Segregation of Alkali and Alkaline Earth Metals at
11(113)[110] Grain Boundary
in Aluminum from First-Principles Calculations
Tokuteru Uesugi
+and Kenji Higashi
Department of Materials Science, Graduate School of Engineering, Osaka Prefecture University, Sakai 599-8531, Japan
The grain boundary segregation energies of Mg, Na, Ca, K and Sr at a symmetric tilt11ð113Þ½110grain boundary were investigated in aluminum using thefirst-principles calculation. The relationship between the grain boundary segregation energies and the volume size factors were examined to understand the role of elastic strain energy in the grain boundary segregation energy. The grain boundary segregation energy decreased with the increase in the volume size factor. It has been explained that the solute atom larger in size than Al, which stores greater strain energy in the bulk, prefers the looser site at the grain boundary plane rather than in the bulk, to release the elastic strain energy. Furthermore, on the basis of the RiceWang model, the effects of grain boundary segregation on the embrittlement at the grain boundary were studied. The embrittlement potency indicates that the Mg, Na, Ca, K and Sr atoms serve as embrittler in the grain boundary.
[doi:10.2320/matertrans.M2012108]
(Received March 21, 2012; Accepted July 3, 2012; Published August 22, 2012)
Keywords: first principles, grain boundary embrittlement, grain boundary segregation energy, volume size factor, RiceWang model
1. Introduction
In aluminum alloys, the segregation of alkali and alkaline earth metals at grain boundaries has been discussed if it is a cause of grain boundary embrittlement.112)In the work by Wert and Lumsden, utilizing Auger electron spectroscopy (AES), in an AlLiCuMgZr alloy containing both 35 ppm Na and 72 ppm K, only K was detected on the intergranular fracture surface and Na was not.1)Horikawaet al.examined
the grain boundary segregation in Al5 at% Mg base alloys containing 10 ppm Sr, Na or Ca using AES, and Sr and Ca were detected on the intergranular fracture surface.2)
Horikawaet al.concluded that the grain boundary embrittle-ment was caused by the segregation of Sr, Na and Ca.2) However, Lynch claimed that the Na-rich particles had caused the embrittlement rather than the Na segregation at the grain boundary, based on detailed microscopic observation.3) Kobayashiet al.showed that the grain boundary segregation of Na occurs in an AlLi base alloy containing 59 ppm Na using AES.4)
The first-principles simulation can provide to understand the mechanism of solute segregation and the bonding strength at grain boundaries in the atomic scale.512) Liu
et al. calculated the grain boundary segregation energy of Mg using first-principles calculation, and on the basis of Rice and Wang’s thermodynamics theory,13) they reported
that the Mg segregation on the grain boundary decreased the grain boundary cohesion for 11ð113Þ½110 tilt grain boundary in aluminum.5)Lu et al. reported that the Na and Ca segregation caused the grain boundary embrittlement for 9ð221Þ½110 grain boundary in aluminum on the basis of Rice and Wang’s thermodynamics theory.6,7) Lu et al. also
reported based on thefirst-principles calculation that the ideal tensile strength of the grain boundary was reduced with both Na and Ca grain boundary segregation.7) The authors have
studied the effect of Ca segregation on the grain boundary cohesion for aluminum11ð113Þ½110grain boundary.8)
In this paper, the grain boundary segregation energies of Mg, Na, Ca, K and Sr were investigated at a symmetric tilt 11ð113Þ½110grain boundary in aluminum, using thefi rst-principles calculation. The relationship between the grain boundary segregation energy and the volume size factor was examined to understand the role of elastic strain energy in the grain boundary segregation energy. Furthermore, on the basis of the RiceWang model, the authors studied the effect of the segregation of Mg, Na, Ca, K and Sr on the grain boundary embrittlement in aluminum.
