S E L E C T I V E D I S R U P T I O N OF
T H E S A R C O T U B U L A R S Y S T E M I N F R O G
S A R T O R I U S M U S C L E
A Q u a n t i t a t i v e S t u d y with Exogenous
Peroxidase as a Marker
B R E N D A E I S E N B E R G a n d R O B E R T S. E I S E N B E R G
From the Department of Physiology, Duke University Medical Center, Durham, North Carolina o~7706. The authors' present address is the Department of Physiology, University of California at Los Angeles, Los Angeles, California 900~4
A B S T R A C T
Skeletal muscles which have been soaked for 1 hr in a glycerol-Ringer solution and then returned to normal R i n g e r solution have a disrupted sarcotubular system. T h e effect is associated with the return to Ringer's since muscles have normal fine structure while still in glycerol-Ringer's. Karnovsky's peroxidase method was found to be a very reliable marker of extracellular space, filling 98.5% of the tubules in normal muscle. It was interesting to note that only 84.1% of the sarcomeres in normal muscle have transverse tubules. T h e sarcotubular system was essentially absent from glycerol-treated muscle fibers, only 2 % of the tubular system remaining connected to the extracellular space; the intact remnants were stumps extending only a few micra into the fiber. Thus, glycerol-treated muscle fibers pro- vide a preparation of skeletal muscle with little sarcotubular system. Since the sarcoplasmic reticulum is not destroyed and the sarcolemma and myofilaments are intact in this prepara- tion, of the properties of the sarcolemma m a y thus be separated from those of the tubu- lar system.
I N T R O D U C T I O N
It has been clear for m a n y years that skeletal muscle fibers must contain a specialized system for linking excitation of the surface m e m b r a n e with contraction in the depths of the fiber (14). Indeed, in frog skeletal muscle H u x l e y and T a y l o r (18) postulated a specialized conduction system lo- cated at the Z line before the fine structure of muscle had been examined in the electron micro- scope. T h e structure of this conducting system is now known (2, 23). I n frog sartorius muscle a system of tubules arises as invaginations of the
sarcolemma and invades the fiber in the plane of the Z disc, branching so as to surround the m y o - fibrils. This system is called the T system or the sarcotubular system. T h e evidence that the tubules are, in fact, invaginations of the sarcolemma is indirect, the aperture of the tubule not having been convincingly observed in this tissue. T h e evidence that the tubular l u m e n is open and thus that the tubular m e m b r a n e is continuous with the sarco- l e m m a is that various extracellular markers fill the lumen of the transverse tubules (5, 15, 19,
22).
451
on November 4, 2015
jcb.rupress.org
Downloaded from
A possible explanation of the difficulty of observing the opening of the tubule in material prepared for observation in the electron microscope is found in the work of Huxley (19), who used ferritin as an extracellular marker. If a muscle is fixed in glutar- aldehyde for 20 min to 2 hr and then exposed to ferritin, no ferritin is found in the tubules. It thus seems likely that glutaraldehyde fixation seals off the opening of the tubules.
Many of the electrical properties of muscle have been explained in terms of the properties of the tubular system. I n particular, the large capacitance of muscle fibers (3, 6, 8), the after potential which follows a single action potential (12), the after potential that follows a train of action potentials (9), and the slow potential change produced by a prolonged pulse of hyperpolarizing current (I) have been attributed to the tubular system. I n addition, the tubule membranes may play a role in some of the permeability properties of muscle fibers, particularly the peculiar potas- sium system (16), called anomalous rectification. I n the absence of a method of selectively remov- ing the sarcotubular system it has been difficult to test any of these ideas directly. Howell and Jenden (17), following an incidental observation of Fujino et al. (11), discovered a treatment which disrupted the transverse tubules. They reported that the sarcotubular system is absent in muscle fibers which have been soaked for 1 hr in a Ringer solu- tion to which 400 mM glycerol had been added, and then returned to normal Ringer solution. The electrical properties of these treated fibers have been examined (4, 12) with a view to localizing the many unusual electrical properties of skeletal muscle. Interpretation of these electrical results and, indeed, the usefulness of this preparation depends on the extent of destruction of the sarco- tubular system in such treated fibers. It is not sufficient to note the presence or absence of a tubular profile since this membrane is not neces- sarily connected to the sarcolemma. The amount of damage is measured here by determining the amount of the sarcotubular system which is still connected to the extraeellular fluid after glycerol treatment. Horseradish peroxidase, rendered visible in the electron microscope by the method of Graham and Karnovsky (13), was used as an extracellular marker in these experiments. Our results show that very little of the sarcotubular system remains connected to the extracellular space in glycerol-treated muscle fibers. Thus, the
properties of treated muscle fibers essentially represent the properties of a smooth cylinder of the surface membrane.
M E T H O D S
Glycerol Treatment
Sartorius muscles, tied to a bar, were placed in glycerol-Ringer's ( l l 5 n ~ NaCl, 2.5 m~ KC1, 1.8 mM CaCl2, 3.0 mM sodium phosphate buffer pH 7.0 and 400 mM glycerol) for 1 hr at room tem- perature (17-20°C). After this treatment, the muscle was rapidly transferred to normal Rin- ger's.
Tetrodotoxin (TTX) (10 -7 g/ml) was added to both glycerol-Ringer's and normal Ringer's to block spontaneous contractions which other- wise often occur when the muscle is first returned to normal Ringer solution (Gage and Eisenberg, unpublished observation). It has been reported (4, 17) that destruction of the transverse tubular system occurs not in the gly- cerol-Ringer solution, but only after the muscle has been returned to normal Ringer's. Some muscles were transferred directly from glyc- erol-Ringer's to fixative to confirm this finding (see Results); in order to have good preservation, it was found necessary to add 400 mM glycerol to the fixative. Muscles fixed this way are referred to as "in glycerol" muscles; muscles fixed (see Standard Fixation) l hr after return to normal Ringer's are called "glycerol-treated" muscles.
Sampling of Tissue
I n these experiments muscles were treated in a variety of ways before, during, and after fixation. The important features common to all techniques were concerned with sampling problems. One of the essential difficulties in relating morphological findings to physiological findings is that morpho- logical results are normally based on samples from a very small part of a few fibers from an undeter- mined location in a muscle, whereas the physio- logical results are based on measurements from many fibers on one surface of the muscle. I n these experiments care was taken to study the morphol- ogy of those fibers normally used in physiological experiments. Only the central third of the muscle was embedded and only those fibers on the deep surface of the muscle were examined. The other surface of the muscle, which lies just under the skin in the frog, is not used in studies using micro-
452 THE JOURNAL OF CELL BIOLOGY - VOLUME 39, 1968
on November 4, 2015
jcb.rupress.org
electrodes because of its dense covering of connec- tive tissue. It is interesting to note that this covering was also found to act as a barrier to penetration of glycerol, peroxidase, and fixative in our experi- ments.
