Influenza-Related Hospitalization in Children
WHAT’S KNOWN ON THIS SUBJECT: In Canada, children 6 months to 9 years old were recommended to receive 2 half-adult (0.25 mL) doses of AS03-adjuvanted pandemic influenza A/H1N1 vaccine. A single dose was highly immunogenic and protective against infection in children, but protection against
hospitalization is unknown.
WHAT THIS STUDY ADDS: Results indicate that a single half-adult dose of AS03-adjuvanted pandemic influenza A/H1N1 vaccine is highly protective against hospitalization in children aged 6 months to 9 years. Protection was substantial 10 days after a single dose (additional marginal increase at 14 days).
abstract
+OBJECTIVE:Young children are generally considered immunologically naive with respect to influenza exposure opportunities; thus, a 2-dose schedule is recommended when a child is first immunized with conven-tional influenza vaccine lacking adjuvant. We estimated the effectiveness of a single pediatric dose of AS03-adjuvanted vaccine against hospitalization for confirmed pandemic influenza A/H1N1 (pH1N1) infection in children aged 6 months to 9 years during the fall 2009 vaccination campaign.
METHODS:In a matched case-control design, case subjects were children hospitalized for pH1N1 infection in the Fall of 2009, in Quebec, Canada. Controls were nonhospitalized children, matched by age and region of residence. Vaccination status in case subjects and controls was ascer-tained in relation to the case subject’s date of illness onset. Vaccine effec-tiveness was estimated through conditional logistic regression.
RESULTS:The overall effectiveness of a single pediatric dose of vac-cine administeredⱖ14 days before illness onset was 85% (95% confi-dence interval [CI]: 61% to 94%), varying according to age category but with wide and overlapping CIs: 92% (95% CI: 51% to 99%) in 6 –23 month-old children, 89% (95% CI: 34% to 98%) in 2– 4 year-olds, and 79% (95% CI:⫺31% to 96%) in 5–9 year-olds. Overall vaccine effective-ness for immunization ⱖ10 days before illness onset was slightly lower at 80% (95% CI: 60% to 90%), with similar variation according to age.
CONCLUSION:In children aged 6 months to 9 years, a single pediatric dose of the AS03-adjuvanted pH1N1 vaccine was highly protective against hospitalization beginning at 10 and 14 days after vaccination.
Pediatrics2011;128:e1084–e1091
AUTHORS:Rodica Gilca, MD, PhD,a,b,cGeneviève Deceuninck, MD, MSc,bGaston De Serres, MD, PhD,a,b,c Nicole Boulianne, MSc,a,bChantal Sauvageau, MD, MSc, FRCPC,a,b,cCaroline Quach, MD, MSc, FRCPC,dFrançois D. Boucher, MD, FRCPC,e,fand Danuta M. Skowronski, MD, MHScg,h
aInstitut National de Santé Publique du Québec, Quebec City,
Quebec, Canada;bPublic Health Research Unit, Quebec University
Hospital Centre, Quebec City, Quebec, Canada;cDepartment of
Social and Preventive Medicine, Faculty of Medicine, Laval University, Quebec City, Quebec, Canada;dInfectious Diseases
Division, Department of Pediatrics, The Montreal Children’s Hospital, McGill University, Montreal, Quebec, Canada;
eDepartment of Paediatrics, Quebec University Hospital Centre,
Quebec City, Quebec, Canada;fDepartment of Pediatrics, Faculty
of Medicine, Laval University, Quebec City, Quebec, Canada;
gEpidemiology Services, British Columbia BC Centre for Disease
Control, Vancouver, British Columbia, Canada; andhSchool of
Population and Public Health, University of British Columbia, Vancouver, British Columbia, Canada
KEY WORDS
pandemic influenza, influenza vaccine, effectiveness, children, hospitalization
ABBREVIATIONS
pH1N1—pandemic influenza A/H1N1
CDC—Centers for Disease Control and Prevention RT-PCR—reverse-transcription polymerase chain reaction ILI—influenza-like illness
VE—vaccine effectiveness CI—confidence interval
TIV—trivalent inactivated influenza vaccine
Drs Gilca, Deceuninck, De Serres, Skowronski, and Sauvageau and Ms Boulianne made substantial contributions to conception and design; Drs Gilca and De Serres and Ms Boulianne performed acquisition of the data; Drs Deceuninck and Gilca conducted data analysis; all authors performed interpretation of the data; and Dr Gilca drafted the article. All authors revised the manuscript critically for important intellectual content and approved the final version of the manuscript.
www.pediatrics.org/cgi/doi/10.1542/peds.2010-3492
doi:10.1542/peds.2010-3492
Accepted for publication Jul 12, 2011
Address correspondence to Rodica Gilca, MD, PhD, Institut National de Santé Publique du Québec, 2400 d’Estimauville, Quebec, Quebec, Canada G1E 7G9. E-mail: rodica.gilca@ssss. gouv.qc.ca
PEDIATRICS (ISSN Numbers: Print, 0031-4005; Online, 1098-4275).
