CONFIDENTIAL
Master Thesis
Optimisation of Treatment Time in Ultrasound
guided High Intensity Focused Ultrasound (HIFU)
in the Treatment of Breast Fibroadenomata.
Mirjam Peek, BSc
ii
Optimisation of Treatment Time in Ultrasound guided High Intensity
Focused Ultrasound (HIFU) in the Treatment of Breast Fibroadenomata.
By Mirjam Peek, BSc S0200441
Master Thesis Technical Medicine Faculty of Science and Technology m.c.l.peek@student.utwente.nl mirjam.1.peek@kcl.ac.uk
Graduation date: December 5th 2014
Graduation Committee:
Chairman: Prof. Wiendelt Steenbergen w.steenbergen@tnw.utwente.nl Medical Supervisor: Dr. Mr. Michael Douek michael.douek@kcl.ac.uk Technical Supervisors
University of Twente: Dr. Ir. Bennie ten Haken b.tenHaken@utwente.nl
Guy’s hospital: Dr. Muneer Ahmed muneer.ahmed@kcl.ac.uk
External Supervisor: Dr. Srirang Manohar s.manohar@utwente.nl Process Supervisor: Drs. Paul van Katwijk p.a.vanKatwijk@utwente.nl
iii
Preface
In December 2013 I started my Technical Medicine graduation on the HIFU-F project at Guy's and St. Thomas' hospitals after a previous three month internship in London. This thesis reports the results of the work I performed in the last 12 months for the master in Technical Medicine.
Firstly, I would like to thank my medical supervisor, Michael Douek, for giving me the opportunity to do my graduation in London. You trusted in me and gave me the responsibility of co-running the HIFU-F trial. You challenged me to get the best out of me and this resulted in three articles, three oral and three poster presentations and a Young Investigators Award. I am looking forward in working with you in the next three years during my MPhil/PhD.
I would like to thank Muneer Ahmed for his supervision and support during the last 12 months. I was always able to go to you for advice and/or questions and you gave me insight in what is possible in the future. The trial started with a lot of struggles but we managed to overcome all obstacles and worked out a steady procedure.
Bennie ten Haken, thank you for the support and advice during my graduation. You made me look at situations from a different perspective which made it easier for me to solve the problem or handle it better. I would like to thank you for the advice you gave me on the future perspective of me as a Medical physician.
I would like to thank Paul van Katwijk and my supervision group (Richte Schuurmann, Gert-Jan Snel and Jordy van Zandwijk), for the useful monthly feedback meetings. Looking back has made me realise how far I have gotten and how much I have learned in the last year. I have learned to be proud of my accomplishments and not to take everything for granted. You gave me advice when I was doubting about my future in Technical Medicine and made me realise that there are more possibilities, it takes just one person to step up as a pioneer. As a result, and with the advice given by Bennie ten Haken, I managed to get in contact with the MST about arranging a Clinical Fellowship at their breast department.
I would like to thank Wiendelt Steenbergen and Srirang Manohar for accepting to be my chairman and external supervisor for this assignment. Even though we did not have much contact, I would like to thank you for the support during this period.
And last but not least, I would like to thank the patients for participating in the trial, without them this thesis would not have been possible and we would not have had these results.
Finally, I would like to thank my family, friends (both in London and in the Netherlands), colleagues and housemates for their support during the last year. You all created a safe environment in which I was able to work on this project fulltime and you made me realise that relaxing every now and then is important as well and you played a great part in this.
iv
Abstract
Introduction
Breast fibroadenomata (FAD) are the most common breast lesions in woman. High intensity focused ultrasound (HIFU) is a promising non-invasive ablative technique for the treatment of these FAD. In HIFU, an ultrasound (US) beam propagates through tissue as a high-frequency pressure wave elevating the temperature within a few seconds without causing damage to the direct adjacent tissues. Two systematic reviews were performed evaluating the current evidence of HIFU and minimally invasive ablative techniques in the treatment of breast cancer. In the "HIFU in the treatment of breast Fibroadenomata" (HIFU-F) trial, circumferential HIFU treatment was performed to isolate the FAD from its blood supply. Outcome measures were volume decrease on US, short-term complication rate, decrease in treatment time and patient recorded outcome measures.
Methods
Two systematic reviews were conducted following the Cochrane Handbook and STROBE statement. Patients (age ≥ 18 years) were recruited with symptomatic palpable FAD which had to be visible on US (graded either benign or indeterminate). Patients were treated using the US-guided - Echopulse device (Theraclion Ltd, Malakoff, France) under local anaesthesia. Two circumferential rings of pulses were applied by deselecting the centre of the FAD. Patients were followed-up at two weeks, three, six and 12 months.
Results
The systematic review demonstrated that very small studies have been conducted to HIFU and other ablative techniques. From December 2013, 25 patients with symptomatic palpable FAD underwent circumferential HIFU treatment. Nine patients opted for HIFU due to pain or discomfort. Average treatment time was 38.5 minutes (SD 12.0 minutes). Circumferential treatment significantly reduced treatment time by an average of 36.4% (SD 18.9%) (T-test, P=0.0001, two tailed). At two weeks short-term complications were erythema (n=6), ecchymosis (n=8), numbness of the skin (n=1), hypo-pigmentation (n=1), dimpling of the skin (n=1), a first-degree skin burn (n=1) and reduced pain in 5/8 patients with resolution in two patients. At three months all local complications had resolved apart from hyper-pigmentation (n=6). Reduction of pain was seen in 7/8 patients with resolution of pain in six. Two weeks post-treatment a volume reduction of 14.3% (SD 24.9%) was seen on US and at three months the reduction was 41.7% (SD 28.6%).
