ARTICLE
Hydrops Fetalis: A Retrospective Review of Cases
Reported to a Large National Database and
Identification of Risk Factors Associated With Death
Matthew E. Abrams, MDa,b, Keith S. Meredith, MDa,b, Paula Kinnard, RNa,b, Reese H. Clark, MDa
aCenter for Research and Education, Pediatrix Medical Group, Sunrise, Florida;bDivision of Neonatology, Phoenix Children’s Hospital, Phoenix, Arizona
The authors have indicated they have no financial relationships relevant to this article to disclose.
ABSTRACT
OBJECTIVES.The objectives were (1) to identify the causes for hydrops fetalis neonates admitted for neonatal intensive care with the diagnosis of hydrops fetalis and (2) to identify the risk factors associated with death.
METHODS.A retrospective review of a large national data set was performed.
RESULTS.There were a total of 253 651 discharges from 162 NICUs in the database; 598 patients were identified with a report of hydrops fetalis. The most common associated diagnoses were congenital heart problems (13.7%), abnormalities in heart rate (10.4%), twin-to-twin transfusion (9%), congenital anomalies (8.7%), chromosomal abnormalities (7.5%), congenital viral infections (6.7%), congenital anemia (5%), and congenital chylothorax (3.2%). Of those 598 neonates, 115 were transferred either to another hospital or to another service, 215 died before discharge, and 267 were discharged from the hospital. One patient did not have a discharge type listed and was not included in the outcome analysis. Mortality rates were highest among neonates with congenital anomalies (57.7%) and lowest among neonates with congenital chylothorax (5.9%). Factors that were associated independently with death in logistic regression analyses were younger gestational age, low 5-minute Apgar score, and need for high levels of support during the first day after birth (higher levels of inspired oxygen support and more often treated with high-frequency ventilation).
CONCLUSIONS.The risk of death among neonates with hydrops fetalis depends on the underlying diagnosis and is highest for those who are born more prematurely and those who are most ill immediately after birth. Information from this large study should prove useful for planning prospective studies and providing prenatal coun-seling to parents with an affected fetus.
www.pediatrics.org/cgi/doi/10.1542/ peds.2006-3680
doi:10.1542/peds.2006-3680
Key Words
neonate, hydrops fetalis, death, lung hypoplasia
Accepted for publication Mar 1, 2007
Address correspondence to Matthew E. Abrams, MD, Pediatrix Medical Group, Phoenix Children’s Hospital, 1919 E Thomas, Building C, Phoenix, AZ 85016. E-mail: matthewabrams@ cox.net
H
YDROPS FETALIS CONTINUESto be a challenging en-tity in neonatal/perinatal medicine.1–7Hydrops, anend-stage process for a number of fetal diseases, results in tissue edema and effusions of multiple body cavities. The cause of hydrops fetalis is multifactorial, and the condition is often associated with high mortality rates, despite improvements in diagnosis and management.1,7,8
Because of early prenatal diagnosis and intervention, Rh disease is now a relatively uncommon cause of hy-drops fetalis in neonates. Underlying mechanisms such as cardiac disease and arrhythmias are relatively straightforward to diagnose, whereas other disorders, such as lysosomal storage diseases, require careful inves-tigation and specialty laboratories.9,10 There are many
case reports in the literature attributing the underlying cause of hydrops to a number of disease entities.2–6,9,11–17
The goals of our study were to understand more com-pletely the underlying etiologic causes of hydrops fetalis in a contemporary data set and to identify the risk factors associated with death.
METHODS
Pediatrix Medical Group health care professionals (doc-tors and nurse practitioners) providing care to neonates admitted for intensive care use a proprietary software system to generate clinical admission, discharge, and daily progress notes. These data are stored in a consoli-dated data set and then deidentified for quality assur-ance, research, and billing purposes. By using the dei-dentified data set, from which several other observations have been reported,18–20 we performed a retrospective
case series review. Cases were identified by searching the diagnosis table in our database for the term hydrops fetalis. We included patients who were discharged be-tween January 1, 1996, and March 1, 2005. Data on fluid accumulation in specific compartments were not reported consistently in the database; therefore, the di-agnosis of hydrops fetalis did not always include a report of fluid in ⬎1 body compartment. We did not capture data on neonates who died in the delivery room and who were not admitted for neonatal intensive care. Clin-ical data on these neonates were recorded during their hospitalizations. Pediatrix Medical Group provides in-tensive care services in 220 hospitals in 32 states and Puerto Rico (10 patients in our sample were from Puerto Rico). The data in the electronic database are used for medical chart documentation, billing, and quality im-provement projects. Clinicians providing care to patients interact with the patients’ data on a daily basis, to gen-erate progress notes and billing information. Each day’s notes are stored with diagnoses made. The local data are consolidated within the Pediatrix Medical Group data warehouse, deidentified, made compliant with Health Insurance Portability and Accountability Act of 1996 regulations, and configured into tables that can be joined and queried for statistical analyses. The Phoenix
Chil-dren’s Hospital institutional review board approved our research with the deidentified data set. Data analysis was performed only within the deidentified database.