Copyright © 2011 by the American Academy of Pediatrics
FINANCIAL DISCLOSURE:Dr Gilca has received research
funding from the Quebec Ministry of Health for this study and research funding from Pfizer for unrelated study; Dr De Serres has received research grants from Sanofi Pasteur and
As with previous influenza pandemics, the 2009 –2010 pandemic of influenza A/H1N1 (pH1N1) showed a more youth-ful epidemiologic profile compared with seasonal strains. Children experi-enced higher attack rates than adults; those younger than 10 years, espe-cially the very young, were more fre-quently admitted to hospital.
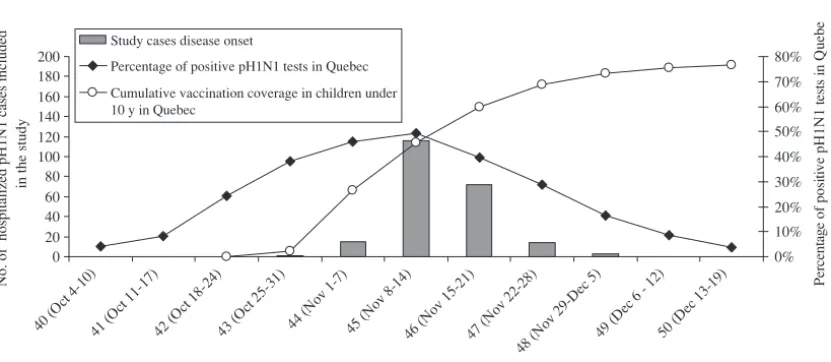
In the province of Quebec (population 7.8 million), the second wave of the 2009 pandemic began the week of Oc-tober 4 (Centers for Disease Control
and Prevention [CDC] week 40),
peaked the week of November 12 (week 45), and was essentially ended by December 19 (week 50) (Fig 1). Vac-cination of children aged 6 months to 4 years started November 2 (week 44) along with that of patients aged 6 months to 64 years with chronic med-ical conditions, and the families of in-fants younger than 6 months or with immunodeficiency. Children 5 to 19 years old without chronic medical con-ditions were vaccinated beginning No-vember 12 (week 45); vaccination then proceeded to all persons aged 65 years and older and to the rest of the population. By the end of CDC weeks 44, 46, and 50, the vaccine coverage in children younger than 10 years
in-creased from 27% to 60% and 77%, respectively.
In contrast to the United States, where adjuvanted vaccine was not available during the pandemic, in Canada, a monovalent AS03-adjuvanted pH1N1 vaccine was selected as the primary formulation and constituted⬃95% of all pandemic vaccine doses adminis-tered.1 Arepanrix (GlaxoSmithKline, Sainte-Foy, Quebec, Canada) is an inac-tivated, split-virion vaccine consisting of a pH1N1 immunizing antigen (as a suspension) and an AS03 adjuvant (as an oil in water emulsion comprising DL-␣-tocopherol, squalene, and poly-sorbate 80).2Pediatric trials with the AS03-adjuvanted vaccine produced in Quebec (n⫽60)3and in Dresden, Ger-many (n⫽ 101)4 demonstrated sub-stantial vaccine-induced antibody re-sponse 21 days after single-dose immunization (0.25 mL containing 1.9
g of hemagglutinin) and half the adult dose of adjuvant, with seropro-tection and seroconversion rates each exceeding 90% among immu-nized participants younger than 10 years.
On the basis of these immunogenicity findings, and balancing also the higher
rate of adverse reactions observed af-ter the second dose of vaccine as well as the limited availability of even a sin-gle dose for all target groups in the initial stages of the immunization cam-paign, a single pediatric dose of the adjuvanted pH1N1 vaccine was recom-mended for children aged 6 months to 9 years in Quebec.5To evaluate this pol-icy recommendation, provincial au-thorities mandated an epidemiologic investigation of the effectiveness of a single dose of adjuvanted pH1N1 vac-cine in preventing hospitalization in children.
METHODS
Participants
Laboratory-confirmed pH1N1 hospital-ization (ⱖ24 hours’ duration) was a notifiable condition during the pan-demic in Quebec. All hospitalized pa-tients with suspected pH1N1 infection were tested for influenza by reverse-transcription polymerase chain reac-tion (RT-PCR), and results were re-corded in the provincial registry. In this case-control study, Quebec resi-dents aged 6 months to 9 years hospi-talized with laboratory-confirmed pH1N1 infection between November 12
0 20 40 60 80 100 120 140 160 180 200
40 (O ct
4-10)
41 (O ct 11
-17)
42 (O ct 18
-24)
43 (Oc t 25-3
1)
44 (No v 1-7
)
45 (N ov 8
-14)
46 (N ov
15-21)
47 (N ov 2
2-28 )
48 (N ov 2
9-D ec 5)
49 (D ec 6
- 12)
50 (D ec 13
-19)
CDC weeks
No. of hospitalized pH1N1 cases included
in the study
0% 10% 20% 30% 40% 50% 60% 70% 80% Per ce n ta g e of pos it ive pH 1N 1 t es ts i n Q u eb e an d c u mu la tiv e p er ce n tage of vacci nat ed ch il dren
Study cases disease onset
Percentage of positive pH1N1 tests in Quebec
Cumulative vaccination coverage in children under 10 y in Quebec
FIGURE 1
pH1N1 vaccination coverage in children younger than 10 years in Quebec, positive pH1N1 tests, and number of study cases (children hospitalized for pH1N1) according to CDC week.