Conclusion
v
Table of Contents
Optimisation of Treatment Time in Ultrasound guided High Intensity Focused Ultrasound (HIFU)
in the Treatment of Breast Fibroadenomata. ... ii
Graduation Committee: ... ii
Preface ... iii
Abstract... iv
Table of Contents ... v
Table of Figures and Tables ... viii
List of Abbreviations ... ix
1. Introduction ... 1
1.1 Fibroadenomata ... 1
Diagnosis ... 1
Management ... 2
1.2 Breast Cancer ... 3
1.3 Ablative Techniques ... 4
1.4 Aim of Thesis ... 6
2. Systematic Review: High Intensity Focused Ultrasound (HIFU) Ablation in the Treatment of Breast Tumours ... 8
2.1 Material and Methods ... 8
Study selection ... 8
Inclusion criteria ... 8
Exclusion criteria ... 8
Data extraction ... 8
Risk of bias in individual studies ... 8
Statistical analysis ... 9
2.2 Results ... 9
Selected studies ... 9
Study characteristics ... 9
Quality assessment ... 13
Clinical outcomes ... 14
2.4 Discussion ... 18
3. Systematic Review: Minimal Invasive Ablative Techniques in the Treatment of Breast Tumours...22
3.1 Materials and Methods ... 22
vi
Inclusion criteria ... 22
Exclusion criteria ... 22
Data extraction ... 22
Risk of bias in individual studies ... 23
Statistical analysis ... 23
3.2 Results ... 23
Selected studies ... 23
Study characteristics ... 24
Quality assessment ... 28
Outcomes ... 29
3.4 Discussion ... 34
4. High Intensity Focused Ultrasound ...38
4.1 The History of HIFU ... 38
4.2 The HIFU Technique ... 38
US wave propagation ... 38
Non-linear wave propagation and cavitation ... 39
4.3 Treatment Devices ... 41
4.4 Treatment delivery ... 41
Focussing of the US beam ... 41
US transducers... 42
5. High Intensity Focused Ultrasound in the Treatment of Breast Fibroadenomata: the HIFU-F trial ...43
5.1 Materials and Methods ... 43
Patients ... 43
Inclusion and exclusion criteria ... 43
HIFU treatment ... 44
Treatment times ... 46
Follow-up ... 46
Case report forms ... 47
Statistical analysis ... 47
5.2 Results ... 47
Study characteristics ... 47
Recruitment ... 47
Study and patient characteristics ... 48
vii
Pain symptoms ... 50
Complications: ... 52
Volume measurements by US ... 53
5.4 Discussion ... 56
6. Conclusions ...61
7. Future Work ...62
8. List of publications and presentations ...65
Publications: ... 65
Abstracts: ... 65
Book chapters: ... 65
Presentations: ... 65
National: ... 65
International:... 65
Awards: ... 65
References ...66
Appendix I. Ablative Techniques in the Treatment of Breast Fibroadenomata ...72
Appendix II. High Intensity Focused Ultrasound for the Treatment of Fibroadenomata (HIFU-F) Study. ...79
viii
Table of Figures and Tables
Figure 1: Results of systematic search of the literature. ... 9
Figure 2: Random effect analysis demonstrating an estimated 30% (95% CI: 0.2-0.4) of patients with no residual tumour after HIFU treatment. ...16
Figure 3: Short-term complications after HIFU treatment. ...17
Figure 4: Cosmetic outcome of HIFU treatment. ...18
Figure 5: Results of systematic search of the literature. ...23
Figure 6: Short-term complications after ablation. ...33
Figure 7: Cosmesis after HIFU, cryo and RFA ablation. ...34
Figure 8: Microbubble oscillations (a) linear oscillation, (b) non-linear oscillation, (c) stable cavitation and (d) inertial cavitation. Figure from Stride et al. (2009) [66] ...40
Figure 9: Echopulse device (l) and VTU unit (r). Figure by Theraclion Ltd (Malakoff, France). ..44
Figure 10: Focal point depth range: (l) minimum and (r) maximum depths of the Echopulse. ....45
Figure 11: Treatment planning of a fibroadenoma with the Echopulse device. ...46
Figure 12: Patient recruitment per month at the multi disciplinary meetings. ...48
Figure 13: Treatment planning in (l) whole lesion ablation and (r) HIFU-F trial: two circumferential rings were treated by deselecting the centre of the lesion. ...49
Figure 14: Macroscopic image of excised fragments of a FAD treated with HIFU. ...50
Figure 15: Pain scale during and right after HIFU treatment. ...51
Figure 16: Short term complications (a + b) two images of ecchymosis at two weeks, (c) hyper-pigmentation at three months and (d) first-degree skin burn at two weeks post-treatment. ...52
Figure 17: US images of first patient (l) pre-treatment (2.1 x 0.9 x 2.0 cm) (m) two week follow-up (1.9 x 0.9 x 1.3 cm) and (r) three months follow-follow-up (1.6 x 0.7 x 1.2 cm). ...53
Figure 18: Residual FAD volume (in %) per patient followed over time. ...55
Table 1: Patient, tumour and treatment characteristics of the included studies. ...11
Table 2: Imaging findings and outcomes of included studies. ...12
Table 3: Histopathology outcomes of included studies. ...13
Table 4: Study quality assessment (a) of cohort studies according to the STROBE statement, (b) included RCT assessed according to the “Risk Bias Tool” in the Cochrane Handbook. ...14
Table 5: Study characteristics and outcomes for (a) RFA, (b) HIFU, (c) cryo-ablation, (d) laser ablation, (e) microwave ablation and (f) the single retrospective study. ...25
Table 6: Quality assessment (a) Study quality assessment of cohort studies and (b) methological characteristics of included RCT. ...28
Table 7: Mean treatment times of ablative techniques...29
Table 8: Post-treatment imaging results seen on MRI and US. ...30
Table 9: Post-treatment H&E and NADH staining findings after surgical excision or core needle biopsy. ...32
Table 10: Study and patient characteristics. ...48
Table 11: Post-treatment pain at two weeks, three and six months. ...52
ix
List of Abbreviations
AJCC American joint committee on cancer ASTRO American society of radiation oncology BCS Breast conserving surgery
BRCA Breast cancer gene CNB Core needle biopsy CRF Clinical report form DCIS Ductal carcinoma in situ
ER Oestrogen
FAD Fibroadenoma
FNAC Fine needle aspiration cytology
FUS Focused ultrasound
HEM Hyper-echoic mark
HER2 Human epidermal growth factor type 2 HIFU High intensity focused ultrasound
HIFU-F High intensity focused ultrasound in the treatment of breast fibroadenomata H&E Haematoxylin and eosin
ISI Increase in signal intensity
MDCT Multi detector computed tomography MDM Multi disciplinary meeting
MDF Maximum difference function MRI Magnetic resonance imaging NADH Nicotinamide adenine dinucleotide PEI Positive enhancement integral
PET-CT Positron emission tomography – computed tomography PIS Patient information sheet
PR Progesterone
RCT Randomized control trial RFA Radiofrequency ablation
SD Standard deviation
SPECT Single positron emission computer tomography
STROBE Strengthening the reporting of observational studies in epidemiology TNM Tumour, node and metastasis classification
US Ultrasound
1
1. Introduction
1.1 Fibroadenomata
Breast fibroadenomata (FAD) are the most common breast lesions in woman and can develop at any age but most often during the second and third decades of their life. They are also not uncommon in post-menopausal women and arise more often after hormone replacement therapy. Breast FAD occur in about 10% of all woman and account for about 50% of performed breast biopsies. Studies revealed that up to 59% of FAD will regress or completely resolve within five years. [1] The average lifetime of a FAD is about 15 years. FAD have been shown to be more common in patients of higher socio-economic classes and in population with darker skin. Age of menarche, menopause and hormonal therapy, including oral contraceptives were shown not to influence the risk of developing these lesions. [2] A negative correlation was found between the risk of developing FAD and the body mass index and number of full-term pregnancies. Consumption of large quantities of vitamin C and cigarette smoking were found to reduce the risk of developing FAD. [2] Transformation of a FAD into a malignant lesion is considered exceptionally rare (0.002 - 0.0125%). [2-4] There is a 1.3 - 2.1 increased risk of developing breast cancer in women with FAD compared with to general population. [2]
FAD are benign lesions that are encapsulated from their surrounding tissues. They can be considered as an aberration of normal development rather than a true neoplasm. On histology, FAD consist of combined proliferation of epithelial and fibroblastic tissue elements which are oestrogen (ER) dependent and slowly growing. [4, 5] FAD are considered to be a benign mixed tumour but recent studies have revealed that only the fibroblastic element is neoplastic, while the epithelial is reactive. Epithelial proliferation appears in a single terminal ductal unit and describes duct-like areas surrounded by fibroblastic stroma. Depending on the ratio between these two elements there are two main histological types: intracanalicular and pericanalicular. Both types are often found within the same FAD. In intracanalicular FAD stromal proliferation pedominates and compresses the ducts and in pericanalucular FAD the fibrous stroma proliferates around the ductal spaces. [5] FAD develop from a lobular origin which explains the high incidence in woman between their twenties and thirties, at their maximum lobular development of the ductal system of the breast. [2] It also explains why the rare cases of cancer developed from FAD are of the lobular type (lobular carcinoma in situ). [2, 4]