2. Calculation Procedure
The first-principles calculations were performed using the Cambridge Serial Total Energy Package (CASTEP).14) CASTEP is an ab initio pseudopotential method code for solving the electronic ground state of periodic systems with the wave functions expanded in a plane-wave basis set, using a technique based on the density functional theory (DFT).15) The electronic exchangecorrelation energy was given by the generalized gradient approximation (GGA-PW91) of Perdew
et al.16) The authors used a norm-conserving Troulier
Martins pseudopotential for Al.17) Ultra-soft
pseudopoten-tials were used for Ca, K and Sr and norm-conserving pseudopotentials were used for Mg and Na.18)Cut-off energy
for the plane-wave basis was 5.61©10¹17J (350 eV) in all
calculations.
The calculated 11ð113Þ½110 symmetric tilt grain boundary was constructed using the coincidence site lattice (CSL) model. The grain boundary structure was modeled in a supercell that was repeated periodically in three dimensions to represent the extended solid. The supercell contained two grain boundaries in order to maintain the periodicity along the direction perpendicular to the boundary plane; a bicrystal was used in this study. When the 11ð113Þ½110 tilt grain boundary breaks ideally, a ð113Þ free surface as a fracture surface is formed. Figure 1 shows a schematic of the supercells for bulk of pure Al (Fig. 1(a)), bicrystal of 11ð113Þ½110 tilt grain boundaries of pure Al (Fig. 1(b)), +Corresponding author, E-mail: uesugi@mtr.osakafu-u.ac.jp
slab containing ð113Þ free surfaces of pure Al (Fig. 1(c)), bulk of Al-based solid solution (Fig. 1(d)), bicrystal of 11ð113Þ½110 tilt grain boundaries with segregated solute atoms (Fig. 1(e)) and slab containingð113Þfree surfaces with segregated solute atoms (Fig. 1(f )). The supercells shown in (a), (b), (c), (d), (e) and (f ) contain 88 Al atoms, 88 Al atoms, 64 Al atoms, 86 Al atoms with two solute X atoms, 86 Al atoms with 2 solute X atoms, and 62 Al atoms with 2 solute X atoms, respectively. The supercell size is twice lager than the one studied in Liu et al.’s work in [110] direction, although the geometry of the supercells is similar; the supercell of 88 atoms in this work and the supercell of 44 atoms in Liuet al.’s work for the grain boundary.5)A solute
X atom was substituted into the boundary plane and surface plane, and a symmetrical equivalent substitution was made at the other boundary plane and surface plane as shown in Figs. 1(e) and 1(f ). The segregation energy is the energy per atom required to exchange a solvent atom at the grain boundary or free surface with a solute atom in the bulk. In order to minimize computational errors, 2 solute X atoms were substituted in the 88-atoms bulk supercell that is of a similar size and geometry to the supercell containing grain boundaries and free surfaces.
In this study, the lattice parameters and atomic positions in the supercells of bulks, grain boundaries, and free surfaces were fully optimized. The stable atomic configurations were obtained through relaxation according to the Hellmann Feynman forces calculated from the first principles. The
lattice parameters at zero pressure were also optimized using a BroydenFletcherGoldfarbShanno (BFGS) minimization algorithm with the stress calculations.19) The convergence
parameters were as follows: total energy tolerance 1.6© 10¹24J/atom, maximum force tolerance 4.8©10¹12N, maximal stress component 0.05 GPa and maximal displace-ment 1©10¹4nm. The segregation energy at a grain boundary and on a surface is much less than the total energies of supercells. Therefore, to obtain reliable values, the bulk, grain boundary, and free surface systems must be equally treated. For this reason, the same set of numerical parameters was used in the CASTEP calculation for the bulk, grain boundary, and free surface. The energy integration over a Brillouin zone was made with ak-point grid according to the MonkhorstPack set of 4©1©6 k-points. The calcu-lated total energies, atomic configurations, and electronic structures were analyzed for the fully relaxed systems.E88AlB
is the total energy of the supercell for bulk of pure Al (Fig. 1(a)).E88AlG is the total energy for grain boundaries of pure Al (Fig. 1(b)).E64AlF is the total energy for free surfaces of pure Al (Fig. 1(c)).E2BXþ86Alis the total energy for bulk of AlX solid solution (Fig. 1(d)).E2Xþ86AlG is the total energy for grain boundaries with segregated solute X atoms (Fig. 1(e)). E2Xþ62AlF is the total energy for free surfaces with segregated solute X atoms (Fig. 1(f )).