Quebec) were eligible as case sub-jects. Those living in the 2 northern-most regions were excluded for prac-tical considerations. Identified case subjects had been hospitalized in 42 facilities (33% in 4 pediatric hospitals). Controls were selected from the pro-vincial health insurance registry, which includes all citizens and immi-grants residing in Quebec. Controls were matched to their case subject by date of birth (⫾3 months for children 6 –23 months and⫾6 months for older children) and by 1 of the 16 administra-tive areas of residence. For each case, 15 potential controls were selected at random from the health insurance
registry. Children who had a
laboratory-confirmed pH1N1 infection before the start of the vaccination campaign, or before the date of vacci-nation of their matched case, were ex-cluded as potential controls.
Trained interviewers administered a standardized telephone questionnaire to parents of case subjects and trols, after receiving their verbal con-sent, between March 25 and June 3, 2010. Up to 5 attempts were made to reach potential study participants, at different times of the day and week, including weekends, until 3 matched controls for each case were reached. Data collected included demographic characteristics, pH1N1 and seasonal 2008 –2009 influenza vaccination status, any influenza-like illness (ILI) whether medically attended or not and defined as fever and cough or sore throat during the first and the second pandemic wave (for case subjects defined as ILI before onset of the pH1N1 infection for which they were admitted), presence of chronic underlying medical conditions as defined by the National Advisory Com-mittee on Immunization,6and other cova-riates as listed in Table 1. The type of
pandemic vaccine and date of vaccina-tion were validated through linkage to the provincial electronic pandemic vaccination registry. The absence of confirmed pH1N1 infection in controls was verified using the provincial regis-try of all confirmed pH1N1 case sub-jects. Among the 4680 potential con-trols provided by the health insurance board, 31 (0.66%) had laboratory-confirmed pH1N1 infection.
This study was conducted as a legally
mandated emergency public health in-vestigation and research ethics board approval was therefore not required.
Vaccination Status
Vaccination status was defined for each hospitalized case subject and corre-sponding 3 matched controls in relation to the date of illness onset of the case (reference date). For the primary analy-sis, a child was considered immunized if the pH1N1 vaccine had been givenⱖ14
(N⫽221), % (N⫽663), % Age
Mean (median), mo 40.6 (33.2) 39.3 (31.6)
6–23 mo 33.9 33.9 NA
2–4 y 39.8 39.8
5–9 y 26.2 26.2
Male gender 56.6 52.3 .27
Symptoms onset
CDC week 43 0.5 NA NA
CDC week 44 6.8
CDC week 45 52.5
CDC week 46 32.6
CDC week 47 6.3
CDC week 48 1.4
Preexisting underlying conditions 24.0 5.0 ⬍.001
ILIb
During wave 1 5.9 2.3 .01
During wave 2 4.1 11.6 .002
Seasonal 2008–2009 influenza vaccine
Received seasonal 2008–2009 influenza vaccine 37.6 22.3 ⬍.001
Not applicable (ⱕ6 mo in January 2009) 17.2 16.7
By underlying conditions
Among children with underlying conditions (53 cases, 33 controls)
54.7 36.4 .9985
Among children without underlying conditions (168 cases, 630 controls)
32.1 21.6 .003
Child care 61.5 64.7 .20
No. of family members
ⱕ3 28.1 39.2
4 50.2 43.6 .05
ⱖ5 21.7 17.0
No. of siblings⬍5 y in family
0 7.2 7.8
1 38.9 51.7 .001
ⱖ2 53.8 40.3
Smoking in the house 11.8 11.8 .9999
Mother education
High school 29.0 26.5
Nonuniversity certificate/diploma 33.5 34.4 .47
University 34.4 37.6
NA indicates not available.
aPvalues were computed by conditional logistic regression, which takes into account the matching between cases and
controls.
days before the reference date; for the secondary analysis, the interval was set at 10 days. Children were classified as unvaccinated if they had received no pH1N1 vaccination before the reference date. Category indicator variables were created inclusive of children who re-ceived the vaccine 0 to 13 days (0 –9 days in the secondary analysis) before the ref-erence date.
