Production of Ni
65Cr
15P
16B
4Metallic Glass-Coated Bipolar Plate for Fuel Cell
by High Velocity Oxy-Fuel (HVOF) Spray Coating Method
Sung-chul Kim
1;*1, Shin-ichi Yamaura
1;*2, Yuta Shimizu
2, Koji Nakashima
2,
Takanori Igarashi
2, Akihiro Makino
1and Akihisa Inoue
11Institute for Materials Research, Tohoku University, Sendai 980-8577, Japan
2Toyohashi Factory, Topy Industries, Ltd., Toyohashi 441-8510, Japan
In this study, the newly designed bipolar plate for proton exchange membrane fuel cells (PEMFC) was produced by spray-coating the Ni65Cr15P16B4metallic glassy alloy on Al plate. The Ni65Cr15P16B4metallic glass was adopted as a coating material because of its excellent
corrosion resistance and the high velocity oxy-fuel (HVOF) spray coating was used for the metallic glass deposition on the Al plates having a bipolar plate flow field.
The corrosion resistance of the Ni65Cr15P16B4glassy alloy film produced by the HVOF spray-coating was studied under simulated PEMFC
environments. As a result, the Ni65Cr15P16B4glassy alloy film showed lower corrosion current density than the high-corrosion-resistant stainless
steel SUS316L. Then, the electricity generation tests with the single cell having the Ni65Cr15P16B4glassy alloy-coated bipolar plates produced in
this study were conducted. As a result, the single cell with the metallic glass-coated bipolar plates showed very high I-V performance as well as the cell with the carbon bipolar plates. The long time durability tests for 24 h were also conducted at the constant current density of 200 mAcm 2. As a result, the single cell with the glass-coated bipolar plates showed no voltage drop during the test. So, it was found in this
study that the Ni65Cr15P16B4glassy alloy-coated bipolar plate produced by the HVOF spray-coating have a potential for practical use for the fuel
cells. [doi:10.2320/matertrans.MAW201006]
(Received April 23, 2010; Accepted June 24, 2010; Published August 25, 2010)
Keywords: proton exchange membrane fuel cell, metallic glass, bipolar plate, high velocity oxygen-fuel spray coating
1. Introduction
Proton exchange membrane (PEM) fuel cell has been widely recognized as one of the most powerful candidates for near future power generating devices of the automobiles and the home appliances. Bipolar plates are one of the most important components of PEM fuel cells and are multifunc-tional as they conduct electricity from cell to cell, they separate the fuel gas from the oxidant gas, and their flow field supplies the gases to the electrodes. Bipolar plates are conventionally made of carbon graphite.1) However, high production cost of graphite bipolar plate inhibits wide spread use of the fuel cells. Moreover, it cannot be used to make a thinner bipolar plate because of its significant brittleness. Thinner bipolar plates will lead to a compact fuel cell, resulting in saving costs and enhancing the usefulness of fuel cells.
Compared to the carbon graphite, the metallic materials have many advantages such as low-cost, good electrical and thermal conductivities, excellent mechanical properties and good workability. However, corrosion may occur when the metallic bipolar plates are used under acidic atmosphere in the fuel cells, which leads to the dissolution of metallic ions. The contamination of the membrane and electrode with metallic ions deteriorates the performance of the fuel cells. Additionally, interfacial contact resistance increases because of the formation of the passive film on the metal surface. So, metallic bipolar plates still have many challenges to overcome.2–5)
Recently, metallic glasses have attracted much attention because of their excellent engineering properties, such as high strength and superior corrosion resistance.6–8)
Further-more, metallic glasses can show super-plastic deformation in a super-cooled liquid state in the temperature range between the glass transition temperature, Tg and the crystallization temperature,Tx. The Ni-based amorphous alloys with non-metal such as Ni-P,9) Ni-B,10) Ni-Si-B11) have been in limelight and reported for three decades. For example, the mechanical properties of the Ni-W-P amorphous alloy thin film deposited on substrates by electro-plating was report-ed.12) Hashimotoet al. reported that the Ni-Cr-P-B quater-nary amorphous alloys possessed excellent corrosion resist-ance.13)Recently, we found that the Ni-Cr-P-B amorphous alloy had a wide super-cooled liquid region,Tx.14)We have studied the metallic glassy alloys for bipolar plate and tried to produce metallic glassy alloy bipolar plates by melt-spinning and subsequent hot-pressing at aroundTg.15–17)
High velocity oxy-fuel (HVOF) spray coating has been widely used to produce thick protective surface film on various industrial applications to reduce wear and corro-sion.18–20) The feedstock powder particles were injected into a high velocity combustion gas jet. Because of high bombardment energy available in HVOF thermal spraying process, collision of the partially molten particles on the substrates results in the formation of a dense coating film.