For studying the volume size factor, rhombohedral super-cells containing 27 atoms were used. The supersuper-cells contained one solute X atom per cell. So, the supercells correspond to about 3.70 at% of solute X. The energy integration over a Brillouin zone was made withk-point grid according to the MonkhorstPack sets of 5©5©5k-points. The total energy was minimized with respect to the atomic positions in the supercell and the lattice parameters of the supercell. The calculated volumes of the supercells were analyzed for the fully relaxed systems. The calculated volumes of the supercells of pure Al and AlX solid solution are³27Aland ³1X+26Al, respectively.
3. Results and Discussions
3.1 Grain boundary segregation
The grain boundary segregation energy can be defined as the difference in total energy between the cell with one solute X atom being substituted at the boundary and the cell with one solute X atom in the bulk solid solution. The grain boundary segregation energy,¦Eb, was obtained by:
Eb¼ ½ðE88AlB E2BX+86AlÞ ðE88AlG E2GX+86AlÞ=2 ð1Þ in which the division by 2 accounts for the number of solute X atoms in the supercell. The grain boundary segregation energies, which are shown in Table 1, are ¹6, ¹32, ¹35, ¹62 and¹66 kJ/mol for Mg, Na, Ca, K and Sr, respectively. These negative values of the grain boundary segregation energies indicate that the solute atom prefers to occupy the 11ð113Þ½110grain boundary.
The grain boundary segregation can be analyzed in the framework of basic statistical mechanics. The simplest model to describe the equilibrium segregation activity in materials was developed by McLean.20) According to the
basic assumptions: the equivalent sites of grain boundary
113
(a) (b) (c) (d)
(e) (f)
] [
] [ ] 110 [
332
[image:2.595.60.276.66.359.2]segregation of a monatomic layer at the grain boundary plane and the ideal solution, i.e., non-interacting solutes, McLean treated the equilibrium grain boundary segregation problems on the basis of FermiDirac statistics with a constant segregation energy, ¦Eb, which can be stated as:20)
cb 1cb ¼
c0
1c0exp Eb
kT
ð2Þ
in whichcbis the grain boundary concentration of the solute,
c0 is the bulk concentration of the solute, kis Boltzmann’s
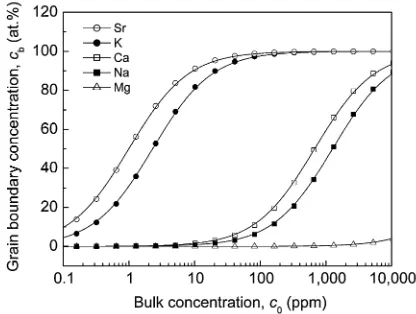
[image:3.595.321.529.70.227.2]constant, and T is the annealing temperature. The grain boundary concentration is solute concentration in the monatomic layer of the grain boundary plane. It is noted thatTis the annealing temperature, but not the measurement temperature, because of the condition of the equilibrium state. The grain boundary concentration as a function of the bulk concentration and temperature can be obtained by means of the grain boundary segregation energy, calculated based on the first principles. Figure 2 shows the grain boundary concentration of solute X (X=Mg, Na, Ca, K and Sr) as a function of the bulk concentration of solute X at 573 K.