I t is clear that the surface fibers could be easily identified in a transverse section cut across the entire muscle. Transverse sections are, however, unsuitable for studies concerned primarily with the transverse tubular system since most fibers will not be sectioned through a Z disc. Longitudinal sec- tions of r a n d o m orientation are also unsuitable because they give little information about the loca- tion of a fiber. Oriented longitudinal sections, cut so that the section extends from the skin surface to the depth of the muscle (see Fig. 1), permit the identification of the location of a fiber. All results presented here are from such sections.
I n order to cut the required sections, precaution had to be taken during the processing of the tissue preceding sectioning. T h e muscle was cut into four strips parallel to the fibers. T h e strips were sepa- rated and cut into 1-2 m m lengths. These pieces were small enough for penetration of the fixatives and reagents, yet each piece could be e m b e d d e d in a flat mold so that sectioning of the Araldite block in a particular oriented plane gave the
desired profile of layers (Fig. 1). I n every experi- ment a surface fiber, and often a few fibers from the next two layers, were examined from each of the four strips.
Dissection and Standard F i x a t i o n
Sartorius muscles from small frogs were dis- sected, particular care being taken not to bruise or otherwise injure the surface fibers. Muscles were tied to a glass bar at " r e s t i n g " length, i.e. that length at which the muscle was just taut. Fixation required 1.5-2.0 hr in a 5 % glutaraldehyde solu- tion to which 0.1 M sodium cacodylate (pH 7.2) and 2 mM CaC12 had been added (23). T h e muscles were left overnight at 4°C in buffer wash (0.1 M sodium cacodylate, p H 7.2, 2 mM CaC12 and 10% sucrose) and were then cut into small pieces (see Sampling of tissue) and postfixed in 10% osmium tetroxide in 0.1 M sodium cacodylate buffer, p H 7.2. I n some experiments thase small pieces of fixed tissue were stained with uranyl acetate, called staining en bloc (7). T h e pieces were ori- ented as described above, and then embedded in Araldite. Sections were cut with glass and dia- m o n d knives on a Porter-Blum microtome (MT-1) ( I v a n Sorvall, Inc., Norwalk, Conn.). Thick sec- tions (1 /~) were stainedwith toluidine blue and ob-
O R I E N T E D P L A N E " R A N D O M " P L A N E
I I I I
3 0 0 / . / . T H I C K C O N N E C T I V E
* . ~. , T I S S U E
FmURE 1 Three-dimensional reeonstmetion of frog sertorius musele showing how seetious were ob-
tained. Four strips (I, II, H I and IV) were eut parallel to the longitudinal axis. NU mierographs in this paper are taken from oriented seetions like that shown in strip III. Longitudinal seetions in a "random" plane, as in strip II, were rejected. The eross seetion of the muscle, used in the front Nee of this figure, was traced from a light mierograph.
BRENDA EISENBERG AND ROBERT S. EISENBERG Selective Disruption of tke Sarcotubular Sy~iem 453
on November 4, 2015
jcb.rupress.org
Downloaded from
served with the light microscope to confirm that the orientation procedures had been correctly performed. T h i n sections for electron microscopy were either unstained, stained with uranyl acetate, or stained with uranyl acetate and then lead citrate (27). Sections were mounted on carbon- coated grids and observed in a Philips EM-200 (Philips Electronics & Pharmaceutical Industries Corp., N e w York) with a single condenser, 20 # aperture, and accelerating voltage of 60 kv.
Peroxidase Method of Marking
Extracellular Space
T h e peroxidase method of G r a h a m and Karnovsky (13) was found to be a cheap, conven- ient, and reliable method of marking the sarcotu- bular system in skeletal muscle (see also 21, 26). N o r m a l untreated sartorius muscles were exposed for 30 min to a Ringer solution to which 0.05% horseradish peroxidase (Sigma Chemical Com- pany, St. Louis, T y p e II) had been added. This solution often also contained T T X . After glutar- aldehyde fixation and buffer wash, small pieces of fixed muscle were placed in a solution containing 0.05% 3,3r-diaminobenzidine tetrahydrochloride (Sigma), 0.01% H202, and 0.05 M Tris-maleate buffer (pH 7.6). Three brief washes in distilled water preceded the 2-hr postfixation at 0°C w k h 1% osmium tetroxide in 0.1 M sodium cacodylate buffer (pH 7.2). Peroxidase itself is not visible in the electron microscope, and neither, presumably, is the reaction product resulting from incubating diaminobenzidine and hydrogen peroxide w i t h peroxidase. T h e electron-opaque material ob- served with the electron microscope is probably osmium which has reacted with this reaction prod- uct (25).
The peroxidase method was used to determine the n u m b e r of tubules still connected with the extracellular space in glycerol-treated muscle fibers. I n these experiments muscles were soaked for 1 hr in T T X glycerol-Ringer's, then for 30 rain in T T X - R i n g e r ' s and finally for 30 rain in T T X - Ringer's to which 0.05% peroxidase had been added. Several muscles were exposed to peroxidase while they still remained in glycerol-Ringer's, to determine whether the sarcotubular system is still connected to the extracellular space in this solu- tion. These muscles were soaked for 30 rain in glyc- erol-Ringer's, then for 30 min in glycerol-Ringer's to which peroxidase had been added, and then transferred to glutaraldehyde fixative. T h e appear-
ance of such in glycerol fibers depended on whether standard fixative or fixative modified by adding 400 mM glycerol had been used. T h e latter fixative was found to give better but not perfect fixation.
Methods of Counting
Unstained or lightly stained thin sections (silver) were used to count the peroxidase-fiUed tubules, since the contrast between the dense peroxidase reaction product and the lightly stained muscle made counting m u c h easier. I n each block of tissue the surface fiber was located, as described above, and micrographs of the fiber were taken. Second- and third-layer fibers were identical in appearance to the surface fibers and were used in counting if the sarcolemma was surround by a dense ring of peroxidase. A montage of each fiber was m a d e at a final magnification of about 20,000. T h e apparent diameter of the fiber was esti- mated and three sets of counts were m a d e on each normal muscle fiber: one for all the filled tubules (called x), one for all the e m p t y tubules (called e), and one for those sites which had no tubules (called n).
Occasionally a tubule was not cut transversely, but longitudinally, so that it was seen for the width of one or more myofibrils (Fig. 4). These tubules were counted according to the n u m b e r of inter- fibrillar spaces they crossed: that is, a tubule extending over one entire fibril and crossing two interfibrillar spaces was counted as two sites.