Patients with FAD usually present themselves in the clinic with a palpable lesion detected during self- or medical examination. FAD are normally solitary, non-tender, smooth, mobile masses of about 1 - 3 cm. [2, 4] The majority are located in the upper outer quadrant of the breast. [2] In 20% of cases, the FAD are multiple or 4 cm or larger (5% of all FAD are 5 cm or larger). In the case of multiple FAD, there is often a strong family history of these lesions. [2] FAD stay at the same size or increase until about 2 - 3 cm, in 15% the FAD regresses spontaneously and in 5 - 10% the lesion progresses. [2, 4]
Diagnosis
2
diagnostic methods are needed to get the correct diagnosis. The second step is imaging. Ultrasound (US) is the main diagnostic imaging method used for the diagnosis of FAD. FAD are visible as oval smooth solid masses with even low-level internal echoes. [2] However, not all FAD have the same characteristics and not all FAD are visible on US images. In 25% of FAD, features like an irregular border are suggestive that the lesion might be malignant. [2, 4] In mammography, FAD are often visible as homogenous well-circumscribed lesions in which calcifications are often observed. [2] This technique is not always used in the diagnosis of breast FAD but can be useful in older woman especially in patients with non-palpable lesions. [4]A fine needle aspiration cytology (FNAC) or core needle biopsy (CNB) is the third step and can be used to get final confirmation of the diagnosis. In FNAC, a thin needle is used to obtain cells of the lesion. This technique is used both in young patients and in patients with needle phobia. The drawback of this technique is that there is a high rate of insufficient obtained tissue material to be examined. CNBs are more reliable but multiple re-insertions are needed and in the case of dense breasts, insufficient breast tissue could be obtained. A third option is vacuum assisted biopsy (VAB), in this technique no re-insertion is needed and a larger amount of breast tissue is obtained. [4] On cytology, FAD are recognised as clusters of spindle cells without inflammatory fat cells; aggregates of cells with a papillary configuration resembling antler horn clusters and/or uniform cells with well-defined cytoplasm lying in rows and columns, these can be found in 96%, 93% and 95% of all FAD, respectively. In one study, it was found that only 82% of CNB proven FAD could be visualised with US. [2] In general, FNAC, CNB and VAB could be performed in all patients, however in woman 25 years no biopsy is performed when US reveals a solid lesion which has benign characteristics due to the low incidence of breast cancer in woman 25 years. [6, 7]
The overall diagnostic efficacy of this triple assessment is approximately 70-80% but an accurate differentiation between a benign and a malignant lesion is provided in 95%. [2, 4]
Management
The management of non-palpable lesions is reassurance with or without a follow-up period of one to three years after diagnosis by CNB or FNAC. For palpable lesions, there are currently three main treatment options available: reassurance (with or without follow-up), vacuum assisted mammotomy (VAM), which officially is not licensed for the treatment of FAD only to obtain the diagnosis of a lesion, or surgical excision. In the case of reassurance, it is advised for patients up to 35 years to use a follow-up protocol in which the patient comes back every six months to determine if the lesion has changed in size. [2]
3
VAM is performed in an outpatient setting under US guidance with subcutaneous local anaesthetic and is less invasive with a better cosmetic outcome compared to surgical excision. Complete resection of the FAD is reported in 75 - 100% of all cases. Disadvantages of VAM are the reduced visibility due to blood, air, local anaesthesia and/or soft tissue oedema. Lesions close to the skin (<0.5 cm) and of a size larger than 3 cm are not suitable for treatment. In some lesions close to the pectoralis major and/or skin local anaesthesia can be injected between the lesion and the skin to increase the distance. Possible complications after VAM are hematoma, skin defect and/or a pneumothorax. [8]In surgical excision the lesion is removed under general anaesthesia. This can be the best option in the case of large or multiple FAD or lesions that have the appearance of a phyllodes tumour (a relatively fast growing potentially malignant tumour). [2] The main advantage is that the whole FAD is completely removed. Possible disadvantages are the scarring, damage to the ducts, cosmetic outcome and chances of anaesthetic and/or operative complications. [2, 4]
A new technique in the treatment of FAD is high intensity focused ultrasound (HIFU) ablation. This a non-invasive ablative technique in which the FAD is treated with focused consecutive repeated US pulses, while surrounding tissues are not damaged.
1.2 Breast Cancer
Breast cancer is the most common cancer in women in the United Kingdom. In 2010, just under 50,000 women were diagnosed with invasive breast cancer and with 11,684 deaths in 2011, it is the second most common cause of death from cancer in women. [9] In men, breast cancer diagnosis and death due to breast cancer accounts for about 1%. [10]
With the wider use of mammographic screening, breast cancers are diagnosed at an increasingly earlier stage. [10-12] Increasing age is the most important risk factor for breast cancer, other risk factors are family health history, major inheritance susceptibility, alcohol intake, breast tissue density, ER level, hormone therapy history, obesity, lack of physical exercise, personal history of breast cancer, personal history of proliferative forms of benign breast disease, race and radiation exposure to the breast. [10, 13] Of all female breast cancers, about 5-10 % may have germ line mutation of the breast cancer 1 and 2 genes (BRCA). Patients carrying the BRCA 1 or 2 gene, also have an increased risk of developing ovarian or other primary cancers. [10, 14] Protective factors for breast cancer are oestrogens use, exercising, early pregnancy, breast feeding, risk reducing mastectomy and oophorectomy or ovarian ablation. [10, 13] When a patient is suspected to have breast cancer the plan is as followed: the diagnosis is confirmed, the stage of the disease is evaluated and the therapy is selected. For the diagnosis the following modalities can be used or a combination of them: mammography, US, magnetic resonance imaging (MRI) and/or breast biopsy. [10]
4
Breast cancer can present itself in a invasive or non-invasive or intraductal form. Infiltrating or invasive ductal cancer is the most common breast cancer histology type and accounts for 70-80% of all cases. Ductal carcinoma in situ (DCIS) is a non-invasive condition. DCIS can progress to become invasive cancer, but the likelihood of this happening is very extensive. Staging is determined according to the tumour size, lymph node status, ER and PR expression levels, HER 2 status, menopausal status and the general health of the patient. The American Joint Committee on Cancer (AJCC) has stratified breast cancer according to the tumour, regional nodal status and distant metastasis (TNM) classification. [10]Surgery in the form of either breast conservation (BCS) or mastectomy followed by adjuvant therapy constitutes the main stay of treatment for early stage breast cancer. [11, 12] In BCS the cancer is removed without removing other breast tissue, this could be done with a lumpectomy, in which the lesion is removed along with a small margin of normal tissue or with a partial mastectomy in which the breast segment with the cancer is removed. Patients who undergo BCS might also have their sentinel lymph node (first lymph node to receive lymphatic drainage from a tumour) removed under the arm to determine if cancer cells have spread to the lymphatic system. [10] In mastectomy, all breast tissue is removed, patients could opt for breast reconstruction with either their own tissue (autologous reconstruction) or an implant (implant based reconstruction). Adjuvant therapy consists of one or a combination of: radiotherapy, in which radiation is used to kill cancer cells and keep them from growing; chemotherapy, in which drugs are taken orally or injected to stop the growth of cancer cells; and/or hormonal therapy, in which hormones are removed or blocked, which prevents the cancer cells from growing. [10]
BCS has proved to be effective and well accepted by patients. However, BCS can be associated with alterations in the size and symmetry of the treated breast and this could lead to a reduced patient quality of life. [15] This would make non-surgical techniques without the removal of breast tissue more attractive. Breast conservation is dependent upon clear margins - defined as no ink visible on resected tumour according to recent American Society for Radiation Oncology (ASTRO) guidelines. [16] However, during surgery the surgeon is unable to visualize the tumour to aid in determining clear margins. This lack of intra-operative target definition results in higher re-operation rates aimed in an attempt to excise residual tumour. There is a clinical need to develop minimally invasive ablative techniques to further reduce re-operation rates by defining the target and the tumour margins intra-operatively. These techniques potentially benefit from the absence of general anaesthesia, a reduced recovery time, because the treatment is under local anaesthesia, absence of scarring and consequently economic benefits. [17] Non-surgical techniques including HIFU; radiofrequency ablation (RFA); cryo-; laser and microwave ablation are under investigation for local treatment of breast tumours. [18]