So, we tentatively produced the Ni-Cr-P-B metallic glass-coated bipolar plates by using the HVOF spray coating technique in this study. The corrosion behaviors of the Ni-Cr-P-B metallic glass under the fuel cell circumstances and the electricity generating properties of the single fuel cell with the metallic glass-coated bipolar plates were also examined.
2. Experimental
A Ni65Cr15P16B4master alloy was prepared by induction-melting the high-purity elements of Ni (99.9%), Ni3P (99%), *1Graduate Student, Tohoku University
*2Corresponding author, E-mail: yamaura@imr.tohoku.ac.jp
Cr (99.9%) and B (99.5%) in a copper crucible under an argon atmosphere. Ni65Cr15P16B4 metallic glassy powder used as a feedstock of HVOF spray-coating was prepared by a high-pressure argon gas-atomization process. The HVOF thermal spray system (PRAXAIR/TAFA, JP-5000) was used to produce Ni65Cr15P16B4 thick film on Al plates having a flow field of bipolar plates. The fuels for combustion were kerosene and O2 and the carrier gas of powder was N2. The film thickness was about 200mm. Prior to the HVOF spray-coating, the Al plates with a flow field were prepared by hot-pressing and their surface was roughened by abrasive blasting to increase the adhesion between the surface alloy film and the Al plate. The thickness of the Al plates was 0.3 mm. Figure 1 shows a schematic illustration of the flow field designed in this work. After the HVOF spraying, the coated samples were slightly deformed because of thermal damage. So, it was necessary to hot-press the coated bipolar plates subsequently at aroundTg (= 660 K) after the HVOF spray coating.
The Amorphicity of the coated samples were examined by X-ray diffractometry (XRD) with a monochromatic Cu-K
radiation at 40 kV and 40 mA. The microstructure of the film was observed by scanning electron microscopy (SEM: JEOL, JEM3000F).
The polarization curves were measured by using a Hokuto Denko HZ-3000 Potentio/Galvanostat controlled by a com-puter. Platinum net was used as a counter electrode and sample potential was measured against saturated calomel electrode (SCE) in 1 M H2SO4 solution at 348 K purged either with H2 gas to simulate the PEMFC anode environ-ment or air to simulate the PEMFC cathode environenviron-ment. In order to avoid the formation of a protective film on the surface of the samples, the cathodic polarization was conducted first and then the anodic polarization was also conducted at a potential sweep rate of 20 mVmin 1.
The I-V characteristics of single cells with the Ni65Cr15P16B4metallic glassy bipolar plates, with Al bipolar plates and with carbon graphite bipolar plates were measured by an automatic fuel-cell test system (Toyo Technica, PEMTest8900). A standard PEM fuel cell was purchased from Electrochem Inc. The MEA area is 5 cm2. Humidi-fication was 100%RH for H2 and O2 at 343 K. The cell temperature was 353 K and the gas flow rate was 0.1– 0.3 Lmin 1.
3. Results and Discussion
Figure 2 shows the outer view of the Ni65Cr15P16B4 metallic glass-coated Al bipolar plate produced by HVOF spray-coating and subsequent hot-pressing. The glass film was deposited on both sides of the plates.
