 0.31; Figure 8) and HbA1c
(MD = 0; 95% CI = -0.02 to 0.02; P = 1.0; Figure
9) between the two groups. Publication bias
No significant publication bias is observed (P > 0.05) based on Begg’s test and Egger’s regres -sion test.
Discussion
Many studies on the effects of metformin on myocardial infarction in animal experimental and human data are inconsistent. Some stud-ies reveal a decrease in myocardial infarct size after metformin treatment [14]. Metformin shows the important ability to prevent the restenosis [25]. Patients with myocardial infarc-tion and diabetes show reduced 30-day all-cause mortality in metformin intervention group compared to control intervention, but
there is not a significant difference of 12-month
all-cause mortality and LVEF between the two groups [26]. In an open-label randomized con-trolled clinical trial, 750 mg metformin pre-treatment leads to less cardiac biomarker release and a favorable outcome at 1-year fol-low-up in patients with metabolic syndrome undergoing elective percutaneous coronary intervention [23].
However in a prospective Metformin in Coronary Artery Bypass Graft (MetCAB), pretreatment of
metformin in 100 patients has no decrease in periprocedural myocardial injury based on
Table 1. Characteristics of included studies
NO. year and Author, country
Metformin group Control group
Follow up time scoresJada Number Age (years) Male (n) index (kg/mBody mass 2)
Diabetes
(n) Methods Number Age (years) Male (n) index (kg/mBody mass 2)
Diabetes
(n) Methods
1 Hartman 2017, Netherlands
191 58.7 ± 11.8 144 26.9 ± 3.8 0 Metformin
hydrochlo-ride (500 mg) twice daily for 4 months.
188 58.8 ± 11.5 140 27.0 ± 3.9 0 Matched
placebo 2 years 4
2
Eppinga 2016, Netherlands
185 58.80 ± 11.82 139 27.0 ± 3.8 0 Metformin (500 mg
bid) during 4 months after primary percu-taneous coronary intervention
185 58.72 ± 11.44 138 27.0 ± 3.9 0 Matched
placebo
4 months 4
3
Al Ali 2016, Netherlands
118 57.9 ± 11.4 95 26.7 ± 3.7 - Metformin 500mg
twice daily initiated directly after PCI
119 58.2 ± 10.7 89 26.9 ± 3.3 - Matched
placebo
4 months 4
4 Li 2014, China 76 62.4 ± 11.0 52 - 26 3 times a day 250 mg of metformin 76 60.7 ± 11.0 53 - 26 Matched placebo 24 h 3
5
Lexis 2014, Netherlands
191 58.7 ± 11.8 144 26.9 ± 3.8 0 Metformin
hydrochlo-ride (500 mg) twice daily for 4 months
188 58.8 ± 11.5 140 27.0 ± 3.9 0 Matched
placebo
outcomes in myocardial infarction [24]. Our
meta-analysis suggests that compared to con-trol intervention for myocardial infarction,
met-formin intervention shows no significant influ -ence on major adverse cardiac events,
HbA1c, but with an increase in LVEDV and
LVESV.
Several factors should be considered for the
[image:5.612.90.521.70.152.2]efficacy of metformin treatment for myocardial
[image:5.612.90.526.165.644.2]Figure 2. Forest plot for the meta-analysis of major adverse cardiac events.
Figure 3. Forest plot for the meta-analysis of reinfarction.
Figure 4. Forest plot for the meta-analysis of LVEF (%).
Figure 5. Forest plot for the meta-analysis of LVEDV (ml).
[image:5.612.93.519.191.283.2]metformin treatment may have a crucial role in its potential cardioprotective effects. Me- tformin administration before or during reper-fusion may be preferred in the experimental
and observational data [14]. In the GIPS-III trial,
metformin is administered directly after percu-taneous coronary intervention and effective plasma levels may be achieved hours later, which reduces the time window to modify isch-emia-reperfusion injury. Ischemic reperfusion
injury can contribute up to 50% to the final size
of myocardial infarction [28]. Metformin given at 500 mg twice daily for four months was
insufficient in the GIPS-III trial, one year treat -ment with metformin dosed at 850 mg twice daily decreases the reduction in LDL particle concentration, small-sized low-density lipopro-tein particles and improves insulin sensitivity in subjects with impaired glucose tolerance [29].