T h e counting was quite reproducible, the pre- cision being better t h a n 1%. I n glycerol-treated muscle fibers the presence of disorganized tubules (see Results) m a d e it impossible to distinguish be- tween e and n sites. Thus, these sites were counted together as (e + n) sites. Each time a filled tubule was found, its depth from the sarcolemma was noted.
R E S U L T S
T h e fine structure of normal frog sartorius muscle has been well described (23). Occasionally most of this structure can be seen in a single longitudinal section (Fig. 2). Such a fortuitous section passes just above a myofibril, cutting through a substantial a m o u n t of the sarcotubular system and sarco- plasmic reticulum. T h e transverse tubule is situ- ated at the Z line and is continuous from one m y o - fibril to the next. T h e tubule branches in two planes; it branches in the transverse plane and forms a ring which surrounds most of the Z disc;
454 THE JOURN~-L OF CELL BIOLOGY - VOLUME 39, 1968
on November 4, 2015
jcb.rupress.org
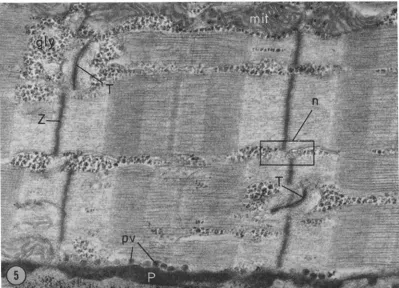
FmURES ~ and 3 Normal muscle longitudinal section with long axis of the fiber oriented horizontally. Fig. 3 has peroxidase reaction product filling the sarcotubular system. (T) transverse sarcotubule. (L) longitudinal sarcotubule.
(t.c.)
terminal cisternae. Zone of intermediate cisternae(i.e.)
indicated by arrow.(1.t.)
longitudinal tubule of reticulum. (f.c.) fenestrated collar. (*) possible frontal section of flattened sarcotubular cisterna filled with peroxidase.(gly)
glycogen granules. Fig. ~, )< 50,000; Fig. 3, X 45,000.455
on November 4, 2015
jcb.rupress.org
Downloaded from
[image:5.612.106.503.61.599.2]and it branches in the longitudinal planes, (Figs. 3 and 12) perhaps so that it can pass near the Z line even w h e n the Z lines of adjacent myofibrils are not in register. T h e longitudinal m e m b r a n e structures which are not continuous w i t h the trans- verse tubules are called the sarcoplasmic reticulum. T h e component of the sarcoplasmic reticulum which is immediately adjacent to the transverse tubule is called the terminal cisterna or lateral sac. T h e characteristic structure formed by two terminal cisternae in close apposition to a transverse tubule is called a triad. T h e terminal cisterna is continuous with the intermediate cisterna, and the latter branches into longitudinal tubules in the outer part of the A band. (This tubu- lar component of the sarcoplasmic reticulum should not be confused w i t h the occasional longi- tudinal elements of the so called "transverse" sarcotubular system, Fig. 3). T h e longitudinal tubules of reticulum then fuse to form the fen- estrated collar in the central region of the A band.
Since our analysis depends on a quantitative estimate of the n u m b e r of tubules accessible to the external medium, it was i m p o r t a n t to use a reli- able marker of extracellular space. Ferritin was not used because it was thought to be rather capricious (24). T h e sodium localization method used on muscle by Zadunaisky (28), which de- pends on the observation of sodium pyroantimon- ate precipitate, was found to be most unreliable in our hands; in only 1 out of 10 experiments (normal muscle) was any precipitate found in the sarco- tubules, and then only 2 0 % of the tubules were filled. However, Figs. 2 and 12, which show the structure of normal muscle, were taken from these negative experiments with pyroantimonate.
O n the other hand, the peroxidase method for marking extracellular space was most successful. T h e peroxidase marker formed a dense ring around all the surface fibers in the muscle. T h e reaction pro- duct w a s n o t found belowthe f o u r t h - o r fifth-layer fibersnor below the sheet of connective tissue which covers the surface of the sartorius and lies just under the skin of the frog. We think that this result is due to the failure of diaminobenzidene to pene- trate. O n l y those fibers which were surrounded with a dense ring of peroxidase were used in this study, but all surface fibers fulfilled this criterion. T h e sarcotubular system was filled w i t h electron- opaque material (Fig. 3, 4, and 5) and was thus continuous w i t h the extracellular medium, as has been described m a n y times previously. Longi-
tudinal branches of the tubular system were seen in about 1% of the sarcomeres, although this n u m b e r obviously will depend greatly on the amount of stagger in the Z lines of adjacent m y o - fibrils. Peroxidase was absent from the sarco- plasmic reticulum, which further confirms a separation of this c o m p a r t m e n t from the sarcotu- bular system. In some places a transverse tubule is not seen in the space between myofibrils near the Z line. Such an absence of a profile of the trans- verse tubule implies that not every myofibril is sur- rounded by a complete ring of transverse tubule. T a b l e I shows the results of counting the various classes of tubules (see Methods) in m a n y muscle fibers. T h e depth of the single muscle fiber in the whole muscle is indicated. Some 8 4 % of the sites where profiles of the transverse tubule are expected to be seen do, in fact, contain tubules filled w i t h peroxidase. This latter n u m b e r is i m p o r t a n t since it forms the basis for the interpretation of the n u m b e r of filled tubules in glycerol-treated muscle fibers. T h e peroxidase method is clearly very reliable, the peroxidase filling some 99 % of all the tubules observed, with little variation from fiber to fiber standard error of the m e a n for nine fibers, 0 . 6 % ; m a x i m u m deviation observed, 3.8%). Thus, the peroxidase method is suitable for deter- mining what fraction of the tubular system is con- nected to the outside in glycerol-treated muscle fibers.
T h e appearance of muscle fibers fixed while they were still in glycerol-Ringer solution, before the sarcotubular system had been disrupted, de- pended on the fixative used. Muscle fibers which were fixed in glutaraldehyde to which 400 mM glycerol had been added were normal in appear- ance (Fig. 5), both the sarcotubular system and the sarcoplasmic reticulum being essentially in- distinguishable from normal. I t was not possi- ble to determine, with any degree of confidence, whether these fibers were shrunken since consider- able variation in fiber size was noted from one normal frog to another. Fixation in standard glutaraldehyde fixative (to which 400 rnM glycerol had not been added) gave fibers which were grossly swollen in appearance, but fibers deeper in the muscle were m u c h more normal. This finding m a y be explained by the osmotic shock involved in transferring a muscle from hypertonic glycerol- Ringer's to a fixative designed for muscles in iso- tonic solutions. Irrespective of the degree of swell- ing of the fiber, the sarcotubular system was intact
4 5 6 T H E JOURNAL OF CELL BIOLOGY • VOLUME 39, 1968
on November 4, 2015
jcb.rupress.org
FIOUan 4 Normal muscle longitudinal section with long axis of the fiber oriented vertically. Peroxidase reaction product fills the sarcotubular system. (x) examples of triads in which the central dement was filled with peroxidase (x-sites). (n) examples of "no tubule sites." Arrow marks triad which happens to be located at the A-I junction. Long lengths of tubule were counted as described in the text. X ~1,000.
