Effect of Concentrated Apple Extract on Experimental Colitis Induced by Acetic Acid
Full text
Figure
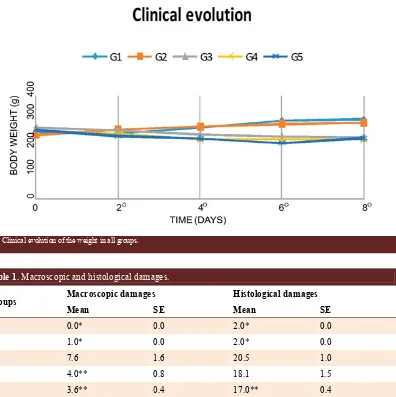
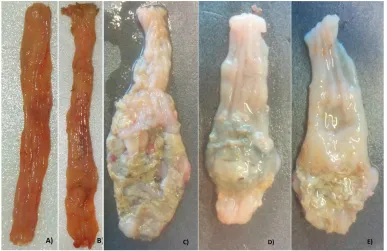
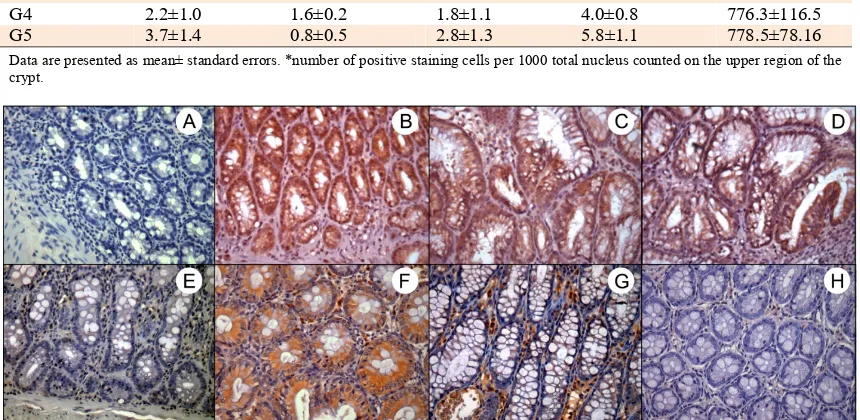
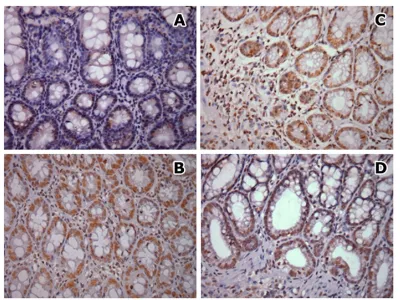
Related documents
As the results table shows, The diet of an equation of GDP, inflation rate in the first and third interrupt negative and positive relationship with economic growth
ADE assessment in observational studies can be based on review of existing practice-based data, such as medi- cal records, laboratory reports, and administrative data, on
Thus, the purpose of the present study was to explore Iranian English language teachers ’ personal styles, namely controlling parent, nurturing parent, adult, adapted child, and
Therefore, in this large population-based cohort study we utilized a joint modeling of longitudinal measures of anthropometric indices and CHD risk, to know whether
First, that of the allocators of resources at the managerial level (Directors of Adult Social Services in local authorities), second, those of older people with knowledge of
A multicentre trial of n = 400 participants admitted to four participating hospitals in Australia will be conducted... Screening will be conducted by a research assistant.
The results of the present study suggest that caution should be exercised when interpreting the results of short-segment nerve conduction studies at the across-elbow segment due to
that copper oxide NPs triggered apoptosis in the human K562 cancer cell line via ROS generation, while no adverse effects were observed against PBMC