1.3 Ablative Techniques
5
and coagulative necrosis. [20, 21] The available HIFU devices are generally integrated with either MRI or US in order to plan treatment and monitor response in real-time. [19, 20] HIFU is capable of providing a completely non-invasive therapy, without causing damage to the direct adjacent tissues, avoiding discomfort and potential complications associated with general anaesthesia and surgery. [20, 21] Disadvantages are the long treatment times and the possible complications (e.g. local pain, redness of the skin, skin burn) . [22]In RFA, a needle electrode is inserted percutaneously under US guidance to deliver an alternating current that generates ionic agitation, localized tissue heating and cell death. [15, 23] The primary source of heat is the tissue surrounding the electrode and not the electrode itself. It is presumed that heating drives extracellular and intracellular water out of the tissue, resulting in coagulative necrosis. [24] Average treatment time is 10 - 30 minutes and moderate complications like discomfort and a skin burn could occur. The procedure can be performed under intravenous sedation, general or local anaesthesia. [22]
Cryo-ablation uses freezing instead of heating. It is accomplished by inserting a cryo-probe under US guidance into the targeted breast tissue. The freezing process involves two phases: freezing and thawing. Four mechanisms destroy the tumour cells: direct damage by (1) intracellular ice formation and (2) osmotic dehydration, indirect damage due to (3) ischemia and (4) immunologic response. The treatment has good precision and control because the formation of an ice ball can be clearly visualized with US. [25, 26] The treatment takes about 15 - 30 minutes with minimal discomfort under local anaesthesia. [22]
In laser ablation, tumours are destroyed using direct heating with low-power laser light energy delivered via thin optical fibers inserted percutaneously under US or MRI guidance. [27] Upon absorption in the tissue, heat is produced, inducing lethal thermal injury. [18] The size and shape of thermal lesions are difficult to predict, however owing to biologic variability, fiber tip charring and changing optical and thermal properties of the tissue during interstitial laser photo-coagulation. [27] Treatment times are approximately 25 - 30 minutes and moderate complications like discomfort and skin burns could occur. The treatment can be performed under intravenous sedation, general and local anaesthesia. [22]
6
1.4 Aim of Thesis
In general, HIFU is capable of providing a completely non-invasive therapy, avoiding potential complications associated with general anaesthesia and surgery. [30]
HIFU has been evaluated for treatment of FAD in only a single clinical trial. Hynynen et al. [31] described a study in which 11 FAD with a volume of 1.9 cm3 (0.7 - 6.5 cm3), were ablated in
nine patients (median 29 years, range 19 - 38 years) to establish the feasibility, safety and effectiveness of HIFU ablation. A complete response was defined as a volume reduction ≥90%, partial response was defined as a volume reduction between 50 - 90% and a volume reduction >50% was defined as a minor response. T1-weighted images showed a partially or nearly completed ablation in 73% (8/11 FAD). Six months post-treatment, T2-weighted images showed a median volume decreased from 1.9 to 1.3 cm3. Pain was marked as slight in four patients,
moderate in two and severe in one patient, and tenderness was common up to ten days and oedema was visible up to two days post-treatment.
Currently there are four trials in which US guided HIFU is used for the treatment of breast FAD. Kovatcheva et al. [32] recruited 27 patients between March 2011 and January 2014 to demonstrate the efficacy of HIFU in the treatment of FAD. Boulanger et al. [33] designed a multicentre trial for the observation of histological changes in FAD following HIFU and recruited 24 patients between October 2011 and February 2014. Hahn et al. [34] recruited 27 patients between December 2013 and January 2016 to evaluate the efficacy of HIFU in the treatment of FAD and Benin et al. [35] designed a feasibility study to determine the safety and efficacy of the HIFU device by recruiting 20 patients between April 2014 and May 2016. None of these trials have published their results thus far. (A review evaluating ablative techniques in the treatment of breast FAD is presented in appendix I.)
For breast cancer HIFU has the potential to improve cosmetic outcomes (by preventing scarring and excessive ductal damage) and allowing earlier administration of systemic therapies due to shorter postoperative recovery times. [30, 36] The treatment also benefits from being able to alter to the shape of the lesion. Two systematic reviews are performed evaluating the current evidence for the clinical outcomes (residual tumour, imaging treatment response and cosmesis) of HIFU and minimally invasive ablative techniques in the treatment of breast tumours. These systematic reviews are presented in chapters two and three. The HIFU technique is described in detail in chapter four.
8
2. Systematic Review: High Intensity Focused Ultrasound (HIFU)
Ablation in the Treatment of Breast Tumours
2.1 Material and Methods
Study selection
A systematic review of the literature was performed using Medline/PubMed library databases to identify all studies published up to December 2013 that evaluated the role of HIFU for the treatment of breast tumours. The MeSH search terms used were High Intensity Focused Ultrasound, HIFU, focused ultrasound ablation and FUS, all in combination with breast. Except for reports in the English language and human subjects, there were no further restrictions. The related articles function was used to broaden the search, and all abstracts, studies and citations obtained were reviewed. References of the articles acquired were also searched by hand. The last search was conducted on December 20th, 2013.
Inclusion criteria
Studies were considered eligible for the systematic review if they addressed the following; (1) studies performed on human subjects with breast tumours; and (2) studies objectively recorded at least one clinical outcome measure of response (cosmetic, imaging and/or histopathology) to HIFU treatment.
Exclusion criteria
Studies that failed to fulfil the inclusion criteria or studies in which the outcomes of interest were not reported or if it was not possible to analyse these from the published reports, were excluded. Conference abstracts, letters, editorials and case reports were also excluded.
Data extraction
Each study was initially evaluated for either inclusion or exclusion. The data extracted from the included studies were: first author, year of publication, study design, number of included patients, mean patient age, tumour type, tumour size, type of guided imaging, frequency, dose, treatment margin used, total treatment time, resection (yes/no), follow-up, cosmetic outcome, imaging outcome, histopathology staining, histopathological outcome, complications, re-treatment of tumours and recurrence. One reviewer, (M.P) extracted data for all selected studies and a second reviewer (M.A) verified the accuracy of the extracted data. In case of a disagreement the senior author (M.D) made the final decision.
Risk of bias in individual studies
9
Statistical analysis
All extracted data was tabulated and presented as means and percentages. Numerators and denominators were provided to address outcomes of included studies. For continuous variables the mean (standard deviation (SD)), median and range are extracted and reported.
The mean proportion of patients with no residual tumour left after HIFU treatment was evaluated by calculating the pooled inverse variance-weighted proportion. Studies with a standard deviation of zero (i.e. in studies with 100% complete ablation) were excluded from the analysis. A random-effects analysis was performed following the suspected high heterogeneity of studies included. All statistical analyses were performed with STATA 12.0 (StataCorp 2011, College station, TX).
2.2 Results
Selected studies
[image:18.612.185.427.381.553.2]A total of 140 articles published up to December 2013 were identified from the literature search (figure 1). After reviewing the abstracts, 101 articles were excluded (because they were not relevant) and 39 articles underwent full text examination. A total of nine articles matched the selection criteria of which six [12, 17, 40-43] were feasibility studies, one was a prospective cohort study [44] and one was a retrospective cohort study. [21] A single RCT [19] was identified in which HIFU followed by mastectomy was compared to mastectomy alone.
Figure 1: Results of systematic search of the literature.
Study characteristics
11
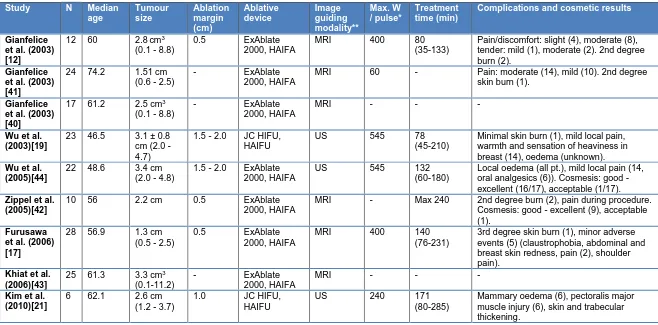
Table 1: Patient, tumour and treatment characteristics of the included studies.Study N Median
age Tumour size Ablation margin (cm) Ablative device Image guiding modality** Max. W / pulse* Treatment time (min)
Complications and cosmetic results
Gianfelice et al. (2003) [12]
12 60 2.8cm3
(0.1 - 8.8)
0.5 ExAblate
2000, HAIFA
MRI 400 80
(35-133)
Pain/discomfort: slight (4), moderate (8), tender: mild (1), moderate (2). 2nd degree burn (2).