Data on estimated gestational age represented the best estimates based on both obstetrical data and neona-tal examination findings. Designation of race was based on the options contained in the database, which were white, black, Hispanic, Native American, and Asian. Az
score was calculated for each patient ([weight of the patient ⫺mean weight of healthy patients of the same gestational age]/SD for the normal population of the same gestational age). A high zscore suggests that the patient’s weight is greater than expected for a neonate of the given degree of gestational maturity. We used this term to estimate the degree of edema.
Our analytical approach to these data was descriptive in nature. Specific database tables within the data ware-house used for this analysis were “patients,” “admis-sions,” and “diagnoses.” All reports of hydrops fetalis collected within the diagnosis table were reviewed. For each patient with a diagnosis of hydrops fetalis, all other associated diagnoses were reviewed for assignment of a potential etiologic cause. In addition, data on causes of death and problems at discharge were reviewed for de-termination of the “primary” etiologic factor associated with hydrops fetalis. Differences in the demographic characteristics of patients who died and those who were discharged home were analyzed by comparing the 2 population samples with univariate analyses. Continu-ous variables (estimated gestational age and birth weight) were evaluated with 2-tailedttests. Categorical variables (eg, race and gender) were evaluated with 2-tailed2tests. Nonparametric data were assessed with
Kruskal-Wallis analysis of variance. After univariate analyses, we used multivariate logistic regression to cal-culate the adjusted odds ratio for death by comparing the neonates who died with those who were discharged home. Transferred patients were not included in this analysis. We incorporated in the logistic regression anal-ysis the variables found in univariate analyses to be different for the treatment groups at a probability of⬍.1. Birth weight and gestational age were entered into the model as continuous variables. Cases with missing val-ues for any of the independent variables were excluded from the analyses. All statistical analyses were per-formed by using JMP 5.0.1a (SAS Institute, Cary, NC).
RESULTS
Study Population
discharge, and 267 (45%) were discharged from the hospital. One patient did not have a discharge type listed and is not included in the outcome analysis. Character-istics of the patients in each outcome group are shown in Table 1.
Diseases Associated With Hydrops Fetalis
Of the 598 patients with a diagnosis of hydrops fetalis, a plausible cause could be found for 441 (73.7%) (Table 2). For 157 (26.3%), a cause could not be determined,
and the degree of evaluation to determine a cause var-ied. Of those 157, 94 (60%) had reports of normal chromosomes, 92 (59%) had negative evaluation results for congenital viral infections, and 97 (61%) had reports of normal cardiac echocardiographic findings; 88 (56%) had reports of all 3. Forty-five (28.6%) of the 157 pa-tients died within 3 days after birth, and 10 (6.4%) were transferred to another hospital atⱕ3 days of age.
