Abiotic mechanisms for biochar effects on soil N
2O emission**
Chaohui He1,2,3, Kiril Manevski3,4 , Mathias Neumann Andersen3,4, Chunsheng Hu2*, Wenxu Dong2, and Jiazhen Li1,2
1University of Chinese Academy of Sciences, 19(A) Yuquan Road, Shijingshan District, Beijing 100049, China 2Key Laboratory of Agricultural Water Resources, Center for Agricultural Resources Research, Institute of Genetics and
Developmental Biology, Chinese Academy of Sciences, 286 Huaizhong Road, Yuhua district, Shijiazhuang 050022, China
3Sino-Danish Center for Education and Research, Estern Yanqihu Campus, 380 Huaibeizhuang, Huairou district,
Beijing 101408, China
4Aarhus University, Department of Agroecology, Blichers Allé 20, 8830 Tjele, Denmark
Received March 25, 2018; accepted August 7, 2019
*Corresponding author e-mail: cshu@sjziam.ac.cn
**This work was financially supported by the National Key
Research and Development Program (2017YFD0800601) (2017-2020) and the Key Program of the Chinese Academy of Sciences (ZDRW-ZS-2016-5-1) (2016-2018).
A b s t r a c t.In this research, sterile soil columns with differ-ent contdiffer-ents of biochar made from apple-tree residues (0, 1 and
5% w/w) at three levels of water filled pore space (40, 60, and
80%) were set up in the laboratory to study nitrous oxide dif-fusion and binding processes. The results indicated that nitrous oxide emission can be effectively mitigated at 5% biochar regard-less of soil water content. However, 1% biochar stimulated nitrous oxide diffusion compared to the other biochar treatments, which was opposite to expectations due to the stronger aeration than adsorption effect, while 0% had a suppression effect between 1
and 5%. Nitrous oxide emissions increased with increasing water filled pore space due to concomitantly decreasing biochar tortu -ousity at high water content. The increase of nitrogen from 1.11 to 1.50% on the biochar surface in the 5% treatment, and from 1.11 to 1.46% in the 100% biochar treatment, suggested that the main abiotic mechanisms for mitigation of nitrous oxide emission is adsorption and subsequent reactions with C = C bonds on apple-tree biochar surfaces since C = O and C-O bonds both increased and C=C/C-C/C-H declined.
K e y w o r d s: gas diffusion, mitigation, nitrous oxide, water
filled pore space
INTRODUCTION
The increasing demand for food and application of
chemical fertilizers have risen nitrous oxide (N2O)
emis-sions (Smith et al., 2008), with agriculture contributing as
much as 60% of the total anthropogenic N2O to the
atmos-phere (Harter et al., 2014). It is well known that N2O is
a potent greenhouse gas with much higher global warming potential than carbon dioxide and methane (IPCC, 2013).
The N2O from agriculture is mainly produced through a se-
ries of microbially-driven reactions in the soil, i.e. nitrifica
-tion, denitrification and dissimilatory nitrate reduction to
ammonia (Baggs, 2011). However, more than two thirds of
the soil N2O emissions come from nitrification and denitri
-fication (Lassey and Harvey, 2007). Moreover, the relative contribution of N2O emissions from each of these reactions
depends on not only the soil characteristics (soil texture, available carbon source, pH, microbial activity, etc.) but also on the environmental conditions (temperature, precipi-tation, etc.). Due to the complexity of the N2O production
reactions and their high spatio-temporal variability, con-trolling the emissions from agricultural soils is still of great challenge (Venterea et al., 2012). Methods are needed to limit or decrease the N2O emissions from agriculture
with-out actually compromising food production.
Biochar has been suggested as a promising tool to de-
crease soil N2O emissions (Liu et al., 2018). It is a form of
charcoal made by the release of energy from plant residues or animal waste during pyrolysis process (<700ºC) under limited oxygen or anoxic conditions, which makes biochar stable and porous (Karhu et al., 2011). Biochar transforms organic into inert carbon (C) not readily available for decomposition by soil microorganisms, thus it sequesters
C and alters the soil nitrogen (N) cycle (van Zwieten et al., 2010; Cayuela et al., 2013). In regard to suppressing soil
N2O emissions, the role of biochar is reported as
inconsist-ent in the sciinconsist-entific literature. A recinconsist-ent meta-analysis by Liu et al. (2018) used more than 200 peer-reviewed scientific studies and found notable benefits of using biochar such as increased symbiotic biological N2 fixation (63%), improved
plant N uptake (11%), decreased soil nitrate leaching (26%) and reduced soil N2O emissions (32%), though up to 4% of
the feedstock N could be emitted as N2O if the pyrolytic
syngas from biochar production is not purified. Similar suppressing effects of biochar on soil N2O emission are
reported by other studies (e.g. see the review of Cayuela
et al., 2014). However, there is little research on the
con-sumption of N2O after it has been produced in the soil. Lin
et al. (2014) investigated the microbial contribution to N2O
emission following biochar addition to a range of soils and
could not attribute the response in produced N2O to a
par-ticular microbial group (e.g. fungi or bacteria), suggesting the presence of abiotic production or consumption routes in biochar-amended soils. Several studies reported a response
of N2O emission to biochar-amended soils due to changes
in soil pH, aeration (Case et al., 2012) and cation exchange
capacity (Novak et al., 2009). Quin et al. (2015) reported
only 16% of the total N2O amount injected into a 100%
biochar column to be emitted and observed changes in the biochar surface functional groups in relation to the water status, suggesting physico-chemical interactions such as
adsorption and reduction-oxidation between N2O and the
biochar applied. It is therefore important to investigate the abiotic mechanisms by which biochar amendment to soil
may affect soil N2O emission in order to further understand
the complex factors governing N2O production and
con-sumption in the soil.