The efficacy of metformin may be determined
by sex-dependent differences in the metabolic and functional response [30]. Metformin thera-py can decrease fatty acid clearance and increase fatty acid plasma levels and myocar-dial fatty acid utilization and oxidation in men, but acts in the opposite in women [31].
This meta-analysis has several potential limita-tions. First, our analysis is based on only 5 RCTs, and more RCTs with larger samples
should be conducted to confirm this issue.
Next, the time, duration and doses of metfor-min treatment in the included RCTs are
differ-ent, which may have an influence on the pool -ing results. Finally, the dose of 500 mg twice
daily may not be sufficient for patients with
myocardial infarction.
Conclusions
Metformin treatment did not show important
beneficial effects for myocardial infarction in
this study.
Disclosure of conflict of interest
None.
[image:6.612.92.522.70.139.2]Address correspondence to: Yong Luo, Department of Cardiology Medical, Jiangjin Central Hospital of Chongqing, NO. 725, Jiang Zhou Road, Dingshan Street, Jiangjin District, Chongqing 402260, China. Tel: 008602347521342; Fax: 0086023023475- 21342; E-mail: 1584637140@qq.com
[image:6.612.92.523.71.391.2]Figure 7. Forest plot for the meta-analysis of NT-proBNP (ng/L).
Figure 8. Forest plot for the meta-analysis of glucose (mmol/L).
[image:6.612.93.521.293.362.2]References
[1] Ibanez B, James S, Agewall S, Antunes MJ, Bucciarelli-Ducci C, Bueno H, Caforio ALP, Crea F, Goudevenos JA, Halvorsen S, Hindricks G, Kastrati A, Lenzen MJ, Prescott E, Roffi M, Val-gimigli M, Varenhorst C, Vranckx P, Widimský P; ESC Scientific Document Group. 2017 ESC Guidelines for the management of acute myo-cardial infarction in patients presenting with ST-segment elevation: the task force for the management of acute myocardial infarction in patients presenting with ST-segment elevation of the European Society of Cardiology (ESC). Eur Heart J 2018; 39: 119-177.
[2] O’Gara PT, Kushner FG, Ascheim DD, Casey DE Jr, Chung MK, de Lemos JA, Ettinger SM, Fang JC, Fesmire FM, Franklin BA, Granger CB, Krumholz HM, Linderbaum JA, Morrow DA, Newby LK, Ornato JP, Ou N, Radford MJ, Tamis-Holland JE, Tommaso CL, Tracy CM, Woo YJ, Zhao DX; American College of Cardiology Foun-dation; American Heart Association Task Force on Practice Guidelines; American College of Emergency Physicians; Society for Cardiovas-cular Angiography and Interventions. 2013 ACCF/AHA guideline for the management of ST-elevation myocardial infarction: executive summary: a report of the American College of Cardiology Foundation/American Heart Asso-ciation Task Force on Practice Guidelines: de-veloped in collaboration with the American Col-lege of Emergency Physicians and Society for Cardiovascular Angiography and Interventions. Catheter Cardiovasc Interv 2013; 82: E1-27. [3] Saaby L, Poulsen TS, Diederichsen AC,
Hos-bond S, Larsen TB, Schmidt H, Gerke O, Hallas J, Thygesen K, Mickley H. Mortality rate in type 2 myocardial infarction: observations from an unselected hospital cohort. Am J Med 2014; 127: 295-302.
[4] Patel MR, Dehmer GJ, Hirshfeld JW, Smith PK, Spertus JA. ACCF/SCAI/STS/AATS/AHA/ASNC 2009 appropriateness criteria for coronary re-vascularization: a report of the American col-lege of cardiology foundation appropriateness criteria task force, society for cardiovascular angiography and interventions, society of racic surgeons, American association for tho-racic surgery, American heart association, and the American society of nuclear cardiology: endorsed by the american society of echocar-diography, the heart failure society of America, and the society of cardiovascular computed tomography. Circulation 2009; 119: 1330-52. [5] Terkelsen CJ, Sørensen JT, Maeng M, Jensen
LO, Tilsted HH, Trautner S, Vach W, Johnsen SP, Thuesen L, Lassen JF. System delay and mor-tality among patients with STEMI treated with
primary percutaneous coronary intervention. JAMA 2010; 304: 763-71.