For the 441 patients with a cause, the most common diagnoses associated with hydrops fetalis were
congeni-TABLE 1 Demographic, Laboratory, and Treatment Data According to Outcome Group
Variable Died Home Transfer Transfer
Service
All Patients
No. of patients (1 patient missing discharge type) 215 267 91 24 598
Maternal age, median (IQR), y 28 (23–33) 28 (23–33) 28 (23–32) 25.5 (22–30.75) 28 (23–33) Gestational age, median (IQR), wka 31 (28–33)b 34 (32–36) 34 (32–37) 33 (32–35) 33 (30–35)
Birth weight, median (IQR), kga 2.1 (1.4–2.6)b 2.7 (2.0–3.3) 2.9 (2.3–3.6) 2.8 (2.1–3.3) 2.5 (1.8–3.1)
zscore, median (IQR)a 1.53 (0.2–3.1)b 1.34 (0.3–2.3) 1.67 (0.7–2.8) 1.48 (0.9–3.3) 1.44 (0.3–2.7)
1-min Apgar scoreⱕ3,n(%)a 165 (76.7)b 103 (38.6) 44 (48.4) 12 (50) 324 (54.2)
5-min Apgar scoreⱕ3,n(%)a 88 (40.9)b 25 (9.4) 16 (17.6) 1 (4.2) 130 (21.7)
Discharge age, median (IQR), da 1 (0–7)b 34 (18–54) 4 (0–15) 24 (5.5–50.5) 14 (1–38)
Inborn,n(%) 201 (93.5) 240 (89.9) 83 (91.2) 20 (83.3) 545 (91.1)
Cesarean section,n(%) 183 (85.1) 223 (83.5) 80 (87.9) 16 (66.7) 502 (83.9)
Male,n(%) 102 (47.4) 147 (55.1) 46 (50.5) 12 (50) 308 (51.5)
Report of prenatal steroid use,n(%) 107 (49.8) 104 (39) 29 (31.9) 9(37.5) 249 (41.6) Multiples,n(%)
Singleton 185 (86) 230 (86.1) 81 (89) 21 (87.5) 518 (86.6)
Twins 29 (13.5) 32 (12) 10 (11) 3 (12.5) 74 (12.4)
Triplets 1 (0.5) 4 (1.5) 0 0 5 (0.8)
Quadruplets 0 1 (0.4) 0 0 1 (0.2)
Race,n(%)
Asian 6 (2.8) 11 (4.1) 3 (3.3) 0 20 (3.3)
Black 12 (5.6) 31 (11.6) 8 (8.8) 2 (8.3) 53 (8.9)
Hispanic 35 (16.3) 36 (13.5) 18 (19.8) 12 (50) 101 (16.9)
Other 22 (10.2) 24 (9) 7 (7.7) 1 (4.2) 55 (9.2)
White 140 (65.1) 165 (61.8) 55 (60.4) 9 (37.5) 369 (61.7)
Pulmonary hypertension,n(%) 44 (20.5) 44 (16.5) 21 (23.1) 2 (8.3) 111 (18.6)
First complete blood count
n 135 226 79 20 460
White blood cell count, median (IQR), 1000 cells perL 9.8 (6.3–16.3) 11 (7.7–15.6) 10.8 (8.3–15.3) 8.95 (5.6–10.9) 10.5 (7.4–15.6) Hemoglobin level, median (IQR), g/dL 13.7 (9.9–16.1) 14.8 (12–17) 14.9 (12.6–17) 14.3 (12.5–17) 14.5 (11.5–16.8) Hematocrit level, median (IQR), % 42 (33.4–50.1) 44 (36.5–50.3) 45 (37.8–49.4) 43.5 (36.2–50.8) 44 (36–50) Platelet count, median (IQR), 1000 cells perLa 116 (55–191)b 176 (113–242) 164.5 (86–237) 147 (108–182) 158 (87–232)
Reticulocyte count, median (IQR), % 8.25 (1.6–13.2) 6.4 (4.9–9.3) 5.95 (1.6–25.6) 10.95 (8.2–13.7) 7.3 (4.6–11.2) Treatments
Surfactant,n(%) 102 (47.4) 121 (45.3) 35 (38.5) 7 (29.2) 265 (44.3)
Fraction of inspired oxygen on day of birth, median (IQR) 1 (1–1)b 0.805 (0.4–1) 1 (0.68–1) 0.74 (0.26–1) 1 (0.5–1)
Support on day of birth,n(%)
No data 8 (3.7) 6 (2.2) 3 (3.3) 1 (4.2) 18 (3)
Room air 5 (2.3) 14 (5.2) 2 (2.2) 3 (12.5) 24 (4)
Hood oxygen 2 (0.9) 16 (6) 5 (5.5) 1 (4.2) 24 (4)
Nasal cannula 0 9 (3.4) 2 (2.2) 0 11 (1.8)
Continuous positive airway pressure 0 13 (4.9) 4 (4.4) 2 (8.3) 19 (3.2)
Ventilator 78 (36.3) 135 (50.6) 57 (62.6) 8 (33.3) 279 (46.7)
High-frequency ventilation 122 (56.7)b 74 (27.7) 18 (19.8) 9 (37.5) 223 (37.3)
Inhaled nitric oxide 9 (4.2) 10 (3.7) 2 (2.2) 1 (4.2) 22 (3.7)
Dopamine or dobutamine 126 (58.6) 136 (50.9) 43 (47.3) 12 (50) 317 (53)
Receiving oxygen at postmenstrual age of 36 wk or discharge
15 (7) 78 (29.2) 13 (14.3) 5 (20.8) 111 (18.6)
Logistic modeling was performed to compare the characteristics of patients who died and those who were discharged home. IQR indicates interquartile range (25th–75th percentile). aSome patients did not have a report of a complete blood count.