The main aim of the study was to investigate the physico-
chemical impact of biochar application on soil N2O
emis-sion and the underlying abiotic mechanisms for soils amended with different rates of biochar, relative to a con-trol without biochar. The objectives were to: i) analyze the
properties of biochar by Scanning Electron Microscope –
Energy Dispersive X-Ray Spectroscopy, ii) conduct X-ray Photoelectron Spectroscopy for a detailed element and
functional group analysis, and iii) analyze the actual N2O
emission data from the soils and propose abiotic
mecha-nism for soil N2O emission.
MATERIALS AND METHODS
Soil samples were taken from Luancheng experimen-tal station, Shijiazhuang Prefecture, Hebei Province, China
(37°53′N, 114°41′E). The climate is continental and
semi-arid, with annual precipitation of 400-550 mm, mostly occurring as rainfall from June to September. The soil is
aquic cinnamon according to the Chinese soil classifica -tion system, corresponding to silty loam soil according
to the USDA soil classification system. The upper 10 cm soil was sampled from a field with auger and roots, stones
and plant debris were removed manually. Biochar from
apple-tree residues was purchased from a commercial company (Shaanxi Yixin Biological Energy Technology Development Co. Ltd., the highest treatment temperature is 520ºC), which was sieved to pass through a 2 mm mesh together with the air-dried soil to achieve homogeneous soil/biochar mixtures, which was then stored in a cool and dry place for later use.
Selected physical and chemical characteristics of the soil were measured before conducting the biochar experiment. Soil pH (1:2.5, soil/water, w/w) was determined after 1-2 min stirring by a glass rod and left for 30 min before using a pH
meter (Mettler Toledo, USA) and soil organic C was ana -lyzed by the potassium dichromate oxidation method
according to Lu (2000). Total C and N contents of the soil were determined by dry combustion analysis using a flash HT elemental analyzer (vario MACRO cube, Elementar,
Germany). Bulk density was measured by the cutting-ring method. Soil porosity was obtained from bulk density and soil particle density. The physico-chemical properties of the soil are summarized in Table 1.
The soil samples were grouped in two main treatments,
each with four replicates, and amended with biochar (first
treatment) at rates of 0, 1 and 5% (dry weight/dry weight), treatments coded as 0% BC, 1% BC and 5% BC. The sam-ples were mixed thoroughly and packed to a bulk density
equal to that under field condition (Table 1). The soil and
biochar mixtures were packed into 19 cm diameter col-umns to a depth of 10 cm. After packing, the soil colcol-umns and the equipment used in the experiment were autoclaved at 130°C for 1 h to attain abiotic condition and sterilized water was added by a syringe in a UV sterilized biological safety cabinet when columns cooled down to obtain three water content levels (second treatment): low (L),
medi-um (M) and high (H), corresponding to water filled pore
space (WFPS) of around 40, 60 and 80% (Table 2). Before autoclaving, the columns were wrapped in tinfoil and tied
Table 1. Physico-chemical characteristics of the sampled soil at Luancheng, China
pH SOC (%) (%)TC (%)TN C/N density Bulk (g cm-3)
Porosity (%)
7.61 0.93 2.93 0.10 9.30 1.30 50.94
SOC is soil organic carbon, TC and TC are total carbon and total
nitrogen, respectively, and C/N is the carbon to nitrogen ratio
Table 2. Water filled pore space in the biochar (BC) amendment
treatments of the study
Water filled
pore space (%) 0% BC 1% BC 5% BC
Low (L) 39.97 40.70 43.91
Medium (M) 59.99 61.04 65.91
tightly to avoid diffusion of water vapor inside. The tinfoil-covered columns were weighted by an electronic scale with maximum capacity of 30 kg before and after autoclaving
and there was no significant water loss/gain during this pro -cess. In addition to the three biochar levels (0, 1 and 5% BC), a 100% biochar level was also included (coded 100% BC) to test for adsorption without altering WFPS.
Each column was placed in a safety cabinet for 60, 90 and 120 min, for low, medium and high WFPS, respectively, when water percolated into the soil/biochar mixtures before
N2O injection. Finally, the biochar×WFPS treatments (0,
1 and 5% BC, each at low, medium and high WFPS) and
the 100% BC treatment were all injected with N2O using
a 100 mL gas mixture of N2O (991 ppm) at the bottom of
each column using a gas tight glass syringe directly through
an injection port. The N2O concentration in the headspace
was measured after injection by a N2O isotope analyzer
(Fig. 1).