[6] Pinto DS, Frederick PD, Chakrabarti AK, Kir-tane AJ, Ullman E, Dejam A, Miller DP, Henry TD, Gibson CM; National Registry of Myocardi-al InfarctionInvestigators. Benefit of transfer-ring ST-segment-elevation myocardial infarc-tion patients for percutaneous coronary intervention compared with administration of onsite fibrinolytic declines as delays increase. Circulation 2011; 124: 2512-21.
[7] Thakkar JB, Zaman S, Byth K, Narayan A, Thiagalingam A, Chow C, Thomas SP, Siva-gangabalan G, Farlow D, Barnett R, Kovoor P. Right ventricular dysfunction predisposes to inducible ventricular tachycardia at electro-physiology studies in patients with acute ST-segment-elevation myocardial infarction and reduced left ventricular ejection fraction. Circ Arrhythm Electrophysiol 2014; 7: 898-905. [8] Ketchum ES, Dickstein K, Kjekshus J, Pitt B,
Wong MF, Linker DT, Levy WC. The Seattle Post Myocardial Infarction Model (SPIM): prediction of mortality after acute myocardial infarction with left ventricular dysfunction. Eur Heart J Acute Cardiovasc Care 2014; 3: 46-55. [9] Velagaleti RS, Pencina MJ, Murabito JM, Wang
TJ, Parikh NI, D’Agostino RB, Levy D, Kannel WB, Vasan RS. Long-term trends in the inci-dence of heart failure after myocardial infarc-tion. Circulation 2008; 118: 2057-62. [10] Weir RA, McMurray JJ, Velazquez EJ.
Epidemi-ology of heart failure and left ventricular sys-tolic dysfunction after acute myocardial infarc-tion: prevalence, clinical characteristics, and prognostic importance. Am J Cardiol 2006; 97: 13F-25F.
[11] Keskin M, Uzun AO, Hayiroglu MI, Kaya A, Cinar T, Kozan O. The association of right ventricular dysfunction with in-hospital and 1-year out-comes in anterior myocardial infarction. Am J Cardiol 2006; 97: 13F-25F.
[12] Gundewar S, Calvert JW, Jha S, Toedt-Pingel I, Ji SY, Nunez D, Ramachandran A, Anaya-Cisne-ros M, Tian R, Lefer DJ. Activation of AMP-acti-vated protein kinase by metformin improves left ventricular function and survival in heart failure. Circ Res 2009; 104: 403-11.
[13] Mellbin LG, Malmberg K, Norhammar A, Wedel H, Ryden L; DIGAMI 2 Investigators. Prognostic implications of glucose-lowering treatment in patients with acute myocardial infarction and diabetes: experiences from an extended fol-low-up of the Diabetes Mellitus Insulin-Glucose Infusion in Acute Myocardial Infarction (DIGA-MI) 2 Study. Diabetologia 2011; 54: 1308-17. [14] Lexis CP, Wieringa WG, Hiemstra B, van
treatment is associated with reduced myocar-dial infarct size in diabetic patients with ST-segment elevation myocardial infarction. Car-diovasc Drugs Ther 2014; 28: 163-71. [15] Rosiak M, Postula M, Kaplon-Cieslicka A,
Trz-epla E, Czlonkowski A, Filipiak KJ, Opolski G. Metformin treatment may be associated with decreased levels of NT-proBNP in patients with type 2 diabetes. Adv Med Sci 2013; 58: 362-8. [16] Hartman MHT, Prins JKB, Schurer RAJ, Lipsic E, Lexis CPH, van der Horst-Schrivers ANA, van Veldhuisen DJ, van der Horst ICC, van der Harst P. Two-year follow-up of 4 months metformin treatment vs. Placebo in ST-elevation myocar-dial infarction: data from the GIPS-III RCT. Clin Res Cardiol 2017; 106: 939-946.