Figure 2 indicates the significant segregation of Sr and K if the bulk concentration of Sr and K is above ³1 ppm. Horikawaet al.reported the grain boundary segregation of Sr in Al5 at%Mg base alloys containing Sr of 10 ppm; Sr was detected on the intergranular fracture surface using AES.2)
This experimental study is consistent with the prediction based on the McLean’s model shown in Fig. 2. Horikawa
et al. also reported the grain boundary segregation of Ca in Al5 at% Mg base alloys containing Ca of 10 ppm.2)
Additionally, Kobayashi et al. showed that the grain boundary segregation of Na occurs in an AlLi base alloy containing 59 ppm Na, using AES.4) These experimental
results of the grain boundary segregation of Ca and Na are not consistent with the prediction based on the McLean model; Fig. 2 indicates the significant segregation of Ca and Na occurring above ³100 ppm of bulk concentration of Ca and Na. This underestimation of the activity of grain boundary segregation in McLean’s model may be caused by the low energy grain boundary of11ð113Þ½110. It has been known that grain boundary segregation cannot easily occur at low energy grain boundaries.21) The 11ð113Þ½110
grain boundary exhibits smaller grain boundary energy of 179 mJ/m2 than the averaged grain boundary energy of
325 mJ/m2in aluminum.22,23)
3.2 Volume size factor
Figure 3 shows the interlayer strain normal to the grain boundary plane as a function of the distance from the grain boundary plane for 11ð113Þ½110 tilt grain boundary in pure Al. The interlayer strain is the relative strain of the interlayer spacing. The interlayer spacing of the nth atomic layer normal to the grain boundary plane, dn, is determined
bydn=(ln+1¹ln¹1)/2, whereln+1is the length between the
(n+1)th andnth atomic layers andln¹1is the length between
the (n¹1)th andnth atomic layers. The interlayer strain,¾, is given as:
¾¼ ðdnd0Þ=d0 ð3Þ
where d0 is the interlayer spacing of a middle layer of the
grain, which is sufficiently distant from the grain boundary. One can see an oscillatory strain profile that has a maximum positive value at the grain boundary plane where solute X atom is substituted and decays into the bulk. This positive large value of the interlayer strain indicates that the environment at the grain boundary plane is looser than that of the bulk. The positive value of the interlayer strain have been observed in other tilt grain boundaries in aluminum and indicates local volume expansion, which corresponds to the Table 1 The grain boundary segregation energies, ¦Eb, and surface
segregation energies,¦Es, of solute X (X=Mg, Na, Ca, K and Sr) in
aluminum11ð113Þ½110tilt grain boundary andð113Þfree surface. The embrittlement potency,¦Eb¹¦Es, is also listed.
Solute X ¦Eb (kJ/mol)
¦Es
(kJ/mol)
¦Eb¹¦Es
(kJ/mol)
Mg ¹6 ¹19 13
Na ¹32 ¹187 155
Ca ¹35 ¹112 77
K ¹62 ¹330 268
Sr ¹66 ¹230 164
Fig. 2 The grain boundary concentration of solute X (X=Mg, Na, Ca, K and Sr) at 11ð113Þ½110 boundary plane as a function of bulk concentration of solute X at 573 K in aluminum.
[image:3.595.46.290.114.201.2] [image:3.595.322.530.283.452.2]grain boundary excess free volume.22)Solute atoms larger in
size than Al would prefer the looser site at the grain boundary plane rather than in the bulk.
The effect of difference in size between the solvent and solute atoms in a solid solution is causing an increase in the elastic strain energy. The elastic strain energy associated with solute atoms can be estimated approximately based on a sphere-in-hole model developed by Eshelby and Friedel, which is based on the isotropic elasticity theory.24,25) Although both the solvent matrix and the solute atom undergo elastic distortion, the major part of the elastic strain energy is stored in the matrix and hence the elastic strain energy,¦Eels, is given as:26)
Eels¼2GðsfÞ 2
3 ð4Þ
in which Gis the shear modulus of the solvent matrix and
³ is the atomic volume of the solvent atom and ³sf is the
volume size factor. The elastic strain energy is given as a function of the volume size factor.