Gianfelice et al. (2003) [41]
24 74.2 1.51 cm (0.6 - 2.5)
- ExAblate
2000, HAIFA
MRI 60 - Pain: moderate (14), mild (10). 2nd degree skin burn (1).
Gianfelice et al. (2003) [40]
17 61.2 2.5 cm3
(0.1 - 8.8)
- ExAblate
2000, HAIFA
MRI - - -
Wu et al. (2003)[19]
23 46.5 3.1 ± 0.8 cm (2.0 - 4.7)
1.5 - 2.0 JC HIFU, HAIFU
US 545 78
(45-210)
Minimal skin burn (1), mild local pain, warmth and sensation of heaviness in breast (14), oedema (unknown).
Wu et al. (2005)[44]
22 48.6 3.4 cm (2.0 - 4.8)
1.5 - 2.0 ExAblate 2000, HAIFA
US 545 132
(60-180)
Local oedema (all pt.), mild local pain (14, oral analgesics (6)). Cosmesis: good - excellent (16/17), acceptable (1/17).
Zippel et al. (2005)[42]
10 56 2.2 cm 0.5 ExAblate
2000, HAIFA
MRI - Max 240 2nd degree burn (2), pain during procedure. Cosmesis: good - excellent (9), acceptable (1).
Furusawa et al. (2006) [17]
28 56.9 1.3 cm (0.5 - 2.5)
0.5 ExAblate
2000, HAIFA
MRI 400 140
(76-231)
3rd degree skin burn (1), minor adverse events (5) (claustrophobia, abdominal and breast skin redness, pain (2), shoulder pain).
Khiat et al. (2006)[43]
25 61.3 3.3 cm3
(0.1-11.2)
- ExAblate
2000, HAIFA
MRI - - -
Kim et al. (2010)[21]
6 62.1 2.6 cm (1.2 - 3.7)
1.0 JC HIFU,
HAIFU
US 240 171
(80-285)
Mammary oedema (6), pectoralis major muscle injury (6), skin and trabecular thickening.
* pre-operatively defined, W = power
12
Table 2: Imaging findings and outcomes of included studies.Study Imaging
modality
Pre-treatment Post-treatment Correlation with
histopathology
< 2 wk 2-12 wk 3-6 M > 6 M
Gianfelice et al. (2003) [12] MRI 1.5T Signa - - - - Gianfelice et al. (2003) [41] MRI 1.5T Genesis Hypo-intense tumour.
- 1 M: little or no
change (92%). - - - Gianfelice et al. (2003) [40] MRI 1.5T Signa
- - - Correlation ISI,
MDF, PEI and % residual tumour.
Wu et al. (2003)[19]
MRI 1.0T Impact
Tumour enhancement.
7-10 D: No enhancement tumour and 1.5-2.0 cm margin.
- - - -
Wu et al. (2005)[44]
MRI 1.0T Impact
- - 3 M: 8.2% ± 6.1
reduction. 6 M: 26.7% ±12.2 reduction. Reduction
12M: 45.2%±22.1 (21pt), 24M: 72.3%±32.1 (17pt), 36M: 80.3%±38.2 (17pt), 48M: 87.3%±42.3 (16pt), 60M: 90.4%±49.1 (5pt).
- Zippel et al. (2005) [42] MRI (unknown) - - - -
Khiat et al. (2006)[43]
MRI 1.5T Signa
In all patients strong enhancement.
- Correlation ISI, PEI
and % residual tumour. Furusawa et al. (2006) [17] MRI 1.5T (unknown) - - - -
Kim et al. (2010)[21] MRI 1.5T Signa Internal enhancements: inhomogeneous (3), internal septal (1), rim (1) and homogeneous (1).
- 2 wk: Iso-intense
signal, no change in tumour size or SI. Thin rim (50%), nodular (33%) and both (17%). Heterogeneous signals on T2w.
4-6M: signal change on T2w.
11-24M: 46% decrease in tumour size (3pt). 11-30M: no change in thin rim enhancement of index tumour.
-
13
Table 3: Histopathology outcomes of included studies.Study Type of
specimen collected Time of specimen collection Type of staining
Complete histopathological response (%)
< 1 M 1-3 M 3-6 M >6 M
Gianfelice et al. (2003)[12]
Resection Unknown H&E - - - -
Gianfelice et al. (2003) [41]
Biopsy 6 M Unknown - - Complete
necrosis (58%) 2nd treatment: total CR (79%) Gianfelice et al. (2003)[40]
Resection 3-21 D H&E CR (24%), RD <10% (53%) and RD 30-75% (24%)
- - -
Wu et al. (2003)[19]
Resection 1-2 wk H&E CR (100%) tumour and margin of 1.80±0.58 cm
- - -
Wu et al. (2005)[44]
Biopsy 2 wk, 3/6/12 M
H&E CR (100%) tumour and adjacent margin Partial fibrosis (100%, 18 pt) Complete fibrosis (100%, 14 pt.) Complete fibrosis (100%, 14 pt.) Zippel et al. (2005) [42]
Resection 7-10 D Unknown CR (20%), microscopic foci (20%), 10% RD (30%) and 10-30% RD (30%)
- - -
Khiat et al. (2006) [43]
Resection 3-21 D Unknown CR (31%), RD <10% (42%), RD 20-90% (27%)
- - -
Furusawa et al. (2006)[17]
Resection 5-23 D H&E CR (54%), <10% RD (36%) 10-15% RD (10%)
- - -
Kim et al. (2010)[21]
Resection + biopsy
3-20 M Unknown - Viable
tumour (50%) No viable tumour (67%) No viable tumour (67%, 4 pt) *CR = complete response, RD = residual disease, D = days, wk = weeks, M = months and H&E =
haematoxylin and eosin.
Quality assessment
14
Table 4: Study quality assessment (a) of cohort studies according to the STROBE statement, (b)included RCT assessed according to the “Risk Bias Tool” in the Cochrane Handbook. a)
Study Study
objectives Inclusion criteria clear Standardized technique Standardized histopathology Standardized imaging Patient follow-up Withdrawals from study Gianfelice et al. (2003)[12]
Y Y N Y Y N* N
Gianfelice et al. (2003)[41]
Y Y N U Y N* N
Gianfelice et al. (2003)[40]
Y Y Y Y Y N* N
Wu et al.
(2005)[44] Y Y Y Y Y Y Y
Zippel et al. (2005) [42]
Y Y Y U Y N* N
Furusawa et al. (2006)[17]
Y Y Y Y Y N* Y
Khiat et al.
(2006) [43] Y Y Y Y Y N* N
Kim et al.
(2010)[21] Y Y Y N Y Y Y
*Follow-up: Studies in which the lesion was resected, follow-up was performed until surgery. b)
Study Adequate
sequence generation Power analysis Concealed allocation
Blinding Incomplete
data addresses Free of other bias Free selective reporting
Wu et al.
(2003)[19] Y N U N N Y Y
Clinical outcomes
Assessment of response to HIFU using imaging:
All studies performed pre- and post-treatment imaging. In two studies [19, 44] (26.9%, 45/167 patients) US colour Doppler was performed prior to the treatment and in one study post-treatment to determine perfusion of the tumour. In one study [44] a SPECT scan was made in 3.6% of all patients (6/167 patients) pre- and post-treatment. Of all patients, 77.8% (130/167 patients) underwent a pre- and post-treatment MRI. [12, 17, 19, 21, 40-44].
