Inhibitive Assesment of Stearamide as a Corrosion Inhibitor for Mild Steel in HCl Solution
Full text
Figure


Related documents
The polarization studies showed that clay is a cathodic-type inhibitor of corrosion one that decreases both cathodic hydrogen reduction reactions and anodic metal dissolution
However, the values of anodic and cathodic Tafel slopes were changed indicating that this behavior reflects the plant extract ability to inhibit the corrosion of composite in 1.0M
than one points to antagonism. In the present case both cathodic and anodic Tafel slopes obtained in the coexistence of the two species are comparable to that obtained in the their
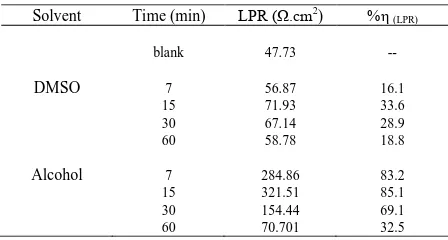
The different polarization parameter such as corrosion potential (Ecorr), corrosion current (icorr), anodic and cathodic tafel slop (a and c) and inhibition efficiency IE i %
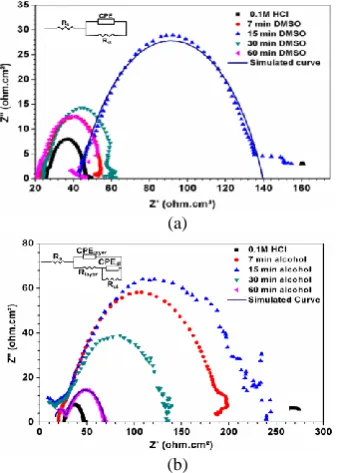
The electrochemical parameters such as: corrosion potential (Ecorr), corrosion current density (icorr), anodic and cathodic Tafel slopes (b a & b c ) derived
There was decrease of only 14 mV/decade in the corresponding values of cathodic Tafel slopes to show that the extract must have acted predominantly by blocking anodic sites,
The electrochemical parameters such as, corrosion potential (Ecorr), corrosion current (icorr), corrosion current density (Icorr), cathodic Tafel constant (bc), anodic Tafel
1) The cathodic and anodic curves obtained exhibit Tafel-type behavior. Addition of extract inhibitors increased both cathodic and anodic over voltages and caused mainly