The atomic volume of a solute atom in a solid solution differs from its volume in a pure state. Electronic interaction causes the atomic volumes to change in non-ideal way. The volume size factors for many metallic solid solutions have been determined and are given in tables published by King.26) These size factors were based on lattice parameter measure-ments of solid solutions as a function of solute concentration. Previously, the authors have been reported that the fi rst-principles results of the size factors are in good agreements with experimental values for aluminum vacancy and magnesium solid solutions.27,28) The volume size factor,
³sf, of solute X in AlX solid solution is defined as:
sf¼XAl
Al ð5Þ
in which ³Al is the atomic volume of solvent Al and³Xis
the effective atomic volume of solute X. The volumes of the supercells of pure Al and AlX solid solutions were calculated from the 27-atom supercells. So, eq. (5) can be rewritten as:
sf¼27Al27 ð1X+26Al27AlÞ ð6Þ
in which ³27Al is the volume of the pure Al supercell
containing 27 Al atoms and ³1X+26Al is the volume of the
solid solution supercell containing 26 Al atoms and one solute X atom.
The volumes of the solid solution supercell containing 26 Al atoms and one X atom (X=Mg, Na, Ca, K and Sr) and the volume size factors are given in Table 2. The volume size factors were derived from eq. (6). The values of the volume size factors of Mg, Na, Ca, K and Sr are 31.4, 93.7, 118.0, 139.9 and 177.6%respectively. King reported that the value of the volume size factor of Mg in Al was 40.1%,26) which was close to the theoretical value based on thefirst principles. These positive values for the volume size factors indicate the size of solute X atoms to be larger than that of the solvent Al atom.
Figure 4 shows the relationship between the grain boundary segregation energy of solute X in aluminum
11ð113Þ½110 tilt grain boundary and the volume size factors of AlX solid solutions (X=Mg, Na, Ca, K and Sr). The grain boundary segregation energy decreases with the increase of the volume size factor; a solute atom larger in size is preferable for grain boundary segregation. Therefore it can be explained that solute atoms larger in size than Al, which store the larger elastic strain energy in the bulk, prefer the looser site at the grain boundary plane rather than in the bulk to release the elastic strain energy.
3.3 Grain boundary cohesion
The effects of solute segregation on the grain boundary cohesion in aluminum were investigated based on the Rice and Wang thermodynamics theory.13) The grain boundary
cohesive energy of a segregated grain boundary, 2£, which is the Griffith work of a brittle boundary separation, plays a key role in the grain boundary embrittlement.13,29,30) According
to the fracture mechanics based on GriffithOrowanIrwin approach,3133) the growth condition of a nucleated micro-crack along the grain boundary is as follows:
ð1¯2Þð·Þ2³a c
E 2£þ£p ð7Þ
where acis a half size of the microcrack, Eis the Young’s
modulus,¯is the Poisson’s ratio,·*is the critical local stress for the grain boundary fracture, and£pis the work of plastic
[image:4.595.307.545.101.392.2]deformation, which is often much larger than 2£. It is pointed out by Jokl et al. that £p is monotonically increasing as
Table 2 The volumes of the solid solution supercell containing 26 Al atoms and one X atom (X=Mg, Na, Ca, K and Sr),³1X+26Al, and the
volume size factors of solute X in Al,³sf.
³1X+26Al
(nm3/cell)
³sf
(%)
Al 0.43427
Mg 0.43932 31.4
Na 0.44934 93.7
Ca 0.45325 118.0
K 0.45678 139.9
Sr 0.46283 177.6
a function of 2£ due to the concomitant processes of dislocations activities and bond breaking near and at the tip of an extending microcrack.34) This indicates that the crack
growth condition given by eq. (7) can be controlled by 2£.29)
The decrease of 2£causes the large decrease of£p. Then the
decrease of£presults in the decrease of·*.