tal heart disease (n⫽82; 13.7%), cardiac arrhythmias (n ⫽62; 10.4%), twin-to-twin transfusion (n⫽54; 9%), congenital anomalies (n⫽52; 8.7%), chromosomal
ab-normalities (n⫽45; 7.5%), congenital viral infections (n ⫽ 40; 6.7%), congenital anemia (n ⫽ 30; 5%), and congenital chylothorax (n⫽19; 3.2%). Five of the
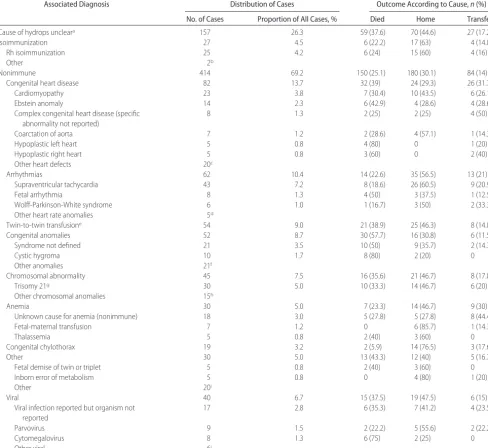
pa-TABLE 2 Causes and Outcomes
Associated Diagnosis Distribution of Cases Outcome According to Cause,n(%)
No. of Cases Proportion of All Cases, % Died Home Transfer
Cause of hydrops uncleara 157 26.3 59 (37.6) 70 (44.6) 27 (17.2)
Isoimmunization 27 4.5 6 (22.2) 17 (63) 4 (14.8)
Rh isoimmunization 25 4.2 6 (24) 15 (60) 4 (16)
Other 2b
Nonimmune 414 69.2 150 (25.1) 180 (30.1) 84 (14)
Congenital heart disease 82 13.7 32 (39) 24 (29.3) 26 (31.7)
Cardiomyopathy 23 3.8 7 (30.4) 10 (43.5) 6 (26.1)
Ebstein anomaly 14 2.3 6 (42.9) 4 (28.6) 4 (28.6)
Complex congenital heart disease (specific abnormality not reported)
8 1.3 2 (25) 2 (25) 4 (50)
Coarctation of aorta 7 1.2 2 (28.6) 4 (57.1) 1 (14.3)
Hypoplastic left heart 5 0.8 4 (80) 0 1 (20)
Hypoplastic right heart 5 0.8 3 (60) 0 2 (40)
Other heart defects 20c
Arrhythmias 62 10.4 14 (22.6) 35 (56.5) 13 (21)
Supraventricular tachycardia 43 7.2 8 (18.6) 26 (60.5) 9 (20.9)
Fetal arrhythmia 8 1.3 4 (50) 3 (37.5) 1 (12.5)
Wolff-Parkinson-White syndrome 6 1.0 1 (16.7) 3 (50) 2 (33.3)
Other heart rate anomalies 5d
Twin-to-twin transfusione 54 9.0 21 (38.9) 25 (46.3) 8 (14.8)
Congenital anomalies 52 8.7 30 (57.7) 16 (30.8) 6 (11.5)
Syndrome not defined 21 3.5 10 (50) 9 (35.7) 2 (14.3)
Cystic hygroma 10 1.7 8 (80) 2 (20) 0
Other anomalies 21f
Chromosomal abnormality 45 7.5 16 (35.6) 21 (46.7) 8 (17.8)
Trisomy 21g 30 5.0 10 (33.3) 14 (46.7) 6 (20)
Other chromosomal anomalies 15h
Anemia 30 5.0 7 (23.3) 14 (46.7) 9 (30)
Unknown cause for anemia (nonimmune) 18 3.0 5 (27.8) 5 (27.8) 8 (44.4)
Fetal-maternal transfusion 7 1.2 0 6 (85.7) 1 (14.3)
Thalassemia 5 0.8 2 (40) 3 (60) 0
Congenital chylothorax 19 3.2 2 (5.9) 14 (76.5) 3 (17.6)
Other 30 5.0 13 (43.3) 12 (40) 5 (16.7)
Fetal demise of twin or triplet 5 0.8 2 (40) 3 (60) 0
Inborn error of metabolism 5 0.8 0 4 (80) 1 (20)
Other 20i
Viral 40 6.7 15 (37.5) 19 (47.5) 6 (15)
Viral infection reported but organism not reported
17 2.8 6 (35.3) 7 (41.2) 4 (23.5)
Parvovirus 9 1.5 2 (22.2) 5 (55.6) 2 (22.2)
Cytomegalovirus 8 1.3 6 (75) 2 (25) 0
Other viral 6j
We limited the data reported to diagnoses with⬎5 patients.