Five g of substrate was collected randomly from the upper surface of the soil columns after autoclaving and at the end of experiment within the safety cabinet and were analyzed for microbial activity by 24 h culturing in the medium composed of peptone (10 g L-1), yeast (5 g L-1),
NaCl (10 g L-1) and agar (15 g L-1). The results showed
no microbial colonies present on the Petri dishes after 24-h incubation.
In order to investigate the possible abiotic mechanisms
that result in N2O suppression due to biochar amendment,
the soil and the biochar from the 5% BC treatment at low,
medium and high WFPS with N2O injection were separated.
The treatment without N2O injection, i.e., a “conventional”
(CK) treatment soil with 5% biochar amendment and
with-out N2O injection, intended to contrast the surface elemental
and functional groups analysis. The separation method fol-lowed the procedure of Joseph et al. (2010) and Lin et al. (2012). Briefly, the samples were dispersed by agitation in
ten volumes of distilled water (1 g :10 mL) and the soil/bio-char mixture suspension was sieved using a 250 µm sieve. Biochar pieces were manually removed from the sieve surface by a tweezer. The isolated biochar samples were then washed several times in a beaker with distilled water, air-dried and stored in a sealing bag at 4°C. The collected
biochar was studied by Scanning Electron Microscopy (SEM), Energy Dispersive X-ray Spectroscopy (EDS) and
X-ray Photoelectron Spectroscopy (XPS) techniques for describing biochar surface structure, relative content of ele-ments and surface functional group changes. The analysis
was conducted by a commercial certified company (Zhong
Ke Bai CE, Beijing, China).
Analysis of variance (ANOVA) was conducted on the data and significant differences between treatments were
determined using t-test. The statistical analysis was
con-ducted using IBM SPSS Statistics 19.0 for Windows (SPSS
Inc., USA).
RESULTS
The SEM morphological description of the biochar in
the 5%BC treatment pooled for the low, medium and high
WFPS samples with injected N2O (LMH 5% BC), as well
as of pure biochar before incorporation into the soil are shown in Fig. 2. There were apparent differences between
LMH5%BC and biochar before application and a layer of
organic molecules and inorganic materials such as minerals
could be seen in LMH 5% BC exterior and internal pores,
signifying probable interactions between the biochar and the soil. The exterior of a typical woody biochar showed smooth surfaces due to high C content and very few mine-
rals on the surface (Fig. 2d, examined at 1K magnification).
Figure 3 shows the elemental spectra of biochar surface
collected from the 5% BC treatment with N2O injection at
low, medium and high WFPS. All the sampled biochar had similar patterns of elemental composition, with C making up the largest proportion of the total amount of elements.
Fig. 2. Morphology of biochar amended at 5% to soil at low, medium and high water contents according to the Scanning Electron Microscopy (SEM) for: (a) external surface after interaction with soil, (b) internal surface covered by organo-mineral matters and (c) external surface and pores having reactions with soil and injected N2O, as well as (d) external surface of pure biochar before incor-porated into soil. Samples shown on a, b and c were examined at 20K, 5K and 1K magnification, respectively.
Other elements included O, Al, Si, K and Ca, whereas Fe, though as a minor part of the total specimen, was observed only at medium water content (Fig. 3b). The few miner-als might have come from the indigenous ash contents in the biochar feedstock or from the compounds formed on the biochar surface when it interacted with the soil mineral matter.
Figure 4 shows the elemental spectra of the 5% BC
treatment without N2O injection at low, medium and high
WFPS. There were no drastic differences in the elemental
composition of the biochar without N2O injection compared
with the biochar with N2O injection (Fig. 3). Yet, the
spec-tral peaks corresponding to Al and Si decreased (Fig. 4b, c), while those of Ca increased notably (Fig. 4c) compared to
the N2O injection treatment, mineral changes probably due
to the soil-biochar-water interactions.
Table 3 shows the results of the XPS analysis for the main elements in the biochar samples collected from 5%
BC treatment without N2O injection (CK treatment), the
5% BC treatment with N2O injection pooled at the three
WFPS (LMH 5% BC) and the 100% biochar with N2O
injection (100% BC). As seen from the table, C was the
most abundant element in all examined samples and had an atomic percentage range of 82-85%, which was in line with the EDS results. The abundance of O was around 14%
for all samples, whereas that of N was low and ranged from 1.11% in CK to 1.50% in LMH 5% BC. However, the Fe
and Al minerals had the lowest abundance of less than 1%. Among the treatments, most of the elements present with
the highest abundance were observed in the LMH 5% BC
treatment.