The Rice and Wang thermodynamics theory suggests that the potency of an impurity in reducing the grain boundary cohesive energy is a linear function of the difference between the grain boundary segregation energy and the surface segregation energy.13)According to the segregation thermo-dynamics, a model for analyzing the grain boundary cohesive energy of a segregated grain boundary was made by Hirth and Rice.35)Rice and Wang re-expressed the equation made
by Hirth and Rice, and concluded that the grain boundary cohesive energy depends on the difference between Gibbs free energy of the grain boundary and the free surface.13)
Within the dilute solute concentration limits, this relation was re-expressed by Rice and Wang as:13)
2£ ¼2£0 ðEbEsÞ ð8Þ
where 2£0 is the grain boundary cohesive energy of a clean
grain boundary, ¦Eb is the grain boundary segregation
energy,¦Esis the surface segregation energy, and¥is a level
of grain boundary segregation presented in a unit of mol/m2. If solute atoms are segregated at the grain boundary plane, the embrittlement potency is determined by ¦Eb¹¦Es.
In the case of ¦Eb¹¦Es < 0, the solute atoms decrease
the Gibbs free energy of grain boundary compared with the Gibbs free energy of a free surface. Hence, the brittle tendency of grain boundary fracture would be reduced. But the positive difference,¦Eb¹¦Es>0, means a decrease in
the Gibbs free energy of a free surface compared with the Gibbs free energy of a grain boundary and thus would reduce the grain boundary cohesion.
The surface segregation energy can be defined as the difference in total energy between the supercell with one solute X atom being substituted in the free surface and the supercell with one solute X atom in the bulk solid solution. The surface segregation energy,¦Es, was obtained by:
Es¼ ½ðE88AlB E2BX+86AlÞ ðE64AlF E2FX+62AlÞ=2 ð9Þ in which division by 2 accounts for the number of solute X atoms in the supercell. The surface segregation energies of the Mg, Na, Ca, K and Sr atoms are shown in Table 1. The embrittlement potency, ¦Eb¹¦Es, is also listed in
Table 1. The values of¦Eb¹¦Esare 13, 155, 77, 268 and
164 kJ/mol for Mg, Na, Ca, K and Sr respectively. All values of ¦Eb¹¦Es were positive. This means that the Mg, Na,
Ca, K and Sr atoms serve as embrittlers in the grain boundary, proportional to the corresponding value of
¦Eb¹¦Es. Horikawa et al. reported that additional 2 ppm
Na, Ca and Sr caused the high temperature embrittlement based on intergranular fracture in Al5 at%Mg alloys in comparison with the transgranular fracture in the based alloy. Horikawa et al. concluded that the embrittlement effect of Na, Ca and Sr is due to their grain boundary segregation.2)
Ourfirst-principles results supported this conclusion: the high temperature embrittlement is caused by the grain boundary segregation of Na, Ca and Sr.
Figure 5 shows the embrittlement potency of Mg, Na, Ca, K and Sr in aluminum11ð113Þ½110tilt grain boundary as a function of the volume size factors. The embrittlement potency is related to both the volume size factor and valence electrons,NV. It is notable that alkali metals such as Na and K
have one valence electron (NV=1), and alkaline earth metals
such as Mg, Ca and Sr have two (NV=2). The embrittlement
potency increases with the increase of the volume size factor and the decrease of valence electrons of segregated solute atoms. The dependence of the embrittlement potency on the volume size factor and valence electrons can be explained by the charge density distribution.
Figure 6(a) shows the charge density distribution in (110) plan for a pure aluminum grain boundary. Charge density distributions in the same plane are shown in Figs. 6(b), 6(c), 6(d), 6(e) and 6(f ) for the grain boundary with Mg, Na, Ca, K and Sr segregation respectively. A lower charge density distribution between atoms means the weaker AlAl or soluteAl bonds. The numbers 0, 1, 2, 3 and 4 refer to the atomic positions of Al shown in Fig. 6. Figure 6 indicates that for Mg, Na, Ca, K and Sr segregation the charge density around the segregated solute atoms is much lower than that around Al 0 in a clean grain boundary. The bond between solute atom and Al 1 (solute atom and Al 2) is weaker than that between Al 0 and Al 1 (Al 0 and Al 2) in the clean boundary. The difference in valence electrons is the main reason for causing the decrease of charge density around solute atoms; Al has three valence electrons (NV=3), while
Na and K have one valence electron, and Mg, Ca and Sr have two.