Figure 3 shows the XRD patterns of the Ni65Cr15P16B4 metallic glassy alloy prepared by three different techniques, melt-spinning (Ribbon), gas-atomizing (Powder) and HVOF spray coating (HVOF). Powder sample was prepared for the feedstock of HVOF spray coating in this work. Melt-spun sample possessed a single glassy phase as indicated in the figure. It is easy to obtain a glassy sample by melt-spinning because its thickness is very thin (about 20mmin thickness). However, the powder sample and the HVOF spray-coated one showed a broad halo peak coming from glassy matrix and some small peaks coming from small crystalline phases. Although it was possible to optimize the experiment conditions to avoid subtle crystallization during the gas-atomizing and the HVOF spray coating, we used these types of the samples having a broad halo peak and small distinct peaks in the XRD observations.
Figure 4 shows a cross sectional view of Ni65Cr15P16B4 metallic glassy surface film deposited on Al plate by HVOF
22.4 mm
Fig. 1 Schematic illustration of the flow field. Fig. 2 Outer view of the Ni
65Cr15P16B4 metallic glass-coated Al bipolar
plate.
Intensity (a. u.)
2θθ
Fig. 3 XRD patterns of the Ni65Cr15P16B4metallic glass samples produced
[image:2.595.106.231.73.215.2] [image:2.595.321.530.275.430.2]spray-coating. The film was so thick that large pinholes connecting the surface and the substrate are not seen in the glassy film, since the thickness of the film was about 200mm. Moreover, the boundary between the film and the substrate is not flat because the abrasive blasting on the Al substrate was conducted before the spray coating to increase the adhesion of the film to the substrate.
Figure 5 shows the polarization curves of both the Ni65Cr15P16B4 metallic glassy as HVOF coated films and the subsequently hot-pressed films. Figures 5(a) and (b) show the polarization curves measured in 1 M H2SO4 solution at 348 K with air bubbling and H2 bubbling, respectively. As seen in Fig. 5(a), the as-HVOF coated Ni65Cr15P16B4glassy film showed slightly higher corrosion current density than the high corrosion-resistant stainless steel SUS316L. However, the glassy film prepared by HVOF coating and subsequent hot-pressing showed lower corrosion current density than the SUS316L in the PEMFC cathode environment at around 0.6 V. Moreover, the glassy film after the subsequent hot-pressing showed lower corrosion current density than the SUS316L in the PEMFC anode environment at around
0:1V. The subsequently hot-pressed film samples showed better corrosion resistance than the as-HVOF coated samples. This may be because the film became denser and the surface of the film became flat after the subsequent hot-pressing, resulting in accurate estimation of the surface area and reduction of defects in the microstructure. High corrosion resistance is one of the essential properties of bipolar plate materials. It was found that the Ni65Cr15P16B4 glassy film prepared in this work showed excellent corrosion resistance. Then the electricity generating tests were conducted with a single fuel cell having the glassy alloy bipolar plates.
Figure 6 shows the I-V curves of the single fuel cell with carbon graphite, Al and Ni65Cr15P16B4 glassy alloy bipolar plates measured at the cell temperature of 353 K at the gas flow rate of 0.1 Lmin 1. The results measured after 50 times repetition for aging the membranes were indicated in the figure. At the cell voltage of 0.5 V, the single cells employing graphite, Al, and Ni65Cr15P16B4 glassy alloy bipolar plates generated the current density of 700, 200, and 750 mAcm 2, respectively. As clearly seen in the figure, the single cell with
the Ni65Cr15P16B4 glass-coated bipolar plates showed better I-V performance than that with Al bipolar plates. This is because the Al bipolar plates are easily subjected to corrosion, resulting in the increase in contact electrical resistance. The I-V performance of the single cell with the Ni65Cr15P16B4 glass-coated bipolar plates is as high as that with the carbon graphite bipolar plates. This means that the
Specimen surface
P
Ni
65Cr
15 16B
4Al substrate
Fig. 4 A cross section of the Ni65Cr15P16B4 metallic glass surface film
deposited on Al plate.