457
on November 4, 2015
jcb.rupress.org
Downloaded from
T A B L E I
Normal Muscle
M u s c l e
F r a c t i o n of F a c t i o n of sites sites a t w h i c h a t w h i c h P e r o x i d a s e t u b u l e s filled t u b u l e s
D e p t h of No. of No. of N o . of sites r e l i a b i l i t y a r e f o u n d , are. f o u n d ,
f i b e r f r o m filled e m p t y w i t h o u t ~ x + e x
s u r f a c e t u b u l e s (x) t u b u l e s (e) t u b u l e s (n) x + e x + e + n x + e + n
A
C
% % %
1 71 4 28 94.7 72.8 68.9
3 87 1 17 98.9 83.8 82.9
4 104 0 26 100.0 80.0 80.0
1 80 0 11 100.0 87.9 87.9
3 32 1 7 97.0 82.5 80.0
1 191 3 40 98.5 82.9 81.6
2 349 6 49 98.3 87.9 86.4
3 162 2 29 98.8 85.0 89.9
4 222 0 24 100.0 90.2 90.2
M e a n , % 98.5 83.7 82.4
S t a n d a r d E r r o r of M e a n , % 0.6 1.7 2.1
M e a n s were c o m p u t e d on, a u n w e i g h t e d basis, i.e. each fiber was given equal weight. If the d a t a from each fiber are weighted according to the n u m b e r of observations made, the means are not c h a n g e d significantly.
a n d filled w i t h peroxidase. T h u s , T a b l e I I includes d a t a f r o m b o t h sets of experiments.
P e r o x i d a s e was a d d e d to the glycerol-Ringer solution before fixation (see M e t h o d s ) , T h e results of c o u n t i n g the n u m b e r of filled tubules, e m p t y tubules, a n d sites w i t h o u t tubules are shown in T a b l e II. T h e d a t a seem to be indistinguishable from those t a k e n from n o r m a l muscles. F o r p u r - poses of c o m p a r i s o n w i t h glycerol t r e a t e d muscle fibers, some of the d a t a in the two tables were l u m p e d together. I n particular, t h e c o m b i n e d figure for the " f r a c t i o n of sites a t w h i c h t h e r e were filled t u b u l e s " is 8 1 % .
Soon after a glycerol-soaked muscle is r e t u r n e d to R i n g e r ' s it becomes o p a q u e a n d often gives spontaneous twitches (unless T T X is used to block muscle action potentials). T h i s glycerol-treated muscle bears no r e s e m b l a n c e to the classical glyc- e r i n a t e d p r e p a r a t i o n of myofibrils, since glycerol- t r e a t e d fibers h a v e relatively n o r m a l resting poten- tials a n d action potentials (4) a n d therefore h a v e a n i n t a c t sarcclemma. F u r t h e r m o r e , these fibers give caffeine c o n t r a c t u r e s 1 (17). L i g h t microscopy reveals a considerable v a r i a t i o n in structure from fiber to fiber: a few fibers a p p e a r n o r m a l while
m a n y h a v e " v a c u o l e s " or swollen spaces b e t w e e n the myofibrils. T h e fine s t r u c t u r e observed w i t h the electron microscope shows a similar r a n g e of fibers : some fibers are n o r m a l in a p p e a r a n c e except for the lack of s a r c o t u b u l a r system, a n d others show evidence of general disruption. I t is n a t u r a l to suppose t h a t the l a t t e r case corresponds w i t h those fibers found to h a v e low m e m b r a n e potentials, b u t direct p r o o f of this identification has n o t b e e n a t t e m p t e d . T h e fine structure is often t h a t shown in Fig. 6. I n this low m a g n i f i c a t i o n survey picture it is e v i d e n t t h a t the muscle striations are n o r m a l a n d t h a t there is only a little swelling b e t w e e n myo- fibrils; the m i t o c h o n d r i a are broken, however. A t h i g h e r m a g n i f i c a t i o n (Figs. 7, 8, a n d 9), the fila- m e n t s a p p e a r n o r m a P a n d the d i s r u p t i o n of the
1A. Sandow and E. S. Geffner (personal communica- tion) have recently measured the tension produced by application of 5 mM caffeine to glycerol-treated toe muscle fibers. They find that there are large caffeine contractures in these fibers, the average peak tension being 62% of normal. It thus seems likely that those parts of the sarcoplasmic reticulum involved in the release of Ca ++ and the filaments are reasonably normal.
4 5 8 T H E J O U R N A L O F C E L L B I O L O G Y • V O L U M E 8 9 , 1 9 6 8
on November 4, 2015
jcb.rupress.org
FIGURE 5 "In glycerol" muscle: treated with peroxidase and fixed with glutaraldehyde + 400 mM glyc- erol. Longitudinal section with long axis horizontally oriented. Peroxidase reaction product (P) seen in the extracellular space outside the sarcolemma, and in the sarcotubular system (T). Muscle is normal in appearance. Note mitochondrion (mit) aDd pinocytotic vesicles (p.v.), glycogen granules (gly), 'no-tubule' site (n). This section was stained with lead citrate and then uranyl acetate, thus producing particularly dark Z lines (X 3~,000).
transverse tubular system can be clearly seen: a typical triad no longer contains three elements since the transverse tubule is usually missing: how- ever, m a n y triads have small remnants of tubules. Some intermediate cisternae remain intact, but most are broken. T h e fenestrated collar seems the least d a m a g e d structure of the reticulum, usually being indistinguishable from normal. T h e fibers shown in Figs. 8 and 9 are more severely altered: the terminal cisternae are damaged and the space between myofibrils is swollen, especially at the A I j unction.