Additional XPS analysis results of the bonding states and the biochar surface functional groups are presented
in Table 4. Only the functional groups including C and N
were detected. The concentration of aliphatics (C=C/C-C/ C-H), which are the major components in all biochars, was
notably higher in CK than LMH 5% BC and BC 100% treat -ments. On the contrary, C in oxidation states (C-O, C = O) increased, indicating surface C change from ordinary bond-ing states to oxidation states due to oxidation reactions or
adsorption of soil organic matter or both. Regarding the N groups, C = N increased dramatically to 77.38% in LMH
5% BC compared to 51.51% in 100% BC and 58.43% in
CK, possibly due to the consumption of N2O.
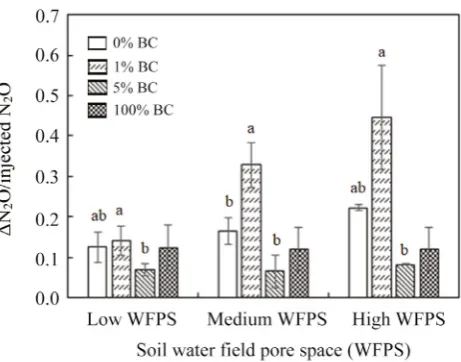
The net increase of N2O as measured in the headspace
of the columns relative to the total injected N2O (ΔN2O/
injected N2O) at the time when it reached the peak
concen-tration is presented inFig. 5. It is apparent from the figure that ΔN2O/injected N2O was markedly lower for the 5% BC
treatment at the three soil water contents. However, oppo-site to the expectations, 1% BC appears to have stimulated
N2O emissions, whereas the N2O suppression effect for 0%
BC was in between that of 1% BC and 5% BC. It can also
be seen on the figure that the ΔN2O/injected N2O increased
with increasing WFPS and this was especially evident for the lower biochar amendments. Since the biochar in the 100% BC treatment was dry and without water additions,
ΔN2O/injected N2O was constant between the different
WFPS, but was lower than that for 0 and 1% BC and higher
than that for 5% BC. Moreover, only a slight increase in ΔN2O/injected N2O could be seen for the 5% BC between
the three WFPS levels.
In addition to ΔN2O/injected N2O, the absolute value of
the net N2O increase in soil water and air was also
comput-ed and the results are shown in Table 5. The dissolvcomput-ed N2O
increased with increasing WFPS for all biochar treatments.
The highest value of dissolved N2O in the water solution
Table 3. X-ray Photoelectron Spectroscopy analysis of the main elements in the biochar samples showing peak binding energy (BE, eV) and atomic percentage (%)
Element CK LMH 5% BC 100% BC
Peak BE (eV) Atomic % Peak BE (eV) Atomic % Peak BE (eV) Atomic %
Al 2p 74.28 0.68 74.88 0.86 74.79 0.60
C 1s 284.78 84.49 284.79 83.10 284.82 82.72
N 1s 400.42 1.11 400.39 1.50 400.06 1.46
O 1s 532.53 13.41 532.66 14.15 532.26 14.92
Fe 2p 719.51 0.31 721.22 0.40 722.00 0.33
CK is conventional treatment of 5% biochar-amended soil without N2O injection, LMH 5% BC is 5% biochar-amended soil with N2O injection at low, medium and high water contents, and 100% BC is 100% biochar treatment with N2O injection.
Table 4. C 1s and N 1s binding energy (BE) and their relative atomic percentage on biochar surface
Element Functional groups CK LMH 5% BC 100% BC
Peak BE (eV) Atomic % Peak BE (eV) Atomic % Peak BE (eV) Atomic %
C 1s A C=C/C-C/C-H 284.58 82.50 284.79 71.02 284.79 72.88
C 1s B C-O&C-NH2 285.84 10.00 286.14 19.65 285.96 21.00
C 1s C C=O&C=N 288.47 7.50 288.93 9.18 289.16 5.43
N 1s A C-NH2 399.46 41.57 399.16 22.62 399.42 48.49
N 1s B C=N 400.60 58.43 400.50 77.38 400.64 51.51
CK is conventional treatment of 5% biochar to the soil without nitrous oxide (N2O) injection pooled for the three WFPS, LMH 5% BC is 5% amended soil with biochar with pooled samples at low, medium and high water filled pore spaces and with N2O injection, and 100% BC is 100% biochar treatment with N2O injection.
Fig. 5. Net increase of N2O in air relative to total injected N2O at peak concentration (ΔN2O/injected N2O) at different soil water
contents and biochar amendments. Error bars denote the standard
was 0.20 μmol for 1% BC, whereas the lowest value was 0.02 μmol for 5% BC. Despite the high solubility of N2O
in water, the values for net N2O increase in air were much
larger, with the highest values of 1.36 and 1.85 μmol for,
respectively, medium and high WFPS in the 1% BC
treat-ment. In the experiment, the total injected N2O was around
4 μmol, hence there was a large amount of N2O sorbed on
the biochar surface according to the small contributions in both water and headspace.
As shown in Fig. 6, the 5% BC treatment had a suppres-sion effect, whereas the 1% BC treatment appears to have
had a stimulating effect on N2O emissions, consistent with
the findings in Fig. 5. N2O peak concentration varied from
1000 ppb to 6000 ppb in biochar-amended soil samples and
the largest N2O peak concentration was seen for 1% BC,
followed by 0, 100 and 5% BC. For all treatments, N2O
increased sharply so that within 5 min the concentration reached its highest values after which it declined gradually. However, 5% BC at low WFPS took almost 3 h to peak, illustrating a more complicated system.