Statistical Analysis
Analyses were based on conditional lo-gistic regression. Vaccine effective-ness (VE) was defined as 1-odds ratio for hospitalization with laboratory-confirmed pH1N1 illness among vacci-nated compared with unvaccivacci-nated subjects. A model containing factors that were associated with hospitaliza-tion risk with aPⱕ.05 in univariate conditional logistic regression and po-tential confounders that modified the point estimate for VE by at least 5% was developed by excluding variables in a stepwise process. Analysis was performed for all age groups and stratified for children aged 6 to 23 months, 2 to 4 years, and 5 to 9 years. Analyses were performed with SAS 9.1 (SAS Institute, Inc, Cary, NC). APvalue of⬍.05 was considered significant.
RESULTS
Participation
Between November 12 and December 19, 2009, a total of 284 pH1N1 hospital-ized case subjects 6 months to 9 years old were identified. Telephone num-bers were available for 278 (99%) but 244 (88%) of them were reached. Of these, 23 were excluded: 8 were living in the 2 northernmost regions; 4 were younger than 6 months at the start of the vaccination campaign; 1 received 2 vac-cine doses; 1 refused participation; 1 was not able to speak English or French; 4 were hospitalized for other reasons with coincident pH1N1 detection; and 4 had symptom onset ⬎14 days before
hospitalization. The final sample in-cluded 221 hospitalized case subjects.
Telephone numbers were available for 78% (n ⫽ 798) of the potential con-trols, but 34 telephone numbers were incorrect and 61 never responded. Among the 704 (88%) potential con-trols reached, 41 were excluded: 26 (4%) refused to participate; 1 had been hospitalized for pH1N1; 1 had a con-firmed pH1N1 infection before the date of vaccination of the matched case subject, 1 had received 2 doses of vac-cine; 12 were excluded because of ex-clusion of the matched case subject. The final sample included 663 age- and region-matched controls.
Baseline Characteristics and Covariates
The characteristics of the hospitalized case subjects and their community controls are shown in Table 1 ( Supple-mental Table 4 for data according to age group). For nearly 60% of the case subjects, symptom onset occurred be-fore CDC week 46; that is,⬍2 weeks
after the start of the vaccination cam-paign (52% in CDC week 45 alone).
pH1N1 Vaccination Status
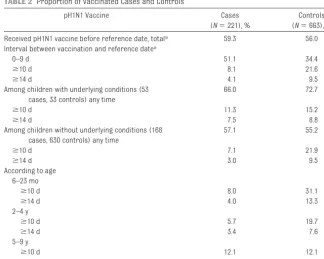
When no reference was made to the date of onset of illness, 62% of case subjects and 82% of controls had re-ceived the pandemic vaccine. In accor-dance with the prioritization schedule, for both case subjects and controls, vaccination was received earlier in children with underlying conditions rather than without underlying condi-tions and in younger (aged 6 –23 months and 2– 4 years) rather than older children (aged 5–9 years) ( Sup-plemental Fig 3). The vaccine was re-ceived before the reference date, by 59.3% of case subjects and 56.0% of controls. Vaccination within 10 days of the reference date was more frequent for case subjects than controls (51.1% and 34.4%), whereas the reverse was seen for intervals ofⱖ10 days (8.1% and 21.6%) or ⱖ14 days (4.1% and 9.5%) (Table 2). A protective effect of
TABLE 2 Proportion of Vaccinated Cases and Controls
pH1N1 Vaccine Cases
(N⫽221), %
Controls (N⫽663), %
Received pH1N1 vaccine before reference date, totala 59.3 56.0
Interval between vaccination and reference datea
0–9 d 51.1 34.4
ⱖ10 d 8.1 21.6
ⱖ14 d 4.1 9.5
Among children with underlying conditions (53 cases, 33 controls) any time
66.0 72.7
ⱖ10 d 11.3 15.2
ⱖ14 d 7.5 8.8
Among children without underlying conditions (168 cases, 630 controls) any time
57.1 55.2
ⱖ10 d 7.1 21.9
ⱖ14 d 3.0 9.5
According to age 6–23 mo
ⱖ10 d 8.0 31.1
ⱖ14 d 4.0 13.3
2–4 y
ⱖ10 d 5.7 19.7
ⱖ14 d 3.4 7.6
5–9 y
ⱖ10 d 12.1 12.1
ⱖ14 d 5.2 7.5
aThe vaccination status for matched controls was determined depending on the date of reference (corresponding to
disease onset of their case).
vaccination was observed starting at day 9 after vaccination (Fig 2).
pH1N1 VE Estimation
Because 60% of the cases occurred ⬍2 weeks after the beginning of the immunization campaign targeting chil-dren, a large number of the 221 sets were concordant (almost exclusively because children were all unvaccinat-ed: among the 175 concordant sets, 99% were unvaccinated, 1% were vac-cinated). In conditional analysis, only case-control sets discordant for the exposure of interest (vaccination) con-tribute to computations.7For vaccina-tionⱖ14 days before the date of
refer-ence, 46 of the 221 sets were
discordant; for an intervalⱖ10 days, 91 sets were discordant ( Supplemen-tal Table 5).