T h e glycerol-treated muscle fiber in Figs. 8 and 9 had been exposed to peroxidase to m a r k the extra- cellular space. T h e peroxidase marker is found in a dense ring around the sarcolemma. T h e sarco- l e m m a had normal substructure (i.e. it consisted of two dense lines) and was seldom broken, even
in the most severely damaged fibers. O n 15 fibers, counts were m a d e of the n u m b e r of tubules filled with peroxidase (see Table I I I ) . T h e average n u m b e r of filled tubules after glycerol treatment was 2.6 4- 0 . 7 % (mean 4- standard error of the mean), compared with 8 1 % in muscles with nor- mal structure (combined data from Tables I and II). T h a t is to say only 2 . 6 % of the sites where tubules might be expected to be found did, in fact, contain filled tubules. Thus, the apparent fraction of the tubular system remaining in the glycerol- treated fibers is 2.6 - - 0.81 = 3.2%. Presumably, 9 7 % of the tubular system no longer contributes to the electrical properties of glycerol-treated mus- cle fibers, nor is involved in excitation-contraction coupling
3 . 2 % is, in fact, an overestimate of the a m o u n t of the tubular system still continuous with the
BRENDA F, ISENBERG AND ROBERT S. EISENBERG Selective Disruption of the Sarcotubular System 459
on November 4, 2015
jcb.rupress.org
Downloaded from
T A B L E I I
Muscles in Glycerol (Tubules Intact)
Muscle
Fraction of Fraction of sites sites at which at which Peroxidase tubules filled tubules Depth of No. of No. of No. of sites reliability, are found, are found,
fiberfrom filled empty without x x + e x
surface tubules (x) tubules (e) tubules (n) x + e x + e + n x + *" + n
A B
% % %
1 36 4 5 90.0 88.9 80.0
1 251 6 65 97.7 79.8 78.0
2 152 0 23 I00.0 86.9 86.9
3 135 1 29 99.3 82.4 81.8
4 199 2 26 99.0 88.5 87.7
1 140 4 19 97.2 88.3 85.9
1 264 6 63 97.8 81.0 79.3
2 146 28 34 83.9 83.7 70.2
2 467 31 92 93.8 84.4 79.1
2 314 41 85 88.5 80.7 71.4
M e a n , °,Vo 94.7 84.5 80.0
S t a n d a r d Error of M e a n , % 1.7 1.0 1.9
M e a n s were c o m p u t e d on a n u n w e i g h t e d basis, i.e. each fiber was given equal weight. I f the d a t a from each fiber are weighted according to the n u m b e r of observations made, the means are not c h a n g e d significantly.
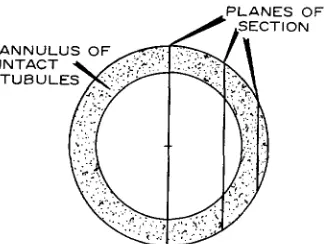
extracellular m e d i u m because of s a m p l i n g errors associated w i t h the d i s t r i b u t i o n of the few tubules r e m a i n i n g in t r e a t e d muscle fibers. Fig. 10 is a h i s t o g r a m showing the location of the few filled tubules. I t is clear t h a t almost all of the tubules r e m a i n i n g are stumps, n o t e x t e n d i n g f a r t h e r t h a n a few m i c r a into the interior of the muscle fiber. T h e i n t e r p r e t a t i o n of this h i s t o g r a m would be complicated if isolated filled tubules were c o m m o n , p a r t i c u l a r l y if t h e y were t b u n d in the d e p t h s of the fiber. F o r t u n a t e l y , this is not the case; indeed, iso- lated filled tubules were almost n e v e r found. Thus, the d i s t r i b u t i o n of r e m a i n i n g tubules c a n b e repre- sented in a c r u d e a p p r o x i m a t i o n as a n a n n u l u s (Fig. I 1), the thickness of w h i c h in relation to the fiber radius represents the fraction of tubules r e m a i n i n g . S u c h a n a p p r o x i m a t i o n incorrectly assumes t h a t every site in the outer p a r t of the fiber has a filled tubule, b u t will give correct results if the p r o b a b i l i t y of finding a filled t u b u l e is inde- p e n d e n t of position in t h e a n n u l u s a n d is c o n s t a n t from fiber to fiber. T h e r e are n o t e n o u g h d a t a a v a i l a b l e to justify m o r e precise models. I n such a simple m o d e l r a n d o m l o n g i t u d i n a l sections will tend to overestimate the relative size of the a n n u -
lus, for two reasons: first, since most sections do not pass t h r o u g h the c e n t e r of the fiber, the a p p a r e n t d i a m e t e r of t h e fiber (actually the c h o r d of the circle) will be less t h a n the a c t u a l d i a m e t e r ; second, since the section will not, in general, cut t h r o u g h the a n n u l u s at r i g h t angles, the a p p a r e n t absolute size of the a n n u l u s will be a n overestimate. T h i s effect is q u a n t i t a t i v e l y a n a l y z e d for idealized geometry in A p p e n d i x I a n d is s h o w n not to b e trivial. I t is likely t h a t the effect is of the o r d e r of 5 0 % in our case; thus, the true figure for the frac- tion of t u b u l a r area r e m a i n i n g c o n n e c t e d to the extracellular space in glycerol-treated muscle fibers is likely to be a b o u t 3 . 2 % X 0.5 = 1.7%.
I t should b e m e n t i o n e d t h a t several v a r i a b l e s w h i c h m i g h t conceivably affect the degree of t u b u - lar d e s t r u c t i o n h a v e n o t b e e n studied in these experiments. I n p a r t i c u l a r , t h e effects of t e m p e r a - ture, stretch, a n d speed of solution c h a n g e were n o t examined.
D I S C U S S I O N
I n the course of these e x p e r i m e n t s a n interesting observation was m a d e c o n c e r n i n g the structure of n o r m a l muscle. Fig. 12 shows a transverse t u b u l e
460 ThE JOURNAL OF CELL BIOLOG~ " VOLUME 39, 1968
on November 4, 2015
jcb.rupress.org
FIGURES 6 and 7 Glycerol-treated muscle. Muscle soaked in Ringer's -4- 400 m M glycerol for one hour, then returned to normal Ringer for 30 min, and then fixed. The A band, I band and Z lines are normal. The mitochondrion is disrupted
(mit).
Arrows point to swellings in intermyofibrillar spaces. Fig. 7 (inset) shows the remnant of a sareotubule (T); terminal cisternae(t.c.)
and glycogen(gly)
are present. Fig. 6: X 17,000; Fig. 7: X 93,000.461
on November 4, 2015
jcb.rupress.org
Downloaded from
I~aURES 8 and 9 Glycerol-treated muscle with disrupted sarcotubular system. Peroxidase reaction pro- duct (P) is seen outside the sarcolemma, but the disruption of the sarcotubular system (arrows) presum- ably prevents peroxidase from filling the remaining "intact" transverse sarcotubules (T) and swollen sareotubules
(S.T.).