DISCUSSION
The N2O emissions were significantly affected by the
WFPS and overall increased from low to high WFPS,
though the net N2O increase was the lowest at 40% WFPS
but rose with increasing water content (Figs 5, 6). The
water content regulated the trend of N2O emissions and had
the greatest impact on 1% BC treatment compared to 0 and
5% BC treatments. Similar responses of N2O emissions
to WFPS have been found in other studies based on data
collected in field or laboratory experiments (Yanai et al., 2007; Chintala et al., 2015; Chang et al., 2016). Most of these studies include microbial participation in nitrification
and denitrification processes. The soils used in the present
study were autoclaved in order to eliminate any microbial impacts. Autoclaving effectively kills most of the microbes,
expect for some thermophiles not relevant for N2O related
processes, and this was supported by the negative tests for sterility for nitrifying and denitrifying bacteria and for chemical indicators in the column cores. Hence, the
sig-nificant increase in N2O emissions with increasing WFPS
is due to abiotic mechanisms, such as altered tortuosity and diffusion pathway. Stark and Firestone (1995) argued that diffusion path lengths become more tortuous and water
films coating surfaces thinner at low water content. When
soil is amended with (porous) biochar, the tortuosity of the
gas flow paths is increased, providing better conditions for
adsorption. These reactions at low WFPS could have been
responsible for the observed low ΔN2O/injected N2O in the
5% BC treatment (Fig. 5), whereas at high WFPS (thus less
tortuous paths) the N2O emissions were higher (Figs 5, 6).
Comparison between 1 and 5% BC (Fig. 6) demonstrated tortuosity can be easily altered by increasing the WFPS in the 1% BC treatment.
Table 5. Dissolved nitrous oxide (N2O) in soil water and net increase of N2O in air. 0%BC, 1%BC and 5%BC denote treatments with 0%, 1% and 5% biochar amendment in the soil. Numbers in
brackets are standard error of the mean (n= 4) and lowercase
let-ters indicate significant differences between the treatments at95%
confidence level
Dissolved in water
(μmol)
(WFPS)
0% BC 1% BC 5% BC
Low 0.03 (±0.01)a 0.03 (±0.01)a 0.02 (±0.00)a
Medium 0.05 (±0.01)b 0.10 (±0.02)a 0.02 (±0.01)b
High 0.09 (±0.00)ab 0.20 (±0.06)a 0.04 (±0.00)b
Net increase in air (μmol)
(WFPS) 0% BC 1% BC 5% BC
Low 0.52 (±0.16)ab 0.59 (±0.15)a 0.29 (±0.06)b
Medium 0.69 (±0.13)b 1.36 (±0.23)a 0.27 (±0.17)b
High 0.92 (±0.03)ab 1.85 (±0.53)a 0.34 (±0.01)b
WFPS – soil water filled pore space.
Fig. 6. The impact of biochar amendments on N2O emissions at (a) low, (b) medium and (c) high water filled pore space (WFPS).
There was a clear response of the observed N2O
con-centrations to the apple-tree biochar amendment rate and,
compared to the CK treatment, ΔN2O in air/injected N2O
was reduced to 5%, corroborating the results of other
labo-ratory and short-term field investigations (Rogovska et al., 2011; Case et al., 2012; Wang et al., 2013; Lan et al., 2015; He et al., 2016; Grutzmacher et al., 2018). Although the
previous studies observed a suppression of N2O by biochar
in the soil, the underlying abiotic mechanism remained poorly understood. While soil pH relates well to biotic mechanisms, redox potential (Eh) is a parameter that could explain (or be involved in the explanation of) abiotic me-
chanisms of N2O change in biochar-amended soils; yet
Eh is characterized by high variability in space and time, irreversibility of redox reactions at the surface of the electrodes, chemical disequilibrium in soils and
polarisa-tion of and/or leakage from electrodes (Whitfield, 1974;
Grundl, 1994; Thomas et al., 2009; Joseph et al., 2015). Alternatively, the present study performed elemental and functional group analysis for 5% apple-tree biochar appli-cation (that is to say, for the most “effective” dosage) under
different WFPS treatments with and without N2O injection,
which is more straightforward and accurate than measuring soil Eh and pH.
Two main potential mechanisms for the mitigation effect
of the 5% BC treatment for N2O emission pointed by this
study are adsorption and subsequent chemical reactions. Biochar surfaces are complex and there will be preferential
sites for adsorption and reduction of N2O. Previous studies
showed that charred materials have strong sorption capa-
city of N2O, even better than that of peat, uncharred wood
and metal oxides (Cornelissen et al., 2013). Therefore, it
is highly probable that N2O was sorbed on the apple-tree
biochar surface, especially in the 5% BC treatment with the
highest specific surface area compared to 1% BC treatment. In fact, slightly less N2O was dissolved in water (Table 5),
indicating a large amount of N2O that was adsorbed on the
surface or further reduced by other pathways. Moreover, the XPS analysis (Table 3) showed an increase of N from
1.11 to 1.50% on biochar surface in the 5% BC treatment, and also from 1.11 to 1.46% in the 100% BC treatment.