[17] Eppinga RN, Hartman MH, van Veldhuisen DJ, Lexis CP, Connelly MA, Lipsic E, van der Horst IC, van der Harst P, Dullaart RP. Effect of met-formin treatment on lipoprotein subfractions in non-diabetic patients with acute myocardial infarction: a glycometabolic intervention as ad-junct to primary coronary intervention in ST el-evation myocardial infarction (GIPS-III) trial. PLoS One 2016; 11: e0145719.
[18] Al Ali L, Hartman MT, Lexis CP, Hummel YM, Lipsic E, van Melle JP, van Veldhuisen DJ, Voors AA, van der Horst IC, van der Harst P. The effect of metformin on Diastolic function in patients presenting with ST-elevation myocardial infarc-tion. PLoS One 2016; 11: e0168340.
[19] Moher D, Shamseer L, Clarke M, Ghersi D, Liberati A, Petticrew M, Shekelle P, Stewart LA; PRISMA-P Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Syst Rev 2015; 4: 1. [20] Jadad AR, Moore RA, Carroll D, Jenkinson C,
Reynolds DJ, Gavaghan DJ, McQuay HJ. As-sessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials 1996; 17: 1-12.
[21] Kjaergard LL, Villumsen J, Gluud C. Reported methodologic quality and discrepancies be-tween large and small randomized trials in meta-analyses. Ann Intern Med 2001; 135: 982-9.
[22] Higgins JP, Thompson SG. Quantifying hetero-geneity in a meta-analysis. Stat Med 2002; 21: 1539-58.
[23] Li J, Xu JP, Zhao XZ, Sun XJ, Xu ZW, Song SJ. Protective effect of metformin on myocardial injury in metabolic syndrome patients follow-ing percutaneous coronary intervention. Cardi-ology 2014; 127: 133-9.
[24] Lexis CP, van der Horst IC, Lipsic E, Wieringa WG, de Boer RA, van den Heuvel AF, van der Werf HW, Schurer RA, Pundziute G, Tan ES, Nieuwland W, Willemsen HM, Dorhout B, Mol-mans BH, van der Horst-Schrivers AN, Wolffen-buttel BH, ter Horst GJ, van Rossum AC, Tijssen JG, Hillege HL, de Smet BJ, van der Harst P, van Veldhuisen DJ; GIPS-III Investigators. Effect of metformin on left ventricular function after acute myocardial infarction in patients without diabetes: the GIPS-III randomized clinical trial. JAMA 2014; 311: 1526-35.
[25] Lexis CP, Rahel BM, Meeder JG, Zijlstra F, van der Horst IC. The role of glucose lowering agents on restenosis after percutaneous coro-nary intervention in patients with diabetes mellitus. Cardiovasc Diabetol 2009; 8: 41. [26] Abualsuod A, Rutland JJ, Watts TE, Pandat S,
Delongchamp R, Mehta JL. The effect of met-formin use on left ventricular ejection fraction and mortality post-myocardial infarction. Car-diovasc Drugs Ther 2015; 29: 265-75. [27] El Messaoudi S, Nederlof R, Zuurbier CJ, van
Swieten HA, Pickkers P, Noyez L, Dieker HJ, Coenen MJ, Donders AR, Vos A, Rongen GA, Riksen NP. Effect of metformin pretreatment on myocardial injury during coronary artery by-pass surgery in patients without diabetes (Met-CAB): a double-blind, randomised controlled trial. Lancet Diabetes Endocrinol 2015; 3: 615-23.
[28] Yellon DM, Hausenloy DJ. Myocardial reperfu-sion injury. N Engl J Med 2007; 357: 1121-35. [29] Goldberg R, Temprosa M, Otvos J, Brunzell J,
Marcovina S, Mather K, Arakaki R, Watson K, Horton E, Barrett-Connor E. Lifestyle and met-formin treatment favorably influence lipopro-tein subfraction distribution in the diabetes prevention program. J Clin Endocrinol Metab 2013; 98: 3989-98.
[30] Lyons MR, Peterson LR, McGill JB, Herrero P, Coggan AR, Saeed IM, Recklein C, Schecht-man KB, Gropler RJ. Impact of sex on the heart’s metabolic and functional responses to diabetic therapies. Am J Physiol Heart Circ Physiol 2013; 305: H1584-91.