aFor 157 patients (26.3%), a cause could not be determined, and the degree of evaluation to determine a cause varied. Of those, 94 (60%) had reports of normal chromosomes, 92 (59%) had negative evaluation results for congenital viral infections, and 97 (61%) had reports of normal cardiac echocardiographic findings; 88 (56%) had reports of all 3. One patient did not have data on discharge type; 45 patients died within 3 days after birth, and 10 were transferred to another hospital atⱕ3 days of age (55 of 157 patients; 35%).
bDuffy antibodies,n⫽1; Kell antibodies,n⫽1.
cCongenital cardiomegaly,n⫽4; aortic valve stenosis,n⫽3; atrioventricular canal,n⫽3; pulmonary valve atresia,n⫽2; pulmonary valve stenosis,n⫽2; truncus arteriosus,n⫽2; double-outlet right ventricle,n⫽1; right ventricular hypertrophy,n⫽1; single ventricle,n⫽1; total anomalous pulmonary venous return,n⫽1.
dAtrial flutter,n⫽4; heart block,n⫽1.
eFive of the patients with hydrops fetalis and a history of twin-to-twin transfusion had reports of fetal siblings who died in utero.
fTeratoma,n⫽4; brain abnormality/hypotonia,n⫽4; dwarf,n⫽3; hydrocephalus,n⫽3; diaphragmatic hernia,n⫽2; anencephaly,n⫽1; cerebral venous fistula,n⫽1; cystic adenomatoid malformation,n⫽1; meningomyelocele,n⫽1; vascular ring,n⫽1.
gSome patients with trisomy 21 had additional anomalies reported (atrioventricular canal,n⫽6; congenital anemia,n⫽2; congenital leukemia,n⫽2; arrhythmia,n⫽1; cystic hygroma,n⫽1). hSpecific abnormality not reported,n⫽6; Turner syndrome/cystic hygroma,n⫽3; trisomy 18,n⫽2; chromosome 13 deletion,n⫽1; XYdel(13)(q14q22),n⫽1; Klinefelter syndrome,n⫽1; ring 18 chromosome,n⫽1.
tients with hydrops fetalis and a history of twin-to-twin transfusion had reports of fetal siblings who died in utero.
Risk Factors Associated With Death
Univariate analyses showed that, compared with neo-nates who were discharged from the hospital, neoneo-nates who died were smaller and more immature (but had higherzscores, which suggests more edema) (Table 1). In addition, neonates who died were sicker in the period immediately after delivery (lower Apgar scores, higher levels of inspired oxygen, and more often treated with high-frequency ventilation) and had lower platelet counts (Table 1). The mortality rate was highest among neonates with congenital anomalies (mortality rate: 57.7%) and lowest among neonates with congenital chylothorax (mortality rate: 5.9%). Factors that were associated independently with death in logistic regres-sion analyses were younger gestational age, low 5-minute Apgar score, and need for high levels of sup-port during the first day after birth (higher levels of inspired oxygen support and more often treated with high-frequency ventilation).
DISCUSSION
Using a national data set, we have described the largest review of underlying causes of hydrops fetalis and iden-tified risk factors for death. The most common diagnoses associated with hydrops fetalis were congenital heart problems, abnormalities in heart rate, twin-to-twin transfusion, congenital anomalies, chromosomal abnor-malities, congenital viral infections, congenital anemia, and congenital chylothorax. Most previous studies had sample sizes of ⬍100 patients, and only 2 of them attempted to identify risk factors associated with death.1,21–24Similar to results reported by previous
inves-tigators,1,22we noted that the prognosis for hydrops
fe-talis depended on the cause. In our data, the mortality rate was highest among neonates with congenital anom-alies (mortality rate: 57.7%) and lowest among neonates with congenital chylothorax (mortality rate: 5.9%). In-fants who died were more likely to be more premature and were sicker after birth (lower 5-minute Apgar scores, higher levels of inspired oxygen support, and more often treated with high-frequency ventilation dur-ing the first day after birth).