In Fig. 9 the swollen tubule in the lower left corner is identifiable by the characteristic scalloped edge where the tubule is attached to the terminal cisterua (t.c.). Intermediate cisternae(i.c.)
are swollen or broken; the fenestrated collar (tic.) is intact. Fig. 8: longitudinal section vertically oriented, >( 41,000. Fig. 9 : longitudinal section diagonally oriented, )< 41,000.on November 4, 2015
jcb.rupress.org
T A B L E I I I
Treated Muscle Fibers (Disrupted Transverse Tubules)
Fraction of sites
at which filled Fraction of tubules* tubules are found conneted to surface No. of
Depth of fiber Apparent No. of filled unfilled sites x' x'/(x' -t- e' "-}- n')
Muscle from surface diameter, ~ tubules (x') (e' + n') x' Jr" e" q- n' x/ (x + e q'- n)
A
B
% %
1 40 7 263 2.6 3.1
1 25 18 536 3.3 3.9
2 15 17 200 7.8 9.5
2 >25~ 6 222 2.6 3.2
2 38 0 128 0 0
2 20 5 253 1.9 2.3
3 >30~ 3 281 1.1 1.3
1 47 3 354 0.8 1.0
1 55 0 392 0 0
1 > 2 0 ~ 7 119 5.6 6.7
2 50 0 56 0 0
2 40 23 253 8.3 10.5
3 25 4 89 4.3 5.2
C 1 > 2 5 , 0 226 0 0
2 40 3 311 1.0 1.2
M e a n , % 2.6 3.2
S t a n d a r d E r r o r of M e a n , % 0.7 0.9
M e a n s were c o m p u t e d o n a n u n w e i g h t e d basis, i.e. each fiber was given equal weight. I f the d a t a from each fiber are weighted according to the n u m b e r of observations made, the means are not c h a n g e d significantly.
* x / ( x + e + n) is t a k e n as 81.1% from c o m b i n e d d a t a in T a b l e s I a n d II.
T h e a p p a r e n t d i a m e t e r is greater t h a n this value, since the edge of t h e fiber was obscured by a grid bar.
w h i c h evidently t h r o w s off two b r a n c h e s o n e i t h e r side of the Z line; these b r a n c h e s t h e n form flat- t e n e d s a r c o t u b u l a r cisternae. Peachey (23) a n d Peachey a n d Schild (24) h a v e r e p o r t e d o t h e r less complex b r a n c h i n g structures of the s a r c o t u b u l a r system. I f structures like these occur fairly often, they m i g h t explain, a t least in part, the finding of E n d o (5) a n d D. K. Hill (15) t h a t most of the I b a n d seems to be accessible to some extracellular markers. Fig. 3 shows peroxidase filling this sarco- t u b u l a r cisterna.
T h e fine s t r u c t u r e of in glycerol muscle fibers is not v e r y consistent; sometimes e n l a r g e d i n t e r m y o - fibril spaces are shown, sometimes not. W e c a n n o t decide w h e t h e r these swellings are caused by " b a d fixation" or w h e t h e r t h e y r e p r e s e n t a t r u e p h e - n o m e n o n . I n all fibers e x a m i n e d t h e t u b u l a r system was i n t a c t a n d the results of the peroxidase
e x p e r i m e n t s show t h a t the t u b u l a r l u m e n was c o n t i n u o u s w i t h the extracellular space. I f glycerol r e m a i n s outside the fiber in a 1 h r soak, the muscle w o u l d shrink a n d the s a r c o t u b u l e w o u l d be swollen as in o t h e r h y p e r t o n i c solutions (10, 20). O n t h e basis of w e i g h t m e a s u r e m e n t s , F u j i n o et al. (11) h a v e suggested t h a t glycerol does n o t p e n e t r a t e , a n d E i s e n b e r g a n d G a g e (4) h a v e i n t e r p r e t e d t h e i r i n t e r n a l resistivity m e a s u r e m e n t s o n this h y p o t h - esis. I n some fibers, however, the t u b u l e s are n o t swollen. I f glycerol crosses the s a r c o l e m m a quickly e n o u g h so t h a t it is essentially in e q u i l i b r i u m after 1 hr, the muscle fiber w o u l d n o t b e s h r u n k e n a n d t h u s swollen tubules w o u l d n o t b e expected.
T h e structure of muscle fibers w h i c h h a v e b e e n r e t u r n e d to R i n g e r solution from glycerol-Ringer's is strikingly different f r o m t h a t of n o r m a l muscle. T h e t u b u l a r system is destroyed; its m o r p h o l o g y is
BRENDA EISENRERG AND RORERT S. EISENRERG Selective Disruption of the Sarcotubular System 463
on November 4, 2015
jcb.rupress.org
Downloaded from
FIGURE tO Histogram to show the location of the few filled tubules remaining after glycerol treatment (see text for details).
,PLANES OF
~ " $SECTION
ANNULUS
OF ~
I I
INTACT -~ / ~ ~ : ; , - ~
T U B U L E S ~
FIGURE 11 A diagram showing location of longitudina section. Note that most longitudinal sections will not pass through the center of the fiber and thus will over- estimate the size of the annulus (stippled).
drastically altered and only a few tubules are left continuous w i t h the extracellular space. I t is important to note that the sarcoplasmic reticulum is not untouched by this treatment: mild d a m a g e occurs in the terminal eisternae and fenestrated collar, and severe d a m a g e occurs in the interme- diate cisternae. Thus, those properties of the muscle which require an intact sarcoplasmic retic- u l u m (e.g. caffeine contractures) would be ex- pected to be somewhat impaired in this prepara- tion.
I t is of particular importance to have an estimate of the area of the sarcotubular m e m b r a n e still left connected to the extracellular m e d i u m and thus possibly functional. I n the Results section, it is
concluded that about 2 % of the tubules are still connected after treatment (the best estimate after making a correction for the " a n n u l u s effect"). I n order to use this figure in interpreting the proper- ties of glycerol-treated muscle fibers, it is necessary to calculate what fraction of the outer m e m b r a n e area this 2 % represents. Peachey (23) estimates that for a 50-/z fiber (our average diameter) the area of the membranes of the transverse tubular system is 3.5 times that of the sarcolemma, and this n u m b e r has recently been modified to 4.5 in order to account for branching of the tubules (24). H o w - ever, we find that 16% of the triadic sites where tubules had been assumed to be present do not, in fact, contain tubules (our n-sites). Therefore, we use a somewhat different figure for the ratio of tubular to surface area in a 50# fiber, n a m e l y 4.5 X 0.84 = 3.8. Hence, the fraction of the tu- bular system remaining in glycerol-treated fibers (2%) represents 3.8 × 2 % - 7.6% of the outer m e m b r a n e area. T h e presence of this m u c h a r e a has some effect on the quantitative interpretation of the electrical properties of treated muscle fi- bers. T h e contribution of tubule area in glycerol- treated fibers is not sufficient to influence the qualitative findings of Gage and Eisenberg (12) concerning excitation-contraction coupling or their conclusion concerning slow electrical phenomena. It must be r e m e m b e r e d that the numerical estimates are based on r a n d o m sampling of surface fibers, whereas the physiological data are based on fibers selected for m e m b r a n e potentials above 70
4 6 4 T H E JOVRNAL OF CELL BIOLOGY • VOLUME 39, 1968
on November 4, 2015
jcb.rupress.org
[image:14.612.170.446.70.254.2] [image:14.612.127.290.301.423.2]FIOURE 1~ Normal muscle showing the complexity in the triadic region. Longitudinal section, fiber axis oriented vertically. The Z line runs horizontally in this figure. The transversely oriented sarcotubule (TI) branches in the longitudinal direction, then bends to form a sarcotubular eisterna (T2) and probably an- other cisterna (*). The terminal eisternae (t.c.) have a particularly speckled appearance, possibly a cal- cium pyroantimonate precipitate. Glycogen granules (gly) are prominent. )< 1~0,000.
