Complex Spinal Paraspinal Fast Flow Lesions in CLOVES Syndrome: Analysis of Clinical and Imaging Findings in 6 Patients
Full text
Figure
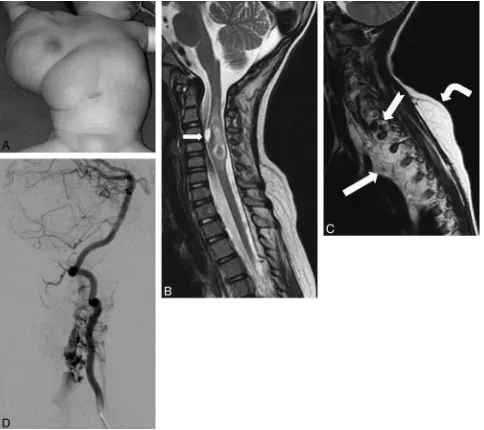
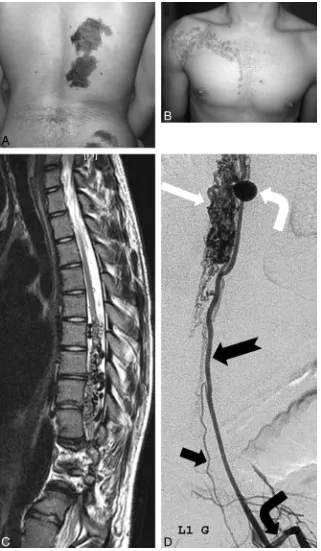
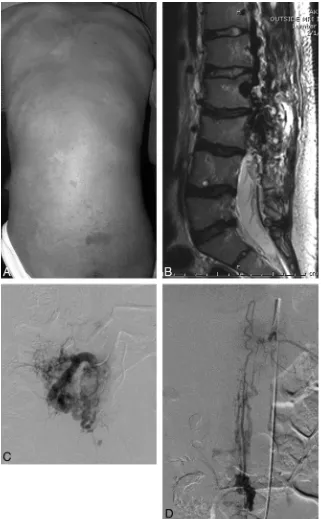
Related documents
IRJP 2011, 2 12, 238-242 F1 to F7 containing Xanthan gum and HPMC K4M showed good release but lower bioadhesive property, while formulation containing Carbopol 934P showed
Grand Valley State University librarians designed and conducted teaching faculty 1 focus groups to gauge their response to a new information literacy (IL) core student
The results showed that the pre-application of both the herbicides (Stomp 330 EC and Dual Gold 960 EC) and Parthenium extract greatly inhibited the germination of the
To assess this natural input, depth wise variation of magnetic susceptibility has been studied for a flood bank section (- 3 m sedimentation by lithogenic. processes) and a pit
The major contributions of proposed scheme are constructing effective sparse representation of DLWT-based local vectors for images, modeling the one-dimensional (1-D) and
We chose randomly the same number of singleton children born at 38 - 40 weeks of gestation (full-term) at our hospital during the same study period with matching
University of Shanxi, Taiyuan, Shanxi 030006, People's Republic of China, and b Institute of Molecular Science, Chemical Biology and Molecular Engineering, Laboratory of the
MG-63 cells were transfected with pcDNA3- GAS5-AS1 or a control vector, qRT-PCR was used to detect the mRNA expression levels of GAS5-AS1 (A) and MMP2 (B), and western blotting