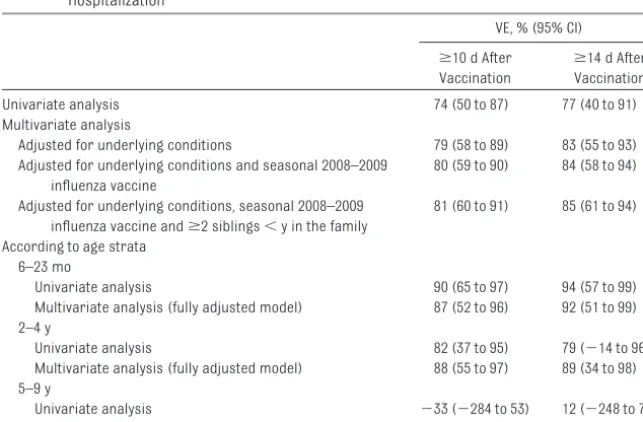
Unadjusted overall pH1N1 VE in pre-venting hospitalization when given at least 14 days before illness onset was 77% (95% confidence interval [CI]: 40% to 91%) (Table 3). Adjusting for under-lying medical conditions, seasonal 2008 –2009 trivalent inactivated influ-enza vaccine (TIV) receipt, and pres-ence ofⱖ2 siblings aged 5 years and younger in the family increased the VE to 85% (95% CI: 61% to 94%). Point es-timates for VE according to age strata were similar, with widely overlapping CIs (Table 3).
Fully adjusted pH1N1 VE in preventing hospitalization when given at least 10
days before illness onset was 81% (95% CI: 60% to 91%).
In different sensitivity analyses (re-stricted to children who did not report ILI; to children without underlying med-ical conditions; excluding children who received the vaccine within 14 [or 10] days of illness onset in the case, or considering them as unvaccinated) (Supplemental Table 6), overall VE estimates remained essentially the same.
There was an association between pre-vious 2008 –2009 TIV receipt and pH1N1 risk. The odds ratio for 2008 –2009 TIV effect on risk of confirmed pH1N1 hos-pitalization in the fully adjusted model
was 2.18 (95% CI: 1.45 to 3.27). When restricted to participants who did not receive pH1N1 vaccine and adjusted for the presence of at least 1 underly-ing medical condition and presence of
ⱖ2 siblings aged 5 years or younger in the family, the odds ratio was 3.88 (95% CI: 1.57 to 9.60). This pattern was maintained when restricted to chil-dren without an underlying medical condition (72 case subjects/283 con-trols): odds ratio⫽6.0 (95% CI: 2.00 to 18.2).
DISCUSSION
To our knowledge, this is the first study to estimate the effectiveness of a
sin--100% -50% 0%
0-1 2 3 4 5 6 7 8 9 10 11 12 13 ≥14
Interval, d
Vacci
n
e ef
fect
iven
FIGURE 2
Exploratory analysis of pH1N1 VE (adjusted for presence of at least 1 underlying medical condition, seasonal 2008 –2009 influenza vaccine receipt, and presence ofⱖ2 siblings agedⱕ5 years in the family) per interval (in days) between vaccination and onset of disease. The vaccination status for matched controls was determined depending on the date of reference (corresponding to disease onset of their case) for each day before the date of reference.
TABLE 3 Univariate and Multivariate Conditional Logistic Regression Analysis of pH1N1 VE Against Hospitalization
VE, % (95% CI)
ⱖ10 d After Vaccination
ⱖ14 d After Vaccination
Univariate analysis 74 (50 to 87) 77 (40 to 91)
Multivariate analysis
Adjusted for underlying conditions 79 (58 to 89) 83 (55 to 93)
Adjusted for underlying conditions and seasonal 2008–2009 influenza vaccine
80 (59 to 90) 84 (58 to 94)
Adjusted for underlying conditions, seasonal 2008–2009 influenza vaccine andⱖ2 siblings⬍y in the family
81 (60 to 91) 85 (61 to 94)
According to age strata 6–23 mo
Univariate analysis 90 (65 to 97) 94 (57 to 99)
Multivariate analysis (fully adjusted model) 87 (52 to 96) 92 (51 to 99) 2–4 y
Univariate analysis 82 (37 to 95) 79 (⫺14 to 96)
Multivariate analysis (fully adjusted model) 88 (55 to 97) 89 (34 to 98) 5–9 y
Univariate analysis ⫺33 (⫺284 to 53) 12 (⫺248 to 78)
gle pediatric dose of adjuvanted pH1N1 vaccine in protecting children aged 6 months to 9 years from confirmed pH1N1 hospitalization. As early as 10 days after immunization, we observed that a single dose conferred an effec-tiveness of 81%, increasing to 85% 14 days after vaccination. These esti-mates of excellent vaccine protection with a single dose of adjuvanted vac-cine in children are consistent with im-munogenicity findings showing sero-protection and seroconversion rates exceeding 90% in children belonging to the same age span.4,8Others have also consistently demonstrated that the ad-juvanted vaccine, although more reac-togenic, was more immunogenic than nonadjuvanted pH1N1 vaccine in chil-dren and adults.9,10 Our VE estimates are slightly lower than the 95.7% and 100% VE against medically attended,
laboratory-confirmed pH1N1
esti-mated for a single pediatric dose of the AS03-adjuvanted pH1N1 vaccine at 10 and 14 days, respectively, after
im-munization from a small
case-control study (n⫽28) in Canada of 28 laboratory-confirmed pediatric cases of pH1N1 (5 hospitalized),11 and 100% VE of an AS03-adjuvanted pH1N1 vaccine against medically at-tended, laboratory-confirmed pH1N1 at 14 days after immunization in chil-dren younger than 15 years (n ⫽