Potential vs SCE, E/ V
Curr
ent Density
,
I
/ A·
cm
-2
Potential vs SCE, E / V
Curr
ent Density
,
I
/ A·
cm
-2
Fig. 5 Polarization curves of the Ni65Cr15P16B4metallic glass films,
As-coated (HVOF) and subsequently hot-pressed samples measured with Air bubbling (a) and with H2bubbling (b).
MEA: 5 cm2
H2, O2gas flow: 0.1 L·min-1
Current Density, I / mA·cm-2
Cell V
oltage,
E
/ V
Fig. 6 I-V curves of the single cell with carbon, Al and Ni65Cr15P16B4
[image:3.595.315.540.74.420.2] [image:3.595.313.540.483.652.2]corrosion resistance of the Ni65Cr15P16B4metallic glass is so high that it can be applied to bipolar plates in practice. Then the electricity generation was conducted for 24 h to examine the stability of the glassy alloy bipolar plates.
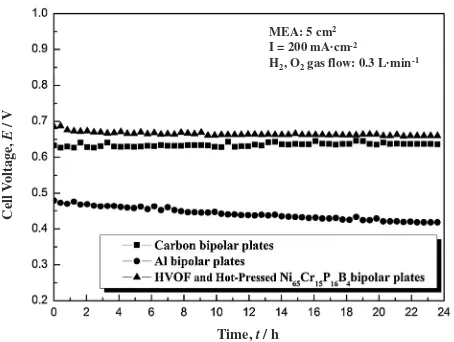
Figure 7 shows the results of the long time durability tests conducted at constant current density of 200 mAcm 2 at 353 K with the single cell having the carbon graphite, Al and the Ni65Cr15P16B4 glassy alloy bipolar plates. The gas flow rate of H2 and O2 was 0.3 Lmin 1. The cell voltage measured with the Al bipolar plates decreased gradually with time because of the increase in contact electrical resistance coming from corrosion during the test. On the contrary, the cell voltage measured with the glassy alloy bipolar plates did not show any significant drop during the test after the voltage decreased slightly in the initial stage of the measurement because of the formation of the passive surface film. Some of the present authors (S.Y. and A.I.) have studied the Ni-based glassy alloys for bipolar plates.15–17) In their previous papers, the melt-spun alloy sheet of 50mm
in thickness and 50–100 mm in width was used to fabricate the metallic glassy alloy bipolar plates by hot-pressing at around Tg. It was reported that the cell performance using those glassy alloy bipolar plates was lower than that with carbon graphite bipolar plates. This may be because it was difficult to mount such a thin bipolar plate on the carbon frame without gas leak and also without the increase in the contact resistance. In this work, metallic glass coated bipolar plates were produced in a bulk form and it was easy to mount those bipolar plates in a single fuel cell. At any rate, we have succeeded to produce the metallic glassy alloy-coated bipolar plates having high corrosion resistance and the single cell employing those metallic glassy alloy-coated bipolar plates shows good I-V performance as well as that with carbon graphite bipolar plates.
4. Conclusions
In this work, the Ni65Cr15P16B4 metallic glass coated bipolar plates were produced by the HVOF spray coating and subsequent hot-pressing. The corrosion behavior of the glassy alloy was investigated with a Potentio/Galvanostat
bipolar plates. The conclusions obtained in this work are as follows.
(1) The corrosion resistance of the Ni65Cr15P16B4 glassy alloy film produced by the HVOF spray coating was studied in a 1 M H2SO4 solution at 348 K. As a result, the as-HVOF coated Ni65Cr15P16B4 glassy alloy film showed larger corrosion current density than the high-corrosion-resistant stainless steel SUS316L. However, the glassy alloy films prepared by the HVOF spray coating and subsequent hot-pressing showed lower corrosion current density than the SUS316L. This is because the film became denser with flat surface, resulting in reducing the corrosive defects and more accurate estimation of sample area.