15
Another study [12] used T1 weighted spin-echo sequences with fat suppression, captured within a dynamic contrast-enhanced protocol.Of the 77.8% (130/167) who underwent post-treatment MRI, in one study (12 patients) [12], the results were not reported. In four studies (80 patients), [17, 40, 42, 43] general descriptive findings were reported without quantitative findings. Contrast-enhancement was seen on pre-treatment scans and no enhancement was seen post-pre-treatment. In four studies [19, 21, 41, 44], 31 patients (81.6%, 50-100%) showed an absence of enhancement at the index tumour and a thin rim of enhancement at the periphery. In seven patients (18.4%, 0-50%) nodular enhancement was seen at the periphery of the tumour, consistent with residual disease.
Two studies [21, 44] recorded reduction in tumour size after HIFU treatment on MRI. After six months, a reduction of 26.7 ± 6.1% was reported and after 11-24 months, a reduction of 46% and 57% (12 months 45.2 ± 22.1% and 24 months 72.3 ± 32.1%) was observed. MRI performed within the first two weeks after treatment showed transient oedema surrounding the target volume [19]. Any change in tumour size as a result of oedema, was not documented.
MRI was performed immediately after HIFU treatment in three studies [17, 42, 43], within the first two weeks in eight studies [12, 17, 19, 21, 40-43] and at an unknown subsequent time in one study [44]. MRI immediately after HIFU treatment showed decreased enhancement, not sufficient enough to determine response to treatment.
Two studies [41, 43] showed a good correlation between the increase in signal intensity (ISI, r = 0.90 and r = 0.75, respectively), maximum difference function (MDF, r = 0.80), positive enhancement integral (PEI, r = 0.86 and r = 0.78, respectively) and the percentage of residual tumour. In one of these studies [43] a stronger correlation was seen (r = 0.93 (ISI) and r = 0.96 (PEI)) when only MRIs seven days or longer post-treatment were included.
In one study [12] with 24 patients, 95% (18/19 patients) of patients who were considered to have successful treatment based on biopsy results demonstrated a lack of enhancement on MRI. 60% (3/5 patients) of patients who were considered to have had a failed treatment because residual tumour was found on biopsy, demonstrated persistent enhancement on MRI after two HIFU sessions.
Histopathological correlations:
16
Complete ablation or no residual tumour was found in 46.2% (55/119, range 17-100%) of all patients, who underwent surgical excision after HIFU treatment. The weighted summary proportion analysis showed an estimated proportion of 30% (95% CI: 0.2 - 0.4) of patients having no residual tumour after HIFU treatment. The weighted proportions are illustrated in a forest plot (figure 2). The I2-statistic of 47.2% confirms the suspected heterogeneity among studies. One study was excluded from the analysis due to 100% ablation.Figure 2: Random effect analysis demonstrating an estimated 30% (95% CI: 0.2-0.4) of patients with no residual tumour after HIFU treatment.
One study recorded complete necrosis of the tumours in all patients [19]. In five out of seven studies [17, 19, 40, 42, 43], patients underwent surgical resection 1 - 3 weeks post-treatment and in one study [21] the patients underwent surgical resection 3 - 11 months post-treatment. The last study [12] did not discuss the time of surgical removal of the breast specimens. Four studies [12, 17, 19, 40] used haematoxylin and eosin (H&E) for histopathological staining, the other three studies [21, 42, 43] did not report the type of staining.
17
In the three studies [21, 41, 44] using CNBs to determine the amount of residual tumour, 90.0% (43/48 patients, range 79 - 100%) of patients showed no residual tumour. Residual tumour was found in the other five patients, however, no quantitative statements were made. In the first study [44], the CNBs were performed after two weeks, in the second after 6 - 7 months [41] and in the last study [21] after 1 - 20 months. In one study [44], H&E was used for histopathological staining, the other two studies [21, 41, 44] did not report the type of staining used.One study [44] reported that after three months partial fibrosis, was seen in the CNBs of all patients (18 patients) and after six and 12 months complete fibrosis was visible in all patients (14 patients).
In two studies [12, 17], the percentage of tumour located in the treatment area was determined. The whole tumour was located in the treatment area in 82.5% (33/40 patients, range 58 - 93%) of patients, in 10.0% (4/40, range 7 - 17%) between 90 - 100% of the tumour was located in the treatment area and in 7.5% (3/40, range 0 - 25%) less than 70% of the tumour was located in the treatment area.
One study [19] measured the ablated margin around the tumour, which was 1.8 ± 0.6 cm.
Post-treatment complications:
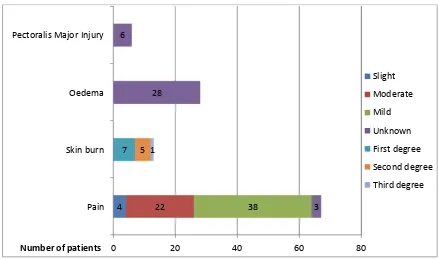
[image:26.612.86.524.365.624.2]Complications were described in seven studies (table 1, figure 3). [12, 17, 19, 21, 41, 42, 44]
Figure 3: Short-term complications after HIFU treatment.
Pain was reported in 40.1% (67/167) of patients and was slight in 6.0% (4/67 patients), moderate in 32.8% (22/67 patients), mild in 56.7% (38/67 patients) and unknown in 4.5% (3/67 patients). Skin burns occurred in 4.2% (seven patients; one superficial, five second and one third-degree burn). Oedema around the tumour was noted in three studies and occurred in at
0 20 40 60 80
Pain Skin burn Oedema Pectoralis Major Injury
4 22 38 3
28 6
7 5 1
Number of patients
Slight
Moderate
Mild
Unknown
First degree
Second degree
18
least 16.8% (28/167 patients). In one study [19] oedema was noted, but the number of patients was not reported. The oedema disappeared within two weeks of the treatment. Pectoralis major injuries were reported in one study and occurred in all six patients (3.6%, 6/167 patients). Other complications were claustrophobia (0.6%; 1/167 patients), redness of the skin (0.6%; 1/167 patients) and tenderness of the breast (0.6%; 1/167 patients).Recurrence:
Recurrence of the tumour was found in two patients (1.2% of total patients, 7.1% of those with follow-up (2/28 patients)) in one study [44]. Both underwent modified radical mastectomy followed by chemotherapy, however one patient died 44 months post-treatment due to metastatic disease.
Cosmesis:
[image:27.612.72.544.320.433.2]Two studies [42, 44] performed an cosmetic analysis (figure 4) after HIFU treatment. Good to excellent cosmetic results were achieved in 92.6% (25/27 patients) and an acceptable result was achieved in 7.4% (2/27 patients). None of the patients were reported to have poor or unacceptable cosmetic results.
Figure 4: Cosmetic outcome of HIFU treatment.
2.4 Discussion
The studies included in this systematic review demonstrate that HIFU has been shown in small series to successfully induce coagulative necrosis in breast tumours. Histopathology showed no residual tumour in 46% of all patients (55/119 patients, range 17 - 100%). Residual tumour of less than 10% was found in 29% (35/119 patients, range 0 - 53%), residual tumour between 10 - 90% in 23% (20/119 patients, range 0 - 33%) and no percentages of residual tumour were mentioned in 2% (2/119 patients, range 0 - 33%) [12, 17, 40, 42, 43]. Post-treatment MRI images showed an absence of contrast enhancement and a thin rim of enhancement at the periphery in 82% of patients (31/38 patients, range 50 - 100%) indicative of coagulative necrosis. [19, 21, 41, 44] MRI of the breast after HIFU treatment [40, 43] showed that there was a positive correlation between the percentage of residual tumour and the ISI, MDF and PEI and that this could be used to determine the extent of residual tumour. The most common complications during and post-treatment were pain (40%), skin burns (4%), oedema (>17%) and pectoralis major injury (4%). Recurrence was reported in two patients in which an increase in tumour size was seen after an initial reduction in US size.