[image:5.595.323.530.71.226.2]between Al 1 and Al 2 produces a fall in the charge density between these atoms. Additionally, one can see a stronger bond between Al 1 and Al 3 (Al 2 and Al 4) in the grain boundary with segregation of the solute atom than between Al 1 and Al 3 (Al 2 and Al 4) in the clean boundary. The distance between Al 1 and Al 3 (Al 2 and Al 4) is also shown in Fig. 7. The distance between Al 1 and Al 3 (Al 2 and Al 4) decreases with the increase of the volume size factor of segregated solute atoms. The decrease of the distance between Al 1 and Al 3 (Al 2 and Al 4) causes the increase in the charge density between these atoms.
The segregation of Mg, Na, Ca, K and Sr atoms causes the grain boundary to expand because of the lager atomic size of these solute atoms than Al. Then the local expansion of the grain boundary results in both weak soluteAl bonds and weak AlAl bonds at the grain boundary. Additionally, fewer valence electrons of Mg, Na, Ca, K and Sr also cause the weak soluteAl bonds. These weaker metallic bonds, which are caused by the lager atomic size and fewer valence electrons of Mg, Na, Ca, K and Sr than Al, are consistent with the dependence of the embrittlement potency on the volume size factor and valence electrons: the embrittlement
potency increases with the increase of the volume size factor and the decrease of valence electrons of segregated solute atoms.
4. Conclusions
The authors investigated the grain boundary segregation energies of Mg, Na, Ca, K and Sr at symmetric tilt 11ð113Þ½110grain boundary in aluminum using thefi rst-principles calculation. The results were as follows.
(1) The grain boundary segregation energies at symmetric tilt11ð113Þ½110grain boundary in aluminum are¹6, ¹32,¹35,¹62 and¹66 kJ/mol for Mg, Na, Ca, K and Sr respectively.
(2) The grain boundary segregation energy decreases with the increase in volume size factor; a solute atom larger in size is preferable for the grain boundary segregation. Therefore, it can be explained that the solute atom larger in size than Al, which stores the larger elastic strain energy in the bulk, prefers the looser site at the grain boundary plane rather than in the bulk, to release the elastic strain energy.
(3) On the basis of the RiceWang model, the effect of grain boundary segregation was studied on the grain boundary embrittlement in aluminum. The embrittle-ment potency is positive for all solute atoms. This means that each of Mg, Na, Ca, K and Sr atoms serves as an embrittler in the grain boundary, proportional to the corresponding value of the embrittlement potency. The embrittlement potency increases with the increase of the volume size factor and the decrease of valence electrons of segregated solute atoms.
(4) The charge density distribution of the grain boundary demonstrates that the Mg, Na, Ca, K and Sr atoms form weak soluteAl bonds and weak AlAl bonds in the grain boundary region. The weaker metallic bonds are caused by the lager atomic size and fewer valence electrons of Mg, Na, Ca, K and Sr than Al. This is consistent with the dependence of the embrittlement potency on the volume size factor and valence electrons.
50 nm 175 nm 300 nm -3
-3
-3
K K
1
2 3
4 Ca Ca
1
2 3
4
Sr Sr
1
2 3
4 Na Na
1
2 3
4 Mg Mg
1
2 3
4 1
2 3
4
(a) (b) (c)
(d) (e) (f)
0
113] [
] [ ] 110 [
332
Fig. 6 Charge density distributions in (110) plan for (a) pure aluminum grain boundary and grain boundary with (b) Mg, (c) Na, (d) Ca, (d) K and (e) Sr segregation. White and light blue balls indicate Al and solute atoms respectively.
Fig. 7 Interatomic distances between Al atoms, dAl, in pure aluminum
[image:6.595.84.513.70.242.2] [image:6.595.64.274.303.460.2]Acknowledgments
This study was partly supported by the Light Metal Educational Foundation Inc., Sumitomo Electric Industries Ltd. and Shimano Corp. The authors are grateful to Mr. Hiroki Fujita and Ms. Kan Tomoko, who were students in the department, for their assistance with the computations.