2182) found in the United Kingdom.12 Our results slightly exceed the 77% VE measured against laboratory-confirmed pH1N1 beginning at ⱖ14 days in children younger than 10 years (n ⫽ 502) with underlying
conditions who received
AS03-adjuvanted pH1N1 vaccine found in a case-control study in the United King-dom.13All these studies used a test-negative case-control design. In other studies not restricted to chil-dren, vaccine protection conferred by adjuvanted vaccine was also very high: published estimates of VE for the AS03-adjuvanted pH1N1 vaccine
in adults or all age patients range from 72% to 97%,13,14and is lower in those with underlying conditions (62%).13
We also observed an increased risk of pH1N1 associated with previous 2008 – 2009 TIV receipt that was more evident when we restricted the analysis to par-ticipants who did not receive the pH1N1 vaccine and to those without chronic conditions. This finding is sim-ilar to what was reported in Canada during the first wave.15,16A study con-ducted by the national influenza senti-nel network during the second wave in ambulatory patients did not see this effect in overall analysis, although it was observed in the pediatric popula-tion.17A case-control study in England during the second wave also found an increased risk of medically attended pH1N1 illness in patients with underly-ing conditions who received the 2009 – 2010 TIV.13 A randomized placebo-controlled trial (using a vaccine different from that primarily used in Canada) in school-aged children in Hong Kong during their first wave also showed a doubling of the risk of pH1N1 infection in vaccinated children com-pared with unvaccinated children.18,19 Decreased immunogenicity after an alum-adjuvanted H5N1 vaccine and af-ter a split-virus nonadjuvanted pH1N1 vaccine has been reported in children who previously received TIV.20,21The im-munologic mechanism underlying this unexpected finding is unknown; an ex-tensive discussion of hypotheses may be found elsewhere.15,19 Although the confirmation of this phenomenon may not have short-term public health im-plications, it could profoundly affect our understanding of the immunopa-thology of influenza and is thus worthy of additional investigation. Similarly, the unexpected but provocative finding of increased pH1N1 risk in the early period after immunization observed in
this study also warrants additional evaluation.
This study has several limitations. Al-though the participation rate among reached case subjects and potential controls was high, we cannot rule out intrinsic differences from those we were unable to reach. It is also pos-sible that the source population of hospitalized case subjects differed on important but unrecognized char-acteristics and as such we cannot rule out residual confounding. More case subjects (especially among younger children) reported having had an ILI during the first wave but more con-trols had an ILI during the second wave. In the analysis restricted to chil-dren without ILI, the VE was unchanged suggesting this was unlikely to have been a major influence on our findings. The statistical power of the study, es-pecially in children aged 5 to 9 years, was limited because most cases oc-curred before the vaccination was of-fered to this group. Small sample size may have also introduced instability and spurious findings in exploratory suba-nalyses. As such, exploratory subanaly-ses cannot be considered conclusive but merit additional evaluation.
This study also had several strengths. It included all pH1N1-hospitalized chil-dren from a substantial population, and all cases were laboratory con-firmed by using RT-PCR. Matching ac-cording to age and area of residence as well as adjustment for young sib-lings in the family, indicative of in-creased influenza exposure opportuni-ties, helped to limit confounding. We also adjusted for the presence of at least 1 chronic condition; however, some conditions may be greater driv-ers than othdriv-ers for vaccination or hos-pitalization. Small sample size by strata precluded control for specific conditions or analysis restricted to children with chronic medical condi-tions. However, in the analysis
Finally, vaccination status and dates of vaccination were confirmed by the provincial registry.