mv. I f t h e r e is some correlation b e t w e e n m e m b r a n e p o t e n t i a l a n d t h e n u m b e r of t u b u l e s r e m a i n i n g , q u a n t i t a t i v e a p p l i c a t i o n of o u r d a t a to physio- logical results could b e in error. W e note t h a t t h e highest p e r c e n t of t u b u l e a r e a r e m a i n i n g is 10.5 % (correcting for the a n n u l u s effect gives 5.3 %), or a b o u t 1 5 % t u b u l a r a r e a referred to t h e sarco- l e m m a area. However, 2 7 % of t h e fibers h a d n o i n t a c t tubules c o n n e c t e d to t h e outside.
Finally, it is necessary to discuss the limitations of this p r e p a r a t i o n . First, in the absence of infor- m a t i o n a b o u t the m e c h a n i s m s of the glycerol t r e a t - m e n t it is unwise to a p p l y this t e c h n i q u e to o t h e r tissues or to modify t h e t e c h n i q u e w i t h o u t due c a u t i o n ; t h e a r e a m e a s u r e m e n t s described here a p p l y only to frog sartorius muscle t r e a t e d as we h a v e described. Secondly, only surface fibers h a v e b e e n studied here; deep fibers a p p e a r to h a v e m o r e tubules r e m a i n i n g . T h i s point, t a k e n in c o n j u n c - tion w i t h t h e finding of a r a t h e r h i g h scatter in resting potentials (4), suggests t h a t e x p e r i m e n t s
w h i c h measure t h e properties of a whole glycerol- t r e a t e d muscle c a n n o t easily be interpreted. Appendix: The Annulus effect
Since the few tubules remaining in the treated muscle fibers all lie very close to the outer m e m b r a n e and since most longitudinal sections do not pass t h r o u g h the center of the fiber, the raw data given in T a b l e I I I will tend to overestimate the n u m b e r of tubules left (Fig. 11). This appendix is devoted to a quantitative analysis of this effect.
Fig. 13 shows the geometry of the system of interest and represents a magnified section of part of the fiber drawn in Fig. 11. T h e length of the line AB (the radius of the fiber) is called a, and the length of the line AC (the inner radius of the annulus) is called a -- d. T h e thickness of then anulus iscalled d, the ap- parent size of the annular region as seen in a r a n d o m longitudinal section is x (i.e., line BC), and the ap- parent diameter is given by 2 z (i.e., twice the length BD). Finally, the displacement of the section from the center of the circle (i.e. line AD) is called w.
Considering triangles ABD and ACD respectively
BRENDA EISENRERG AND ROBERT S. EISENBERG Selective Disruption of the Sarcotubular System 465
on November 4, 2015
jcb.rupress.org
Downloaded from
A
- - - - w
I
~-~ O - ~
FmtTUE 18 T h e geometry of a q u a d r a n t of a mnsele fiber in cross section showing location of section. See text for definitions used in t h e analysis of t h e a n n u l u s effect.
it c a n be seen t h a t :
W2~ a 2 -- Z 2
w 2 + ( z - x ) ~ = ( a - - d ) 2
T h e s e e q u a t i o n s c a n be solved to give t h e t r u e fractional size of t h e a n n u l u s (i.e., d/a) in t e r m s of t h e a p p a r e n t fractional size (x/z) a n d t h e ratio of t h e a p - p a r e n t d i a m e t e r of t h e section to t h e t r u e d i a m e t e r
(z/a).
d 1 F1 + . . . .
a
m
\ a / \ z / , , z / , , a / aor a p p r o x i m a t e l y (if x / z is sufficiently small)
(1)
a Z (2)
S o m e i d e a of t h e t r u e v a l u e of d/a in o u r case c a n
R E F E R E N C E S
1. ADRIAN, R. H., a n d W. H. FREYOANG. 1962. T h e p o t a s s i u m a n d chloride c o n d u c t a n c e of frog m u s c l e m e m b r a n e . J. Physiol., (London).
163:61.
2. ANDERSSON-CEDERGREN, F,. 1959. U l t r a s t r n c t u r e of m o t o r e n d plate a n d sarcoplasmic c o m - p o n e n t s of m o u s e skeletal muscle fiber as re- vealed b y t h r e e - d i m e n s i o n a l reconstructions f r o m serial sections. J. Ultrastruct. Res. (Suppl. 1) 5.
be f o u n d b y s u b s t i t u t i n g average values in e q u a t i o n (2). If t h e a v e r a g e d i a m e t e r of t h e muscle fibers u s e d is a b o u t 50#, as transverse sections indicate, a n d t h e a v e r a g e a p p a r e n t d i a m e t e r is 36#, t h e n u m b e r (3.2%) g i v e n in T a b l e I I I as t h e fraction of t h e t r a n s - verse t u b u l a r s y s t e m r e m a i n i n g c o n n e c t e d to t h e outside is a n overestimate, t h e t r u e n u m b e r b e i n g
d/a = 0.032 X (36/50) 2 = 1.7%.
T h u s , t h e best e s t i m a t e for t h e a m o u n t of t u b u l a r a r e a r e m a i n i n g in treated fibers is a b o u t 1 . 7 % of n o r m a l .
O u r correction is only a coarse a p p r o x i m a t i o n since t h e figure for t h e true d i a m e t e r is likely to be in serious error because of t h e severe v a r i a t i o n in di- a m e t e r of t h e fibers f r o m m u s c l e to muscle, a n d also since o u r whole analysis requires m u s c l e fibers to b e circular in cross section, w h i c h r e q u i r e m e n t few fibers e v e n a p p r o x i m a t e l y fulfill.
