Cytokine and Cellular Inflammatory Sequence in Enteroviral Meningitis
Masatoki Sato, MD*; Mitsuaki Hosoya, MD*; Ken Honzumi, PhD*; Mikako Watanabe, MD§; Norio Ninomiya, MD§; Shiro Shigeta, MD‡; and Hitoshi Suzuki, MD*
ABSTRACT. Objective. To clarify the sequence of cy-tokines and inflammatory cells in enteroviral meningitis.
Methods. Cerebrospinal fluid (CSF) was collected from 86 patients who received a diagnosis of enteroviral meningitis after detection of the enteroviral genome in the CSF using polymerase chain reaction. Twenty-one of 86 patients had repeated lumbar punctures. Cytokine concentrations were measured acutely and in 32 samples collected during recovery.
Results. The proinflammatory cytokines (interleukin [IL]-6, IL-8, and interferon-␥) were detected at signifi-cantly higher concentrations during the acute phase when enteroviral genomes were present. Proinflamma-tory cytokines decreased to normal levels in the recovery phase when enteroviral genomes disappeared. Anti-in-flammatory concentrations (IL-10 and transforming growth factor-1) were significantly higher in the recov-ery phase than in the acute phase. Of the 86 CSF samples collected in the acute phase, 11 had no pleocytosis (<10 white blood cells/mm3). In 7 of those 11 CSF samples,
IL-6 and IL-8 levels were as high as those in the 75 samples with pleocytosis (>10 white blood cells/mm3).
Seven patients were considered to be in the initial stage of their illness when production of proinflammatory cy-tokines were high but leukocytes had not yet infiltrated the cerebrospinal cavity.
Conclusions. The inflammatory process observed in human enteroviral meningitis is comparable with that observed in animal models: 1) infection induces proin-flammatory cytokine production, followed by infiltra-tion of white blood cells into the infected area, and 2) inflammation is terminated by the anti-inflammatory
cy-tokines that are produced when pathogens are
eliminated.Pediatrics2003;112:1103–1107;viral meningi-tis, enterovirus, cytokine, inflammatory cells.
ABBREVIATIONS. WBC, white blood cell; IL, interleukin; TNF, tumor necrosis factor; IFN, interferon; TGF, transforming growth factor; CSF, cerebrospinal fluid; PCR, polymerase chain reaction; ELISA, enzyme-linked immunosorbent assay.
I
nflammation is initiated by local production of several soluble mediators. Many different cyto-kines and chemocyto-kines control the inflammatory response via activation and migration of white blood cells (WBCs).1 The physiologic functions of thosecytokines were inferred from the biological activity observed in vitro2,3and confirmed by in vivo exper-iments.4 – 6The results of those experiments indicated that the production of proinflammatory cytokines (interleukin [IL]-1, tumor necrosis factor [TNF]-␣, IL-6, and interferon [IFN]-␥) stimulated by infection induce the migration of WBCs into the infected area and that inflammation is terminated by anti-inflam-matory cytokines (IL-10, IL-4, and transforming growth factor (TGF)-1) that are produced after elim-ination of the microorganisms. In humans, however, the induction of inflammation by cytokines at the initial stage of infection has not been demonstrated. In the present study, we measured the concentra-tions of several proinflammatory and anti-inflamma-tory cytokines in the cerebrospinal fluid (CSF) of patients with naturally occurring enteroviral menin-gitis in the acute and recovery phases and deduce the role of cytokines in the inflammatory process.
METHODS Samples
We observed an outbreak of viral meningitis caused by echo-virus type 30 from June to December 1997. CSF samples were collected from 86 patients with signs and symptoms suggestive of meningeal involvement at Jyusendo General Hospital in the Fuku-shima Prefecture, Japan. CSF samples were transferred to the Department of Pediatrics, Fukushima Medical University, imme-diately after collection and stored at ⫺80°C until use. All 86 patients received a diagnosis of enteroviral meningitis after detec-tion of the enteroviral genome in the CSF using polymerase chain reaction (PCR; Table 1). Of the 86 patients, 48 had neutrophil-predominant pleocytosis (ⱖ10 WBCs/mm3), 27 had
lymphocyte-predominant pleocytosis, and the remaining 11 had no pleocytosis (⬍10 WBCs/mm3) in the CSF. In Jyusendo General Hospital,
repeated lumbar punctures were performed to confirm decreasing CSF WBC counts (⬍50 WBCs/mm3) as a marker of the end of
inflammation in the central nervous system. Parents of 21 patients with pleocytosis in the CSF in the acute phase consented to re-peated lumbar punctures to confirm sufficiently decreasing WBCs in the CSF (Table 1). Twenty-one patients had 2 lumbar punctures, 9 patients had 3, and 2 patients had 4. Of the 21 patients who received multiple lumbar punctures, there was no detectable en-teroviral genome in 32 of the CSF samples using PCR, and all 21 patients therefore were considered to be collected in the recovery phase, although pleocytosis remained in 20 of the samples. No differences were observed in the characteristics of these 21 pa-tients and the other 65 papa-tients (Table 1). CSF samples collected from 14 patients with acute leukemia in complete remission dur-ing the same period were used as controls. Informed consent was obtained from patients or their parents for cytokine concentration measurements in the CSF samples collected. The ethics committee in Jyusendo General Hospital approved the study.