In the context of vaccination given dur-ing the course of an epidemic, rapid onset of protection with a single dose of vaccine is desirable. For children never immunized against influenza, 2 doses of vaccine, seasonal or pan-demic, are generally recommended. However, the rapid unfolding of the fall pH1N1 wave would not have permitted a second dose to exert its effect in time, suggesting that this recommen-dation may not always be practical and the incremental benefit of a second dose may be doubtful when immuniza-tion coincides with an epidemic. The estimated VE of a single dose of
adju-randomized clinical trials during sea-sons with good match to circulating in-fluenza strains. Rather than, or in addi-tion to, a direct effect of adjuvant, our results may also reflect well-matched pH1N1 antigen to circulating virus dur-ing the pandemic wave in Quebec (99.6% characterized as matched to the vaccine component). However, our VE exceeds that estimated through case-control and cohort designs for seasonal influenza in children.22,23Immunogenicity data in chil-dren 6 months to 9 years old adminis-tered a single dose of nonadjuvanted pH1N1 vaccine also showed seropro-tection rates exceeding 90%,22 but the clinical implications of that have yet to be confirmed through effec-tiveness studies.
diatric dose of the AS03-adjuvanted pH1N1 vaccine given to children aged 6 months to 9 years is highly protec-tive against hospitalization, begin-ning as early as 10 days after immu-nization. These findings suggest that the decision to administer 2 doses of influenza vaccine to children is con-text dependent: during a pandemic this should take into account the stage of the evolving epidemic, avail-able immunogenicity data, and prac-tical considerations around vaccine supply in relation to need across at-risk groups.
ACKNOWLEDGMENT
This research was funded by the Que-bec Ministry of Health.
REFERENCES
1. Health Canada. Summary Basis of Decision (SBD) for AREPANRIX H1N1. Available at: www.hc-sc.gc.ca/dhp-mps/alt_formats/ p d f / p r o d p h a r m a / s b d s m d / p h a s e 1 -d e c i s i o n / -d r u g - m e -d / s b -d _ s m -d _ 2 0 1 0 _ arepanrix_h1n1_132070-eng.pdf. Accessed April 20, 2011
2. Health Canada. Product information leaflet Arepanrix H1N1 AS03-Adjuvanted H1N1 Pan-demic Influenza Vaccine—Version 4 ap-proved 20 April 2010. Available at: www.hc-sc.gc.ca/dhp-mps/prodpharma/legislation/ interimorders-arretesurgence/prodinfo_ vaccin-eng.php. Accessed April 20, 2011 3. GlaxoSmithKline Inc. A study to evaluate the
safety and immunogenicity of A/California/ 7/2009 (H1N1)v-like vaccines GSK2340274A and GSK2340273A in children 6 months to less than 9 years of age. Available at: http:// download.gsk-clinicalstudyregister.com/ files/3d226ebd-7b95-4336-bb27-12dc16b231b9. Accessed April 20, 2011
4. GlaxoSmithKline Inc. Safety and immunoge-nicity study of GSK Biologicals’ pandemic in-fluenza candidate vaccine (GSK2340272A) in children aged 6 to 35 months. Available at: http://gsk.sylogent.com/files/27868.pdf. Accessed April 20, 2011
5. Sauvageau C, Gilca V. Avis complémentaire concernant la vaccination contre le virus pandémique influenza A (H1N1) 2009 pour les enfants de 6 mois a` 9 ans. Québec,
Canada: Institut national de santé publique du Québec; 2009. Available at: http://www. i n s p q . q c . c a / p d f / p u b l i c a t i o n s / 1 0 2 5 _ AvisVaccH1N1Enfants6-9Ans.pdf. Accessed April 20, 2011
6. NACI. Statement on seasonal trivalent inac-tivated influenza vaccine (TIV) for 2009-2010.CCDR RMTC. 2009;35(ACS-6):1– 41 7. Rothman KJ, Greenland S, Lash TL.Modern
Epidemiology. 3rd ed. Philadelphia, PA:
Wolters Kluwer Health; 2008
8. Carmona A, Omenaca F, Tejedor JC, et al. Immunogenicity and safety of AS03-adjuvanted 2009 influenza A H1N1 vaccine in children 6 –35 months. Vaccine. 2010; 28(36):5837–5844
9. Waddington CS, Walker WT, Oeser C, et al. Safety and immunogenicity of AS03B adj u v a n t e d s p l i t v i r i o n v e r s u s n o n -adjuvanted whole virion H1N1 influenza vaccine in UK children aged 6 months-12 years: open label, randomised, parallel group, multicentre study.BMJ. 2010;340: c2649
10. Nicholson KG, Abrams KR, Batham S, et al. Immunogenicity and safety of a 2-dose schedule of whole-virion and AS03A-adjuvanted 2009 influenza A (H1N1) vaccines: a randomised, multicentre, age-stratified, head-to-head trial.Lancet Infect Dis. 2011;11(2):91–101
11. Van Buynder PG, Dhaliwal JK, Van Buynder JL, et al. Protective effect of single-dose ad-juvanted pandemic influenza vaccine in chil-dren.Influenza Other Respi Viruses. 2010; 4(4):171–178