However, the 1% BC treatment promoted N2O emissions
regardless of WFPS (Figs 5, 6), which is in contrast to expectations and previous studies. This is probably due to improved soil aeration by the biochar incorporated in the soil, which causes faster gas diffusion owing to the porous structure.
Sorbed N2O can also be reduced by organic molecules
of biochar or soil organic compounds on the biochar surface (van Zwieten et al., 2009). Avdeev et al. (2005) re- ported a range of aromatic and aliphatic compounds
oxi-dized by N2O and their calculations indicated that an O
atom is transferred through the 1,3-dipolar cycloaddition
of N2O to the C=C bond. Subsequently, the resulting
inter-mediate decomposed to a ketone and released N2 to the
gas phase. The schematic diagram of chemical reaction is shown below:
.
Biochar contains a large number of aromatic and ali-phatic groups on the surface. As the XPS analysis showed (Table 4), C = O and C-O both increased and C=C/C-C/C-H decreased in the 5% BC, indicating that these compounds
may be partly responsible for the reduction in N2O
emis-sions observed in this treatment. Alternatively, studies have shown that reductive metals such as Fe can be catalytically
active for the decomposition of N2O (Moraghan and Buresh,
1977; Carabineiro et al., 2008) and Fe has already been
reported as central for orchestrating the N-transformations
in biochar-amended soils (Hans et al., 1996; Alowitz and Scherer, 2002). Sang et al. (2005) proposed the following
reaction mechanism as being the most likely to fit their experimental data for the reduction of N2O:
N2O + FeO ⇌ N2O ‒ FeO
N2O – FeO ⇌ OFeO + N2
N2O + OFeO ⇌ N2O – OFeO
N2O – OFeO ⇌ O2 – FeO + N2
O2 – FeO ⇌ FeO + O2 .
Studies have also demonstrated that up to 84% of N2O could
be rapidly reduced to N2 in alkaline conditions (pH = 8) by
ferrous iron (Moraghan and Buresh, 1977). Considering the
observed Fe in the element analysis (Fig. 3b), the adsorbed
N2O on apple-tree biochar surface and the alkaline
environ-ment, the above reactions might have taken place in the
present experiment. Even though the amount of N2O in air
and water can be computed, how to distinguish the effect of adsorption and chemical reaction is not straightforward because adsorption is temporal and might be accompanied by desorption. Finally, it should be mentioned that the soil
columns stood 60 to 120 min prior N2O injection, giving
insights on a short-time scale interactions, therefore, future studies may investigate the same reactions occurring after longer time in the organomineral layers of more aged bio-achar (e.g. Quin et al., 2015).
CONCLUSIONS
This study performed elemental and functional group analysis of soil amended with different rates of apple-tree biochar application under varying WFPS treatments with
and without N2O injection in order to investigate the abiotic
mechanisms of soil N2O emissions. The results can be
sum-marized as follows:
1. The N2O emissions were significantly lowered by
apple-tree biochar in the 5% BC treatment regardless of
water filled pore space, though the effect was more evident
2. The observed sharp increase in N2O emissions at high
WFPS might be due to the changed tortuosity and diffu-sion pathway of soil amended with porous biochar, which in turn provides suitable conditions for adsorption.
3. Indeed, the biochar surface elemental and functional groups analysis revealed adsorption and subsequent chemi-cal reactions as the most possible abiotic mechanisms for
N2O consumption.
Conflict of interest: The Authors do not declare conflict
of interest.
REFERENCES
Alowitz M.J. and Scherer M.M., 2002. Kinetics of nitrate,
nitrite, and Cr(VI) reduction by iron metal. Environ. Sci. Technol., 36(3), 299-306. https://doi.org/10.1021/es011000h Avdeev V.I., Ruzankin S.F., and Zhidomirov G.M., 2005.
Molecular mechanism of direct alkene oxidation with
nitrous oxide: DFT analysis. Kinetics and Catalysis, 46(2), 177-188. https://doi.org/10.1007/s10975-005-0066-z Baggs E.M., 2011. Soil microbial sources of nitrous oxide: recent
advances in knowledge, emerging challenges and future direction. Current Opinion in Environmental Sustainability, 3(5), 321-327. https://doi.org/10.1016/j.cosust.2011.08.011 Carabineiro S.A., Fernandes F.B., Silva R.J.C., Vital J.S.,
Ramos A.M., and Fonseca I.M., 2008. N2O reduction by
activated carbon over iron bimetallic catalysts. Catalysis Today, 133-135, 441-447. https://doi.org/10.1016/j.cattod. 2007.11.019
Case S.D.C., McNamara N.P., Reay D.S., and Whitaker J., 2012. The effect of biochar addition on N2O and CO2 emis-sions from a sandy loam soil – The role of soil aeration. Soil
Biol. Biochem., 51, 125-134. https://doi.org/10.1016/j. soilbio.2012.03.017
Cayuela M.L., Sanchez-Monedero M.A., Roig A., Hanley K., Enders A., and Lehmann J., 2013. Biochar and denitrifi -cation in soils: when, how much and why does biochar
reduce N2O emissions? Scientific Reports 3, 1732.