25 2
0% 10% 20% 30% 40% 50% 60% 70% 80% 90% 100%
19
This is the first systematic review that describes the clinical efficacy of HIFU in the treatment of breast tumours and therefore, no comparisons with the outcomes of other systematic reviews could be made. Comparing the imaging results, studies, perhaps surprisingly show that US-guided HIFU treatment [19, 21, 44] appears to give better results than MRI-US-guided studies [12, 17, 40-43]. Although only three studies [19, 21, 44] (two research groups) have performed HIFU under US guidance, two of these [19, 44] had an efficacy of 100% and the third [21] had an efficacy of 67%, although the latter included only six patients. More studies are needed to get an idea of the efficacy of US-guided HIFU. It is likely that patients are selected for US treatment and patient selection may be responsible for this observation. A positive correlation was found between the increase of signal intensity and the percentage of residual tumour tissue. [40, 43] Before HIFU treatment, a strong enhancement of the whole tumour was observed in dynamic contrast-enhanced MRI. Post-treatment, no enhancement was seen when the tumour was completely necrosed. When residual tumour was left behind, MRI scans showed a thin rim of enhancement. However, some benign processes such as oedema, fibrosis, necrosis and inflammation can mimic malignant contrast and therefore the time interval between treatment and imaging procedure, as well as the shape of the enhancement curves, must be taken into account. Malignant tissues after HIFU continue to show an irregular border, a rapid enhancement and an early distinct washout phase. [43]20
complete or incomplete ablation has been achieved within either days or months after HIFU treatment. It is possible that sampling after longer periods of time may demonstrate more extensive fibrosis or even additional necrosis related to isolation of any residual tumour from its blood supply.The extra margin of normal tissue ablated around the target tumour in the included studies varies from 0.5 to 1.5 - 2.0 cm. In two studies [19, 44], the margin was between 1.5 - 2.0 cm, and these are the only two studies having complete necrosis of the tumour in 100% of cases. Both were based on US-guided HIFU treatment. The only other US-guided study [21] applied a margin of 1.0 cm and achieved complete ablation in 66.7% of cases. In the MRI-guided studies [12, 17, 40-43], the margins treated around the tumour were between 0 - 1.0 cm and complete ablation was obtained in fewer cases. This wider treated margin of surrounding tissue could explain the high percentages of complete ablation achieved in the US-guided studies. The width of tissue surrounding the tumour that should be included in the treatment field is not clear from this review of the literature and may potentially not be identical for MRI and US-guided techniques; further research is required. The histopathology and imaging results after HIFU treatment were directly compared in three studies. [19, 41, 44] Two studies with a complete ablation of 100% histologically also showed a complete ablation on MRI. However, in one other study [41] the MRI gave one false positive and two false negatives results when compared to histopathology as the gold-standard. This suggests that MRI is an accurate predictor of complete ablation following HIFU treatment.
The treatment times of HIFU are a major disadvantage of the technique. However, only five studies [12, 17, 19, 21, 44] have reported treatment times, these range between 78 and 171 minutes for a lesion of 1.3 - 3.4 cm. It is imperative to make this treatment a viable alternative not only to patients clinically unfit for surgery that treatment times must be reduced.
21
The cosmetic result after HIFU treatment was good to excellent in 93% (25/27) of patients asked and acceptable in 7% (2/27). [42, 44] Due to the fact that in six studies HIFU was followed by surgical resection, the cosmetic outcome of HIFU could not be assessed. Lesion resorption is a long process and can take up to six months after HIFU treatment. [41, 44] This could prove to be a challenge during follow-up and may also have a psychological impact on patients believing that they might still have a lesion or even recurrence. [19] Therefore, it is important to inform patients that lesions may remain palpable for a long period and that this does not constitute recurrence. Furthermore, HIFU treatment might also prove challenging for interpretation of future breast imaging, if radiologists are not made aware of the HIFU treatment.All cohort studies reviewed were performed in different ways, varied in outcome measures and consistency of reporting of results, and results could therefore not, be directly compared with each other in an any quantitative analysis. There are significant variations in, for example, the times of further imaging and subsequent biopsies as well as the lesions included in the studies, the mode of the HIFU treatment, different MRI devices and MRI sequences. Heterogeneity was also found in the width of the surrounding tissue treated, the ablation dose and the frequency of the treatment. Furthermore, inclusion criteria differed in the distance between the lesion and the skin, chest wall and the nipple. Finally, the median amount of patients per study was 16.7, and two studies [21, 42] have a number of patients of ten or fewer. Strict standardization within the setting of RCT’s is needed to compare HIFU with breast surgery and to compare MRI-guided HIFU with US-guided HIFU and, in particular, large prospective studies are needed.
Compared with breast surgery, HIFU offers several potential advantages, including reduced recovery time and hospital stay, decreased complication risks and the ability to perform the treatment under local anaesthesia in an outpatient setting [48, 49]; all these factors could lead to a significant cost reduction, but require formal assessment. Furthermore, compared to the use of VAM for therapeutic interventions, HIFU has the advantage of not requiring any puncture of the skin, preventing the risk of infection and haematoma development. HIFU has the added benefit over VAM, that visualization of the tumour does not become increasingly obscured during the treatment due to excessive bleeding. [50] Studies [12, 17, 19, 21, 40-42, 44, 49] still use adjuvant therapy after HIFU treatment to acquire successful removal of the tumour. HIFU could potentially achieve complete removal of the tumour without the need of adjuvant therapy, like radiotherapy, if the ideal margin that needs to be included in the treatment is known.
22
3. Systematic Review: Minimal Invasive Ablative Techniques in the
Treatment of Breast Tumours
3.1 Materials and Methods
Study selection
A systematic review of the literature was performed using PubMed/Medline library database to identify all studies published up to January 2014 that evaluated the role of ablative techniques for the treatment of breast tumours. The MeSH terms used were ablative techniques, ablative interventions, ablative therapy, thermal ablation, high intensity focused ultrasound, radiofrequency ablation, laser ablation, cryo-ablation and microwave ablation in combination with breast. Except for reports in the English language and human subjects, there were no further restrictions. All obtained abstracts, studies and citations were reviewed. The related articles function was used to broaden the search, and all abstracts, studies and citations obtained were reviewed. References of the articles acquired were also searched by hand. The last search was conducted on February 18th, 2014.
Inclusion criteria
Studies were considered eligible for the systematic review if they addressed the following: (1) studies performed on human subjects with breast tumours, (2) studies using a non-surgical ablative technique including RFA, HIFU ablation, cryo-ablation, laser ablation or microwave ablation as a treatment for breast tumours, (3) studies objectively recorded response to treatment using imaging and histopathology, (4) studies objectively recoded treatment times, complication and/or recurrence rates (5) studies with 20 patients or over included.
Exclusion criteria
Studies that failed to fulfil the inclusion criteria or studies in which the outcomes of interest were not reported or if it was not possible to analyse these from the published reports, were excluded. Conference articles, letters, editorials and case reports were excluded. Studies using laser ablation as a scalpel were also excluded. In the case of studies with overlapping study populations, the most recent study with histopathological outcomes was included. When full text was not available, the study was also excluded.
Data extraction
23
Risk of bias in individual studies
The “Risk of bias” tool presented in the Cochrane Handbook [51] was used to determine the suitability of RCTs selected for inclusion in the quantitative analysis. The study quality of cohort studies was assessed according to the recommendations of the STROBE statement. [52] Seven items of the STROBE statement were considered relevant for quality evaluation. Studies with a score of less than four were excluded. Two reviewers (M.P and M.A) performed the assessment independently. In case of disagreement, a consensual decision was made.