12. Hardelid P, Fleming DM, McMenamin J, et al. Effectiveness of pandemic and seasonal fluenza vaccine in preventing pandemic in-fluenza A(H1N1)2009 infection in England and Scotland 2009-2010.Euro Surveill. 2011; 16(2):pii⫽19763. Available at: www. e u r o s u r v e i l l a n c e . o r g / V i e w A r t i c l e . aspx?ArticleId⫽19763. Accessed April 20, 2011
13. Andrews N, Waight P, Yung CF, Miller E. Age-specific effectiveness of an oil-in-water ad-juvanted pandemic (H1N1) 2009 vaccine against confirmed infection in high risk groups in England.J Infect Dis. 2011;203(1): 32–39
14. Ortqvist A, Berggren I, Insulander M, Sve-nungsson B. Effectiveness of an adjuvanted monovalent vaccine against the 2009 pan-demic strain of influenza A(H1N1)v, in Stock-holm County, Sweden.Clin Infect Dis. 2011; 52(10):1203–1211
16. Janjua NZ, Skowronski DM, Hottes TS, et al. Seasonal influenza vaccine and increased risk of pandemic A/H1N1-related illness: first de-tection of the association in British Columbia, Canada.Clin Infect Dis. 2010;51(9):1017–1027 17. Janjua NZ, Skowronski D, De Serres G, et al. Analysis of the Association Between 2008-09 Trivalent Influenza Vaccine (TIV) and Pan-demic A/H1N1 Risk During the Fall 2009 [ab-stract]. Presented at the Options for the Control of Influenza VII (Abstract #4405), Hong Kong SAR, China; 2010
18. Cowling BJ, Ng S, Ma E, Cheng C, Wai W, Fang
V. Protective efficacy of seasonal influenza vaccination against seasonal and pandemic influenza virus during the 2009 in Hong Kong.Clin Infect Dis. 2010;51(12):1370 –1379 19. Skowronski DM, Janjua NZ, Hottes TS, De Serres G. Mechanism for seasonal vaccine effect on pandemic H1N1 risk remains un-certain.Clin Infect Dis. 2011;52(6):831– 832 20. Nolan T, Richmond PC, Formica NT, et al.
Safety and immunogenicity of a prototype adjuvanted inactivated split-virus influenza A (H5N1) vaccine in infants and children. Vaccine. 2008;26(50):6383– 6391
21. Nolan T, McVernon J, Skeljo M, et al. Immu-nogenicity of a monovalent 2009 influenza A(H1N1) vaccine in infants and children: a randomized trial.JAMA. 2010;303(1):37– 46 22. Jefferson T, Rivetti A, Harnden A, Di Pietran-tonj C, Demicheli V. Vaccines for preventing influenza in healthy children.Cochrane Da-tabase Syst Rev. 2008;(2):CD004879 23. Kelly H, Jacoby P, Dixon GA, et al. Vaccine
effectiveness against laboratory-confirmed influenza in healthy young children: a case-control study. Pediatr Infect Dis J. 2011; 30(2):107–111
(Continued from first page)
GlaxoSmithKline for unrelated studies; Ms Boulianne has received research funding from GlaxoSmithKline and Sanofi Pasteur for unrelated studies; Dr Sauvageau has received research grants, honoraria, and travel reimbursements from Wyeth for unrelated studies and honoraria and travel reimbursements from GlaxoSmithKline and Merck for conferences and unrelated studies; Dr Quach has received research grants from Merck for unrelated studies; Dr Boucher has received research funding from Novartis for an unrelated study; and Dr Skowronski was principal investigator on a clinical trial for which influenza vaccine was provided free by Sanofi Pasteur.
DOI: 10.1542/peds.2010-3492 originally published online October 10, 2011;
2011;128;e1084
Pediatrics
Sauvageau, Caroline Quach, François D. Boucher and Danuta M. Skowronski
Services
Updated Information &
http://pediatrics.aappublications.org/content/128/5/e1084
including high resolution figures, can be found at:
References
http://pediatrics.aappublications.org/content/128/5/e1084#BIBL
This article cites 14 articles, 1 of which you can access for free at:
Subspecialty Collections
b
http://www.aappublications.org/cgi/collection/infectious_diseases_su Infectious Disease
following collection(s):
This article, along with others on similar topics, appears in the
Permissions & Licensing
http://www.aappublications.org/site/misc/Permissions.xhtml
in its entirety can be found online at:
Information about reproducing this article in parts (figures, tables) or
Reprints
http://www.aappublications.org/site/misc/reprints.xhtml
DOI: 10.1542/peds.2010-3492 originally published online October 10, 2011;
2011;128;e1084
Pediatrics
Sauvageau, Caroline Quach, François D. Boucher and Danuta M. Skowronski
Rodica Gilca, Geneviève Deceuninck, Gaston De Serres, Nicole Boulianne, Chantal
Hospitalization in Children
Effectiveness of Pandemic H1N1 Vaccine Against Influenza-Related
http://pediatrics.aappublications.org/content/128/5/e1084
located on the World Wide Web at:
The online version of this article, along with updated information and services, is
http://pediatrics.aappublications.org/content/suppl/2011/10/04/peds.2010-3492.DC1
Data Supplement at:
by the American Academy of Pediatrics. All rights reserved. Print ISSN: 1073-0397.