Effects of isopropanol on collagen fibrils in new parchment
Full text
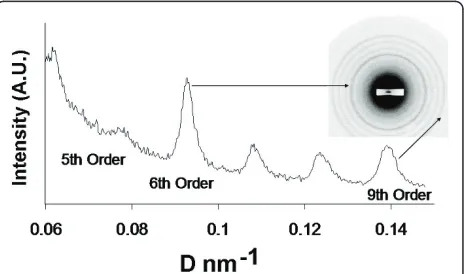
Figure


Related documents
(2011) ‘Seeking Help for Intimate Partner Violence: Victims’ Experiences when approaching the criminal justice system for IPV-related support and protection in an Australian
At vores informanter har været meget negative omkring deres hverdag, kan derfor have noget at gøre med, at de alle er afviste eller grundet Dublin-forordningen skal sendes tilbage
The concentrations of the elements in submerged and emergent plants from Lake Qattieneh differed depending on the sampling site and on the species (Table 6).. Site 61 located in
reported the first published case of an adult toxic epidermal necrolysis patient with ureteropelvic mu- cosa damage, haematuria, extensive ureteral mucosal sloughing, and acute
This paper proposed a novel audio watermarking scheme which is based on the Discrete Wavelet Transform also called as (DWT), the Singular Value Decomposition (SVD),
The normal manual welding method was used throughout the experiment, where the welding machine was perfectly fitted with the earth conductor had full and strong contact with load;
The proposed artificial neural network model have been simulated using neural networks toolbox in MATLAB. The model takes four inputs Vent O 2 concentration, SO 2 concen-
When the teachers help learners develop awareness about their own thinking and learning processes, the teachers are helping learners think about the effectiveness of the