REFERENCES
1) J. A. Wert and J. B. Lumsden:Scr. Metall.19(1985) 205209. 2) K. Horikawa, S. Kuramoto and M. Kanno: Acta Mater. 49(2001)
39813989.
3) S. P. Lynch:Scr. Mater.47(2002) 125129.
4) T. Kobayashi, M. Niinomi and K. Degawa:J. JILM37(1987) 816 823.
5) X. Liu, X. Wang, J. Wang and H. Zhang:J. Phys. Condens. Matter17 (2005) 43014308.
6) G. H. Lu, A. Suzuki, A. Ito, M. Kohyama and R. Yamamoto:Philos. Mag. Lett.81(2001) 757766.
7) G. H. Lu, Y. Zhang, S. Deng, T. Wang, M. Kohyama, R. Yamamoto, F. Liu, K. Horikawa and M. Kanno:Phys. Rev. B73(2006) 224115. 8) T. Uesugi and K. Higashi:Mater. Sci. Forum654656(2010) 942945. 9) W. T. Geng, A. J. Freeman and G. B. Olson:Mater. Trans.47(2006)
21132114.
10) M. Yamaguchi, M. Shiga and H. Kaburaki:Mater. Trans.47(2006) 26822689.
11) M. Yuasa and M. Mabuchi:Mater. Trans.52(2011) 13691373. 12) R. Z. Wang, S. Tanaka and M. Kohyama:Mater. Trans.53(2012) 140
146.
13) J. R. Rice and J. S. Wang:Mater. Sci. Eng. A107(1989) 2340.
14) M. D. Segall, P. J. D. Lindan, M. J. Probert, C. J. Pickard, P. J. Hasnip, S. J. Clark and M. C. Payne:J. Phys. Condens. Matter14(2002) 2717 2744.
15) P. Hohenberg and W. Kohn:Phys. Rev.136(1964) B864B871. 16) J. P. Perdew, J. A. Chevary, S. H. Vosko, K. A. Jackson, M. R.
Pederson, D. J. Singh and C. Fiolhais:Phys. Rev. B46(1992) 6671 6687.
17) N. Troullier and J. L. Martins:Phys. Rev. B43(1991) 19932006. 18) D. Vanderbilt:Phys. Rev. B41(1990) 78927895.
19) T. H. Fischer and J. Almlof:J. Phys. Chem.96(1992) 97689774. 20) D. McLean: Grain Boundaries in Metals(Clarendon Press, Oxford,
1957) pp. 116149.
21) A. P. Sutton and R. W. Balluffi: Interface in Crystalline Materials, (Oxford, Clarendon Press, 1995).
22) T. Uesugi and K. Higashi:J. Mater. Sci.46(2011) 41994205. 23) J. P. Hirth and J. Lothe:Theory of Dislocations, second edition (Wiley,
New York, 1982) p. 839.
24) J. D. Eshelby:J. Appl. Phys.25(1954) 255261. 25) J. Friedel:Adv. Phys.3(1954) 446507. 26) H. W. King:J. Mater. Sci.1(1966) 7990.
27) T. Uesugi, M. Kohyama and K. Higashi: Phys. Rev. B 68 (2003) 184103.
28) T. Uesugi, M. Kohyama and K. Higashi:Mater. Sci. Forum426432 (2003) 599603.
29) J. Kameda and C. J. McMahon: Metall. Mater. Trans. A11(1980) 91 101.
30) M. Yamaguchi:Metall. Mater. Trans. A42(2011) 319329. 31) A. A. Griffith:Phil. Trans. Roy. Soc. A221(1921) 163198. 32) G. R. Irwin: J. Appl. Mech.24(1957) 361364.
33) E. Orowan:Rep. Prog. Phys.12(1949) 185232.
34) M. L. Jokl, V. Vitek and C. J. McMahon:Acta Metall.28(1980) 1479 1488.