There are some limitations of a retrospective review of any data set that is accumulated as part of medical chart documentation. Retrospective studies are limited by incomplete data. Test or autopsy results that returned after infants died, were transferred, or were discharged might not have been entered into the database. In addi-tion, early prenatal diagnosis may lead to early termina-tion of the pregnancy. We did not obtain informatermina-tion regarding the timing and severity of prenatal
presenta-tion or intervenpresenta-tions. Has et al25reviewed 30 cases with
hydrops fetalis diagnosed between 10 and 14 weeks of pregnancy. All pregnancies with nonimmune hydrops resulted in abortion, intrauterine fetal death, or termi-nation of the pregnancy. Therefore, it is possible that more infants had a diagnosis than our analyses showed, and mortality rates among neonates with hydrops fetalis might be higher if we considered perinatal death.
We caution against using our data to make decisions regarding the timing of delivery. Immaturity (young gestational age at delivery) was an important risk factor associated with death. Delivering fetuses early to treat worsening hydrops may not improve survival rates. The diagnosis and management of hydrops fetalis continue to be challenges for perinatologists and neonatologists. Mortality rates are high, and treatment options are lim-ited. The data on the outcomes for neonates who have hydrops fetalis and are admitted for neonatal intensive care should be helpful for planning prospective studies of specific interventions and for counseling parents.
REFERENCES
1. Huang HR, Tsay PK, Chiang MC, Lien R, Chou YH. Prognostic factors and clinical features in liveborn neonates with hydrops fetalis.Am J Perinatol.2007;24:33–38
2. Czernik C, Stiller B, Hubler M, Hagen A, Henrich W. Hydrops fetalis caused by a large intrapericardial teratoma.Ultrasound Obstet Gynecol.2006;28:973–976
3. Kadrofske M, Parimi P, Myers M, Kumar ML, Abughali N. Nonimmune hydrops fetalis and hepatitis in a neonate with congenital human immunodeficiency virus infection. Pediatr Infect Dis J.2006;25:952–954
4. Khositseth A, Samankatiwat P, Withurawanit W, Khowsathit P. Pacing in preterm with hydrops fetalis due to congenital complete heart block. Asian Cardiovasc Thorac Ann. 2006;14: 428 – 431
5. Proust S, Philippe HJ, Paumier A, Joubert M, Boog G, Winer N. Mirror pre-eclampsia: Ballantyne’s syndrome—two cases [in French].J Gynecol Obstet Biol Reprod (Paris).2006;35:270 –274 6. Kollamparambil TG, Jani BR, Aldouri M, Soe A, Ducker DA.
Anti-C(w) alloimmunization presenting as hydrops fetalis.Acta Paediatr.2005;94:499 –501
7. Favre R, Dreux S, Dommergues M, et al. Nonimmune fetal ascites: a series of 79 cases. Am J Obstet Gynecol.2004;190: 407– 412
8. Bukowski R, Saade GR. Hydrops fetalis.Clin Perinatol.2000;27: 1007–1031
9. Trainor B, Tubman R. The emerging pattern of hydrops fetalis: incidence, aetiology and management.Ulster Med J.2006;75: 185–186
10. Burin MG, Scholz AP, Gus R, et al. Investigation of lysosomal storage diseases in nonimmune hydrops fetalis.Prenat Diagn.