T h i s r e s e a r c h was carried o u t in t h e l a b o r a t o r y of Dr. M . J . Moses, D e p a r t m e n t of A n a t o m y , w i t h facilities s u p p o r t e d b y g r a n t s to h i m f r o m U S P H S (GM-06753) a n d t h e A m e r i c a n C a n c e r Society (E-213). It is a pleasure to t h a n k b o t h Dr. M o s e s for m a k i n g this r e s e a r c h possible a n d Dr. P e t e r W . G a g e for t h e m a n y c o m m e n t s w h i c h helped to clarify t h e physiological implications of this work.
A p r e l i m i n a r y report of this w o r k a p p e a r e d in
Science 160:1243, 1968.
Note."
W e h a v e recently b e e n i n f o r m e d of a p a p e r i n t h e R u s s i a n literature b y S. A. K r o l e n k o , S. Y A . A d a m y a n , a n d N. E. Shvinka, 1967, entitled V a c u o - lization of skeletal muscle fibers. 1. V a e u o l i z a t i o n after effiux of low m o l e c u l a r non-electrolytes (sic). Tsitologiya 9:1346. T h i s p a p e r describes t h e m o r p h o l - ogy of glycerol-treated m u s c l e fibers o b s e r v e d w i t h t h e light microscope. A f u r t h e r paper, f r o m t h e s a m e l a b o r a t o r y , o n t h e fine s t r u c t u r e as seen in t h e electron m i c r o s c o p e is to a p p e a r , we are told, in t h e J u l y 1968 issue of Tsitologiya.
Received for publication 20 May 1968, and in revised form 17 July 1968.
3. EISENBERG, R. S. 1967. T h e e q u i v a l e n t circuit of single c r a b muscle fibers as d e t e r m i n e d b y i m -
p e d a n c e m e a s u r e m e n t s w i t h intracellular electrodes. 3". Gen. Physiol. 50:1785.
4. EISENBERO, R . S., a n d P. W . GAOE. 1967. F r o g
skeletal m u s c l e fibers: c h a n g e s in electrical properties after d i s r u p t i o n of transverse t u b u -
lar system. Science. 158:1700.
5. ENDO, M. 1966. E n t r y of fluorescent dyes into t h e
466 THE JOURNAL OF CELL BIOLOGY • VOLLrME ~9, 1"968
on November 4, 2015
jcb.rupress.org
sarcotubular system of the frog muscle. J .
Physiol., (London). 185:224.
6. FALK, G., and P. FATT. 1964. Linear electrical properties of striated muscle fibers observed with intracellular electrodes. Proe. Roy. Soc. (London), Set. B. 160:69.
7. FARQUHAR, M. G., and G. E. PALADE. 1965. Cell junctions in amphibian skin. J. Cell Biol.
26:263.
8. FATT, P. 1964. An analysis of the transverse electrical impedance of striated muscle. Proe. Roy. Soc. (London) Set. B. 159:606.
9. FP,~YGANO, W. H., J m , D. A. GOLDSTEIN, and D. C. HELLAM. 1964. The afterpotential that follows trains of impulses in frog muscle fibers.
J. Gen. Physiol. 47:929.
10.
FREYGANO,
W. H., D. A. GOLDSTEIN, D. C. HELLAM, and L. D. PEACHEY. 1964. The rela- tion between the late after-potential and the size of the transverse tubular system of frog muscle. J. Gen. Physiol. 48:235.11. FuJINO, M., T. YAMAGUCHI, and K. SUZUKI. 1961. "Glycerol effect" and the mechanism linking excitation of the plasma membrane with contraction. Nature. 192:1159.
12. GAOE, P. W., and R. S. EISENBERQ. 1967. Action potentials without contraction in frog skeletal muscle fibers with disrupted transverse tubules.
Science. 158:1702.
13. GRAHAM, R. C., and M. J . KARNOVSKY. 1966. The early stages of absorption of injected horseradish peroxidase in the proximal tubules of mouse kidney : ultrastructural cytochemistry by a new technique, o r. Histochem. Cytochem.
14:291.
14. HILL, A. V. 1948. O n the time required for dif- fusion and its relation to processes in muscle.
Proc. Roy. Soc. (London) Ser. B. 135:446. 15. HILL, D. K. 1964. The space accessible to al-
bumin within the striated muscle fiber of the toad. J. Physiol., (London). 175:275.
16. HODOKIN, A. L., and P. HoRowmz. 1960. T h e effect of sudden changes in ionic coneentra-
tions on the membrane potential of single muscle fibers, d. Physiol., (London). 153:370. 17. HOWELL, J . N., and D. J . JENDEN. 1967. T-
tubules of skeletal muscle: morphological alterations which interrupt excitation contrac- tion coupling. Federation Proe. 26:553.
18. HUXLEY, A. F., and R. E. TAYLOR. 1958. Local activation of striated muscle fibers. J. Physiol.
(London). 144:426.
19. HUXLEY, H. E. 1964. Evidence for continuity between the central elements of the triads and extracellular space in frog sartorius muscle.
Nature. 202:1067.
20. HUXLEY, H. E., S. PAGE, and D. R. WILKm. 1963. An electronmicroscopic study o f muscle in hypertonic solutions. J. Physiol., (London).
169:312. Appendix of Dydynska, M. and Wilkie, D. R.
21. KARNOVSKY, M. J . 1965. Vesicular transport of exogenous peroxidase across capillary endo- thelium into the T-system of muscle. J. Cell Biol. 27:49A.
22. PAGE, S. 1964. The organization of the sareo- plasmic reticulum in frog muscle, or. Physiol., (London). 175:10P.
23. PEACHRY, L. D. 1965. The sarcoplasmic reticu- lure and transverse tubules of the frog's sar- torius, or. Cell Biol. 25 (Pt. 2):209.
24. PEACHEY, L. D., and R. F. SCHILD. 1968. T h e distribution of the T-system along sarcomeres of frog and toad sartorius. 3". Physiol., (London).
194:249.
25. RERSE, T. S., and M. J. KARNOVSKY. 1967. Fine structural localization of a blood-brain barrier to exogenous peroxidase. J. Cell Biol. 34:207. 26. SOMMER, J . R., and E. A. JOHNSON. 1968.
Purkinje fibers of the heart examined with peroxidase. J. Cell Biol. 37:507.
27. VENABLE, J . H., and R. COCCESHALL. 1965. A simplified lead citrate stain for use in electron microscopy. J. Cell Biol. 2~ (Pt. 1)$ 407. 28. ZADUNAISKY, J . A. 1966. The location of sodium
in the transverse tubules of skeletal muscle.
or. Cell Biol. 31:C1 I.
BRENDA EISENBERG AND ROBERT S. EISENBERG Selective Disruption of the Sarcotubular System 467