Statistical analysis
All extracted data were tabulated and presented as means and percentages. Numerators and denominators were provided to address outcomes of included studies. For continuous variables the mean (SD), median and range should also be extracted and reported.
3.2 Results
Selected studies
[image:32.612.154.458.443.663.2]A total of 1532 articles published up to January 2014 were identified from the literature search (figure 5). Two articles were identified by searching the references of selected articles. After reviewing the abstracts, 1446 articles were excluded and 88 articles underwent full text examination. A total of 11 articles matched the selection criteria of which six [27, 44, 53-56] were feasibility studies, two [57, 58] were pilot studies, one [26] was a phase I study and one [19] was a RCT. One article [11] included four sub-studies: one phase I, one phase II and two randomized studies in which microwave ablation was compared to breast (conserving) surgery. Only one sub-study (phase II) matched all selection criteria and was included into the systematic review.
24
Study characteristics
25
Table 5: Study characteristics and outcomes for (a) RFA, (b) HIFU, (c) cryo-ablation, (d) laser ablation, (e) microwave ablation and (f) the single retrospective study.(a)
Author Patients Age Size (cm)
Treatment time (min)
Follow-up
Histopathology Imaging Complications and cosmesis
Manenti et al. (2009)
[55]
34 53 ± 5 (49-62)
1.9 ± 0.6 (1.7-2.0)
27 ± 3.7 (25-35)
- H&E (4 wk): No RD 94%. NADH (4 wk): No RD 97%.
MRI (3.0T Achieva Philips) 1 wk: 91% no enhancement, 4 wk: 97% no enhancement.
Skin burn & hyper-pigmentation (1). Cosmesis: excellent (28), good (5), poor (1). Ohtani et
al. (2011)
[58]
41 59 (38-92)
1.3 (0.5-1.8, MRI)
9 (6-15) - General: No RD 88%.
H&E: No RD 12.5% NADH (1-2 M): No RD 100% (12/12)
MRI (3.0T Signa GE medical) 1-2 M: 25/26 (96%) no enhancement
Skin burn (1).
Oura et al. (2007) [56]
52 55 (37-83)
1.3 (0.5-2.0)
12 (5-25) Every 2-3 M
Cytology (3-4 wk): 42% no RD.
MRI (1.5T Siemens Magnetom) 1-2M: 100% no RD, US 2-3 M: 42% no RD, 100% no vascular flow.
Skin burn (1). Cosmesis: excellent (43), good (6), fair (3).
Yamamoto et al. (2011) [54]
29 (30) 55.9 (38-78)
1.3 (0.5-1.9)
11.4 (6-20) 17 (2-41) M
H&E (3-4 wk): 10%, NADH (3-4 wk): 92%
MRI 3-4 wk: no hyper-vascularity in ablated zone 100%, mean size 3.8x3.3cm.
[image:34.792.69.742.106.354.2]26
(b)
Author Patients Age Size (cm)
Treatment time (hr)
Follow-up
Histopathology Imaging Complications
and cosmesis
Wu et al. (2003) [19]
23 46.5 ± 1.7 3.1 ± 0.79 (2.0-4.7) 78 (45-210)
- H&E (1-2 wk): No RD 100% Margin 1.8 ± 0.58 (1.5-2.2) cm
MRI (1.0T Impact) 7-10 days: 100% (3/3) no enhancement, extra margin of 1.5-2.0 cm.
Skin burn (1), mild local pain (1), oedema
Wu et al. (2005) [44]
22 (23) 48.6 (36-68) 3.4 (2.0-4.8) 132 (60-180) 54.8 (36-72) M Biopsy results (2 wk, 3, 6, 12 M): H&E: no RD 100%
US (Q-2000): 86% absence blood flow, volume decrease: 6M (n=17) 26.7 ± 12.2%, 12M (n=17) 45.2 ± 22.1%, 60M (n=5) 90.4 ± 4.91%. MRI (1.0T Impact): 100% (5/5) no enhancement.
Mild local pain (14), oedema. Cosmesis: good - excellent (16), acceptable (1).
* D=days, wk=weeks, M=months, RD=residual disease, H&E=haematoxylin and eosin, MRI = magnetic resonance imaging and US = ultrasound. (c)
Author Patients Age Size (cm)
Treatment time (min)
Histopathology Imaging Complications
and cosmesis
Sabel et al. (2004) [26]
29 Median 52.5 (34-77)
1.2 ± 0.5 (0.6-2.0)
10.3 (10-12)
H&E (14D): 85% no RD, 4x DCIS in normal tissue
US: during treatment:
Size ice ball: 4.8x3.4x3.3 cm3
(high freezing cycle 8-10 min), 3.8x2.7x2.7 cm3 (6 min).
-
Tafra et al. (2003)
[57]
24 Mean
61 (41-78)
1.2 ± 0.4 (0.7-2.0)
15.8 ± 7.6 (median 14)
Unspecified: 83% negative margins, diameter 1.4 ± 0.5 cm (+14%)
US during treatment:
Ice ball: 3.9 ± 0.3 (3.2-4.4) cm (high freeze cycle 8 min) Margin: 0.8 ± 0.2 (0.5-1.1) cm
Small seroma (2)
* D = days, wk = weeks, M = months, RD = residual disease, H&E = haematoxylin and eosin and US = ultrasound. (d)
Author Patients Age Size (cm)
Treatment time (min)
Histopathology Imaging Complications
and cosmesis
Mumtaz et al. (1996) [27]
20 (24) 57 (34-79)
2.0 (0.4-3.3)
8.3 H&E (5D): 65% no RD, 25% beyond margin, 10% missed. NADH (5D): 100% (2/2), Size: 1.0 (0.5-1.5) cm (-50%)
MRI 4hr, 24/48 hr:
65% centric, 25% eccentric, size 1.0 (0.7-1.8)cm (-50%).
Severe pain (2)
27
(e)
Author Patients Age Size (cm)
Treatment time (min)
Histopathology Imaging Complications and
cosmesis
Dooley et al. (2010) [11]
25 57.2 (no range) 1.76 (0.7-2.8) 159-206 (8), 108-148 (9), 82.8-97.2 (2)
H&E (17D): 68% necrotic tumour in relation to necrotic and viable tumour, 8% no RD.
US, prior to resection: size 1.84 (0.7-3.8) cm (+5%), PR* (16%), SD (52%), PD (32%)
Mild pain (9), erythema (9), oedema (5), first-degree burn (2), third-degree burn (1), severe pain (1).
* CR = complete response, PR = partial response, decrease tumour volume ≥ 50%, SD = stable disease, decrease <50% and PD = progressive disease, increase tumour volume >25%, D = days, RD = residual disease, H&E = haematoxylin and eosin and US = ultrasound.
(f)
Author Patients Age Size (cm)
Treatment time (min)
Histopathology Imaging Complications and cosmesis
(pre- vs. post-treatment)
Manenti et al. (2013) [53]
40 (RFA) 73 ± 5 (64-82)
- 15 ± 3.7 (12-23)
NADH (30-45D): 93% no RD
MRI (unknown) 88% (1 wk) and 93% (4 wk) no
enhancement. RFA area: 2.7 ± 0.1 x 4.2 ± 0.1cm
Cosmesis: excellent 23 vs. 34, good: 10 vs. 3,
acceptable: 5 vs. 1 and poor: 1 vs. 2.
40 (cryo) 73 ± 5 (64-82)
- 25 NADH (30-45D): 95% no RD
MRI (unknown) 90% (1 wk) and 95% (4 wk) no
enhancement. Size ice ball: 1.6 ± 0.1 x 3.1 ± 0.1 cm
Cosmesis: excellent: 26 vs. 37, good: 8 vs. 2,
acceptable: 7 vs. 1 and poor: 0 vs. 0.