https://doi.org/10.1038/srep01732
Cayuela M.L., van Zwieten L., Singh B.P., and Jeffery S., Roig A., and Sánchez-Monedero M.A., 2014. Biochar’s role in mitigating soil nitrous oxide emissions: A review and meta-analysis. Agric. Ecosys. Environ., 191, 5-16.
https://doi.org/10.1016/j.agee.2013.10.009
Chang J., Clay D.E., Clay S.A., Chintala R., Miller J.M., and Schumacher T., 2016. Biochar reduced nitrous oxide and carbon dioxide emissions from soil with different water and temperature cycles. Agronomy J., 108(6), 2214-2221. https://doi.org/10.2134/agronj2016.02.0100
Chintala R., Owen R.K., Schumacher T.E., Spokas K.A., McDonald L.M., Kumar S., Clay D.E., Malo D.D., and Bleakley B., 2015. Denitrification kinetics in biomass-and
biochar-amended soils of different landscape positions. Environ. Sci. Pollution Res. Int., 22(7), 5152-5163. https:// doi.org/10.1007/s11356-014-3762-2
Cornelissen G., Rutherford D.W., Arp H.P., Dorsch P., Kelly C.N., and Rostad C.E., 2013. Sorption of pure N2O to
bio-chars and other organic and inorganic materials under anhydrous conditions. Environ. Sci. Technol., 47(14), 7704-7712. https://doi.org/10.1021/es400676q
Grundl T., 1994. A review of the current understanding of redox capacity in natural, disequilibrium systems. Chemosphere, 28(3), 613-626.
https://doi.org/10.1016/0045-6535(94)90303-4
Grutzmacher P., Puga A.P., Bibar M.P.S., Coscione A.R., Packer A.P., and de Andrade C.A., 2018. Carbon stability
and mitigation of fertilizer induced N2O emissions in soil
amended with biochar. Sci. Total Environ., 625, 1459-1466. https://doi.org/10.1016/j.scitotenv.2017.12.196
Hans C.B.H., Christian B.K., Hanne N.K., Ole K.B., and Jan S., 1996. Abiotic nitrate reduction to ammonium: key role of green rust. Environ. Sci. Technol., 30(6), 2053-2056. Harter J., Krause H.M., Schuettler S., Ruser R., Fromme M.,
Scholten T., Kappler A., and Behrens S., 2014. Linking
N2O emissions from biochar-amended soil to the structure and function of the N-cycling microbial community. ISME
J., 8(3), 660-674. https://doi.org/10.1038/ismej.2013.160 He L.L., Zhao X., Wang S.Q., and Xing G.X., 2016. The effects
of rice-straw biochar addition on nitrification activity and
nitrous oxide emissions in two Oxisols. Soil Till. Res., 164, 52-62. https://doi.org/10.1016/j.still.2016.05.006
IPCC, 2013. Climate Change 2013: The Physical Science Basis.
Cambridge University Press, Cambridge, UK and New
York, USA.
Joseph S., Husson O., Graber E., van Zwieten L., Taherymoosavi S., Thomas T., Nielsen S., Ye J., Pan G., Chia C., Munroe P., Allen J., Lin Y., Fan X., and Donne S., 2015. The elec-trochemical properties of biochars and how they affect soil redox properties and processes. Agronomy, 5(3), 322-340. https://doi.org/10.3390/agronomy5030322
Joseph S.D., Camps-Arbestain M., Lin Y., Munroe P., Chia C.H., Hook J., Van Zwieten L., Kimber S., Cowie A., Singh B.P., Lehmann J., Foidl N., Smernik R.J., and Amonette J.E., 2010. An investigation into the reactions of biochar in soil. Soil Res., 48, 501-515. https://doi.org/10.1071/sr10009 Karhu K., Mattila T., Bergström I., and Regina K., 2011. Biochar
addition to agricultural soil increased CH4 uptake and water holding capacity – Results from a short-term pilot field
study. Agric., Ecosys. Environ., 140(1-2), 309-313. https:// doi.org/10.1016/j.agee.2010.12.005
Lan Y., Meng J., Yang X., Jiang L.L., Liu Z.Q., Liu S.N., and Chen W.F., 2015. Effects of different straw incorporation
ways on N2O emission and soil physicochemical properties
of brown soil (in Chinese). Chinese J. Ecol., 34(3), 790-796. Lassey K. and Harvey M., 2007. Nitrous oxide: the serious side
of laughing gas. Water Atmosphere, 15(2), 10-11. Lin X., Spokas K., Venterea R., Zhang R., Baker J., and Feyereisen
G., 2014. Assessing microbial contributions to N2O impacts
following biochar additions. Agronomy, 4(4), 478-496. https://doi.org/10.3390/agronomy4040478
Lin Y., Munroe P., Joseph S., Kimber S., and Van Zwieten L., 2012. Nanoscale organo-mineral reactions of biochars in
ferrosol: an investigation using microscopy. Plant Soil, 357(1-2), 369-380. https://doi.org/10.1007/s11104-012-1169-8 Liu Q., Zhang Y., Liu B., Amonette J.E., Lin Z., Liu G., Ambus
P., and Xie Z., 2018. How does biochar influence soil N
cycle? A meta-analysis. Plant and Soil, 426(1), 211-225. https://doi.org/10.1007/s11104-018-3619-4
Lu R.K., 2000. Methods of Soil and Agro-Chemical Analysis (in
Moraghan J.T. and Buresh R.J., 1977. Chemical reduction of nitrite and nitrous oxide by ferrous iron. Soil Sci. Soc. Am. J., 41, 47-50.