2004;24:653– 657
11. Bellini C, Boccardo F, Campisi C, Bonioli E. Congenital pul-monary lymphangiectasia.Orphanet J Rare Dis.2006;1:43 12. Kondo T, Kitazawa R, Noda-Maeda N, Kitazawa S. Fetal
hy-drops associated with spontaneous premature closure of ductus arteriosus.Pathol Int.2006;56:554 –557
sacrococcygeal teratoma by magnetic resonance imaging.Magn Reson Imaging.2006;24:977–978
14. Sueters M, Middeldorp JM, Lopriore E, et al. Timely diagnosis of twin-to-twin transfusion syndrome in monochorionic twin pregnancies by biweekly sonography combined with patient instruction to report onset of symptoms.Ultrasound Obstet Gy-necol.2006;28:659 – 664
15. Velaphi S, Reubenson G, Nakwa F. Meconium peritonitis and parvovirus B19 infection associated with hydrops fetalis.Ann Trop Paediatr.2006;26:371–375
16. Arnon S, Tamary H, Dgany O, et al. Hydrops fetalis associated with homozygosity for hemoglobin Taybe (␣38/39 THR dele-tion) in newborn triplets.Am J Hematol.2004;76:263–266 17. Baena N, De Vigan C, Cariati E, et al. Turner syndrome:
eval-uation of prenatal diagnosis in 19 European registries.Am J Med Genet.2004;129A:16 –20
18. Clark RH, Bloom BT, Spitzer AR, Gerstmann DR. Reported medication use in the neonatal intensive care unit: data from a large national data set.Pediatrics.2006;117:1979 –1987 19. Garges HP, Moody MA, Cotten CM, et al. Neonatal meningitis:
what is the correlation among cerebrospinal fluid cultures,
blood cultures, and cerebrospinal fluid parameters?Pediatrics.
2006;117:1094 –1100
20. Garite TJ, Clark R, Thorp JA. Intrauterine growth restriction increases morbidity and mortality among premature neonates.
Am J Obstet Gynecol.2004;191:481– 487
21. Lallemand AV, Doco-Fenzy M, Gaillard DA. Investigation of nonimmune hydrops fetalis: multidisciplinary studies are nec-essary for diagnosis: review of 94 cases. Pediatr Dev Pathol.
1999;2:432– 439
22. Ismail KM, Martin WL, Ghosh S, Whittle MJ, Kilby MD. Eti-ology and outcome of hydrops fetalis. J Matern Fetal Med.
2001;10:175–181
23. Mascaretti RS, Falcao MC, Silva AM, Vaz FA, Leone CR. Char-acterization of newborns with nonimmune hydrops fetalis ad-mitted to a neonatal intensive care unit.Rev Hosp Clin Fac Med Sao Paulo.2003;58:125–132
24. Wy CA, Sajous CH, Loberiza F, Weiss MG. Outcome of infants with a diagnosis of hydrops fetalis in the 1990s.Am J Perinatol.
1999;16:561–567
25. Has R. Non-immune hydrops fetalis in the first trimester: a review of 30 cases.Clin Exp Obstet Gynecol.2001;28:187–190
COOLING CAP IS APPROVED
“A head-cooling cap that can prevent or reduce brain damage in infants starved of oxygen at birth won federal approval. The Food and Drug Admin-istration said the device, called the Cool-Cap, could cut rates of death and disability among the estimated 5000 to 9000 such children born each year. The device, made by the Olympic Medical Corporation of Seattle, works by maintaining a flow of chilled water through a cap placed on a newborn’s head.”
DOI: 10.1542/peds.2006-3680
2007;120;84
Pediatrics
Matthew E. Abrams, Keith S. Meredith, Paula Kinnard and Reese H. Clark
Database and Identification of Risk Factors Associated With Death
Hydrops Fetalis: A Retrospective Review of Cases Reported to a Large National
Services
Updated Information &
http://pediatrics.aappublications.org/content/120/1/84 including high resolution figures, can be found at:
References
http://pediatrics.aappublications.org/content/120/1/84#BIBL This article cites 25 articles, 2 of which you can access for free at:
Subspecialty Collections
http://www.aappublications.org/cgi/collection/genetics_sub Genetics
sub
http://www.aappublications.org/cgi/collection/fetus:newborn_infant_ Fetus/Newborn Infant
following collection(s):
This article, along with others on similar topics, appears in the
Permissions & Licensing
http://www.aappublications.org/site/misc/Permissions.xhtml in its entirety can be found online at:
Information about reproducing this article in parts (figures, tables) or
Reprints
DOI: 10.1542/peds.2006-3680
2007;120;84
Pediatrics
Matthew E. Abrams, Keith S. Meredith, Paula Kinnard and Reese H. Clark
Database and Identification of Risk Factors Associated With Death
Hydrops Fetalis: A Retrospective Review of Cases Reported to a Large National
http://pediatrics.aappublications.org/content/120/1/84
located on the World Wide Web at:
The online version of this article, along with updated information and services, is
by the American Academy of Pediatrics. All rights reserved. Print ISSN: 1073-0397.