(2) The I-V characteristics of the single fuel cell having the Ni65Cr15P16B4 glassy alloy-coated bipolar plates pro-duced in this study were examined at 353 K with pure H2and O2 gas flow. As a result, the single cell with the metallic glass-coated bipolar plates showed very high I-V perform-ance as well as the cell with the carbon bipolar plates. The long time durability tests were conducted for 24 h at the constant current density of 200 mAcm 2. As a result, the single cell with the glass-coated bipolar plates showed no voltage drop during the test as well as that with the carbon graphite bipolar plates. The single cell with the Al bipolar plates showed a significant voltage drop due to the increase in contact electrical resistance. So, it was found in this work that the Ni65Cr15P16B4 glassy alloy-coated bipolar plate produced by the HVOF spray-coating and subsequent hot-pressing was found to have a potential for practical use for the fuel cells.
Acknowledgements
This work was supported by the Ministry of Education, Culture, Sport, Science and Technology (MEXT) of Japan, Grant-in-Aid for Scientific Research (C), 21560716, 2009 and was partly supported by the financial aid from the Global COE program of Tohoku University.
REFERENCES
1) J. Larminie and A. Dicks: Fuel Cell Systems Explained, (John Wiley & Sons Ltd., West Sussex, 2003, translated into Japanese language) pp. 120–122.
2) J. Wind, R. Spaeh, W. Kaiser and G. Boehm: J. Power Sources105
(2002) 256–260.
3) S. Joseph, J. C. McClure, R. Chianelli, P. Pich and P. J. Sebastian: Int. J. Hydrogen Energy30(2005) 1339–1344.
4) M. P. Brady, H. Wang, B. Yang, J. A. Turner, M. Bordignon, R. Molins, M. Abd Elhamid, L. Lipp and L. R. Walker: Int. J. Hydrogen Energy32(2007) 3778–3788.
5) A. Pozio, F. Zaza, A. Masci and R. F. Silva: J. Power Sources179
(2008) 631–639.
6) W. L. Johnson: JOM54(2002) 40–43. 7) J. Schroers: JOM57(2005) 35–39.
8) K. Asami, K. Hashimoto, T. Masumoto and S. Shimodaira: Corros. Sci.
16(1976) 909–914.
9) A. Inoue, A. Kitamura and T. Masumoto: J. Mater. Sci.18(1983) 753– 758.
10) A. Inoue, A. Kitamura and T. Masumoto: Mater. Trans., JIM20(1979) 404–406.
I = 200 mA·cm
H2, O2gas flow: 0.3 L·min-1
Time, t / h
Cell V
oltage,
E
/ V
[image:4.595.56.283.70.241.2]11) A. Inoue, H. Yamamoto and T. Masumoto: Mater. Trans., JIM 30
(1989) 677–683.
12) J. Ahmad, K. Asami, A. Takeuchi and A. Inoue: Mater. Trans. 44
(2003) 705–708.
13) H. Yoshioka, K. Asami, A. Kawashima and K. Hashimoto: Corros. Sci.
27(1987) 981–995.
14) M. Yokoyama, S. Yamaura, H. M. Kimura and A. Inoue: Mater. Trans.
48(2007) 3176–3180.
15) A. Inoue, T. Shimizu, S. Yamaura, Y. Fujita, S. Takagi and H. M. Kimura: Mater. Trans.46(2005) 1706–1710.
16) M. Yokoyama, S. Yamaura, H. M. Kimura and A. Inoue: Int. J. Hydrogen Energy33(2008) 5678–5685.
17) S. Yamaura, M. Yokoyama, H. M. Kimura and A. Inoue: J. Phys.: Conf. Ser.144(2009) 012001.
18) H. S. Ni, X. H. Liu, X. C. Chang, W. L. Hou, W. Liu and J. Q. Wang: J. Alloy. Compd.467(2009) 163–167.
19) F. Otsubo and K. Kishitake: Mater. Trans.46(2005) 80–83. 20) H. J. Kim, K. M. Lim, B. G. Seong and C. G. Park: J. Mater. Sci.36