A Possible Angular Quantization as a Complement to the Conventional Radial Quantization in the Hydrogen Atom and Aqueous Systems
Full text
Figure
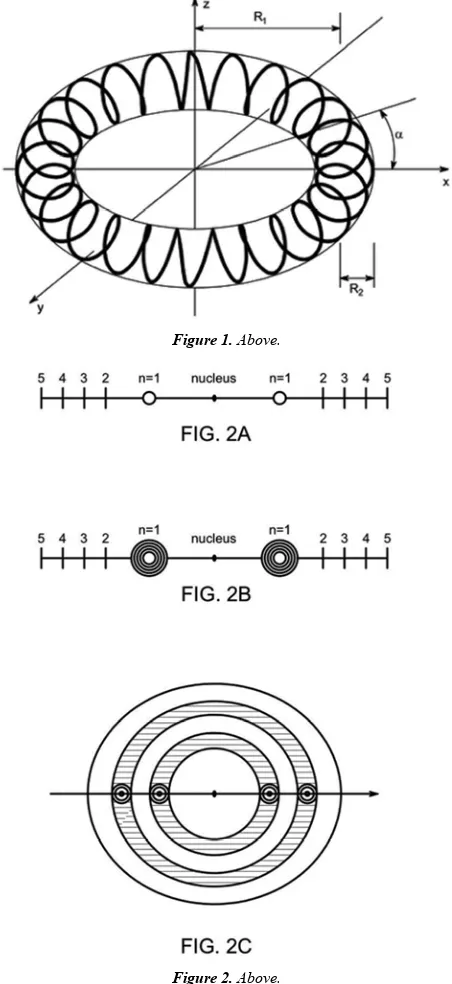
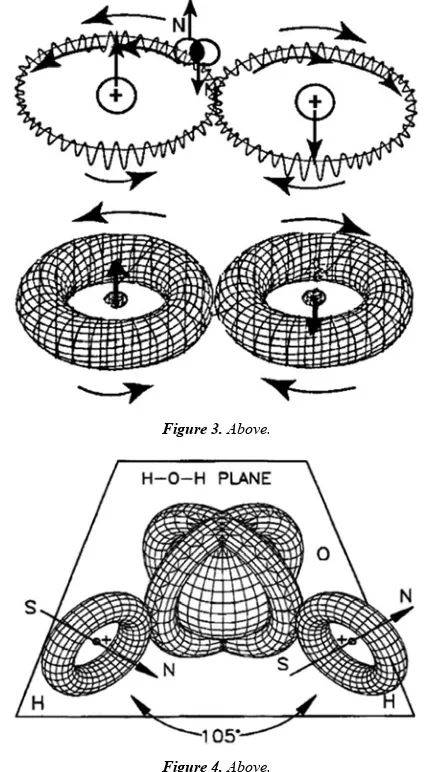
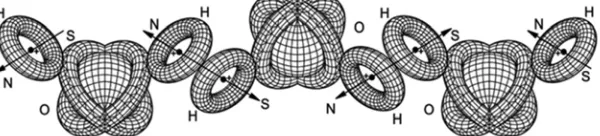
Related documents
For hydrogen (in the absence of a magnetic field), the energy level depends on the principle quantum number n. In ground state an atom cannot emit
The radial wave function R and the spherical harmonics Y determine the probability density for the various quantum states. The total wave function depends on n, ℓ, and
So an external magnetic field should have an effect on atoms, spectral lines are “fingerprint” characteristics of atoms, in an external magnetic field, each spectral line should
• This means that when the proton absorbs the energy, the proton magnetic field changes from aligned to opposed (low energy to high energy) - it flips its spin state.. • In order
Given the energies of the lines in an atomic spectrum, it is possible (although sometimes very di cult) to determine the energy levels of an atom. Energy-level diagrams are used
Connect the other end of the negative lead to a good ground on the engine block or body of the vehicle with the discharged battery, and as far away as possible from the battery..
It is therefore proper to state, “An electron is located within this volume with this probability at this time,” but not, “An electron is located at the position (x, y, z) at
Patients are routinely discharged from home health services when: the patient has reached his maximum potential to a point where he can manage in the judgment of the physician