https://doi.org/10.2136/sssaj1977. 03615995004100010017x Novak J.M., Busscher W.J., Laird D.L., Ahmedna M., Watts D.W.,
and Niandou M.A.S., 2009. Impact of biochar amendment on fertility of a southeastern coastal plain soil. Soil Sci., 174(2), 105-112. https://doi.org/10.1097/ss.0b013e3181981d9a Quin P., Joseph S., Husson O., Donne S., Mitchell D., Munroe P.,
Phelan D., Cowie A., and Van Zwieten L., 2015. Lowering
N2O emissions from soils using eucalypt biochar: the importance of redox reactions. Scientific Reports, 5, 16773.
https://doi.org/10.1038/srep16773
Rogovska N., Laird D., Cruse R., Fleming P., Parkin T., and Meek D., 2011. Impact of biochar on manure carbon stabi-lization and greenhouse gas emissions. Soil Sci. Soc. Am. J., 75(3), 871-879. https://doi.org/10.2136/sssaj2010.0270 Sang C., Kim B.H., and Lund C.R.F., 2005. Effect of NO upon
N2O decomposition over Fe/ZSM-5 with low iron loading.
J. Physical Chem. B, 109(6), 2295-2301. https://doi.org/10.1021/jp048884m
Smith P., Martino D., Cai Z., Gwary D., Janzen H., Kumar P., McCarl B., Ogle S., O’Mara F., Rice C., Scholes B., Sirotenko O., Howden M., McAllister T., Pan G., Romanenkov V., Schneider U., Towprayoon S., Wattenbach M., and Smith J., 2008. Greenhouse gas miti- gation in agriculture. Phil. Trans. R. Soc. B, Biological Sci., 363(1492), 789-813. https://doi.org/10.1098/rstb.2007.2184 Stark J.M. and Firestone M.K., 1995. Mechanisms for soil-
moisture effects on the activity of nitrifying bacteria. Appl.
Environ. Microbiol., 61(1), 218-221.
Thomas C.R., Miao S.L., and Sindhoj E., 2009. Environmental factors affecting temporal and spatial patterns of soil redox potential in Florida Everglades Wetlands. Wetlands, 29(4), 1133-1145. https://doi.org/10.1672/08-234.1
van Zwieten L., Kimber S., Morris S., Downie A., Berger E., Rust J., and Scheer C., 2010. Influence of biochars on flux of N2O and CO2 from ferrosol. Australian J. Soil Res.,
48(6), 555-568. https://doi.org/10.1071/sr10004
van Zwieten L., Singh B., Joseph S., Kimber S., Cowie A., and
Chan Y.K., 2009. Biochar and emissions of non-CO2
greenhouse gases from soil. In: Biochar for Environmental
Management Science and Technology (Eds J. Lehmann, S.
Joseph). Earthscan Press, UK.
Venterea R.T., Halvorson A.D., Kitchen N., Liebig M.A., Cavigelli M.A., Grosso S.J.D., Motavalli P.P., Nelson K.A., Spokas K.A., Singh B.P., Stewart C.E., Ranaivoson A., Strock J., and Collins H., 2012. Challenges and opportu-nities for mitigating nitrous oxide emissions from fertilized cropping systems. Frontiers in Ecology the Environment, 10(10), 562-570. https://doi.org/10.1890/120062
Wang Z.Y., Zheng H., Luo Y., Deng X., Herbert S., and Xing B.S., 2013. Characterization and influence of biochars on
nitrous oxide emission from agricultural soil. Environ. Pollution, 174, 289-296. https://doi.org/10.1016/j.envpol. 2012.12.003
Whitfield M., 1974. Thermodynamic limitations on the use of the platinum electrode in Eh measurements. Limnology and Oceanography, 19(5), 857-865.
https://doi.org/10.4319/lo.1974.19.5.0857
Yanai Y., Toyota K., and Okazaki M., 2007. Effects of charcoal
addition on N2O emissions from soil resulting from
rewet-ting air-dried soil in short-term laboratory experiments. Soil
Sci. Plant Nutr., 53(2), 181-188.