PCR
Nested PCR was performed for detection of the enterovirus in the CSF as described previously.7Primer sequences used in this
study were F1 (CAAGCACTTCTGTTTCCCCGG), F2
(TCCTCCG-From the Departments of *Pediatrics and ‡Microbiology, Fukushima Med-ical University, School of Medicine, Fukushima, Japan; and §Jyusendo General Hospital, Koriyama, Japan.
Received for publication Jan 25, 2002; accepted Apr 17, 2003.
Reprint requests to (M.S.) Department of Pediatrics, Fukushima Medical University, School of Medicine, Hikarigaoka 1, Fukushima 960-1295, Japan. E-mail: toki422@guitar.ocn.ne.jp
GCCCCTGAATGCG), and R1 (ATTGTCCACCATAAGCAGCCA) for the enterovirus. RNA was extracted from 250L of whole CSF using an RNA extraction kit (Nippon Gene, Tokyo, Japan). After RNA extraction, cDNA was synthesized (42°C, 30 minutes) from the resuspended RNA using 2.5 U of Moloney murine leukemia virus reverse transcriptase (Toyobo, Osaka, Japan) and reaction mixture containing 50 mM KCl, 10 mM TRIS-HCl (pH 8.3), 5 mM MgCl2, 0.2 mM each dNTP, 1mol each of primer F1 and primer
R1, and 20 U of RNase inhibitor (Toyobo). The cDNA product was amplified in 50L of reaction mixture containing 50 mM KCl, 10 mM TRIS-HCl (pH 8.3), 5 mM MgCl2, 0.2 mM each dNTP, 0.2
mol each of primer F1 and primer R1, and 1.25 U of Taq DNA polymerase (Perkin-Elmer, Norwalk, CT). Thirty cycles were per-formed in a thermal cycler (Perkin-Elmer) as follows: denaturation for 1 minute at 93°C, annealing for 1 minute at 55°C, and extension for 2 minutes at 72°C. The second PCR was performed as above, using the second primer pair (F2 and R1) and 2L of the first PCR product. The nested PCR product was run on a 2% agarose gel containing ethidium bromide and photographed under ultraviolet light. To eliminate and detect laboratory contamination leading to false-positive PCR results, negative controls were included for each step of the assay.
Cytokine Assay
IL-6, IL-8, IFN-␥, IL-10, and TGF-1 concentrations in the CSF samples were determined using monoclonal antibody enzyme-linked immunosorbent assay (ELISA) kits (IL-6 ELISA, IL-8 ELISA, IFN-␥ELISA, and IL-10 ELISA; Endogen, Inc, Woburn, MA; and TGF-1 ELISA, R & D Systems, Minneapolis, MN). The minimum detectable concentrations were 1 pg/mL (IL-6), 2 pg/mL (IL-8), 2 pg/mL (IFN-␥), 3 pg/mL (IL-10), and 7 pg/mL (TGF-1). The intra- and interassay coefficients were⬍10%. We measured the concentrations of proinflammatory cytokines (IL-6, IL-8, and IFN-␥) and anti-inflammatory cytokines (IL-10 and TGF-1) in 21 CSF samples in the acute phase and 32 CSF samples in the recovery phase collected from 21 patients who received re-peated lumbar punctures. IL-6 and IL-8 concentrations were also measured in 65 CSF samples collected from patients with entero-viral meningitis in the acute phase and 14 control CSF samples obtained from patients with leukemia. The cytokine concentration in the CSF samples that was below the detectable limit of the ELISA kit was defined as 100pg/mL for statistical analysis.
Statistical Analysis
Wilcoxon rank-sum test was used for the data analysis.P⬍.05 was considered to be significant.
RESULTS
Sequential Appearance of Proinflammatory and Anti-inflammatory Cytokines in the CSF
We compared the concentrations of several cyto-kines in the CSF samples collected during the acute and recovery phases from 21 patients who received repeated lumbar punctures. The CSF concentrations in the acute phase were calculated to be log (2.48⫾
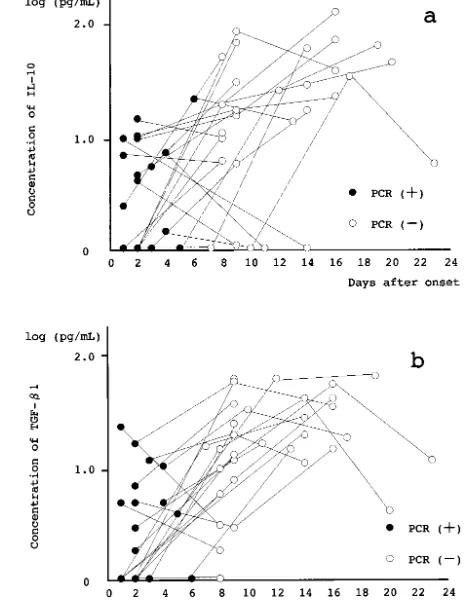
0.55) pg/mL (IL-6), log (3.12 ⫾ 0.52) pg/mL (IL-8), and log (1.80⫾ 0.39) pg/mL (IFN-␥). Those in the recovery phase were calculated to be log (0.39⫾0.43) pg/mL (IL-6), log (1.13⫾0.28) pg/mL (IL-8), and log (0.49 ⫾ 0.41) pg/mL (IFN-␥). The proinflammatory cytokine concentrations in the acute phase were sig-nificantly higher than those in the recovery phase (IL-6:P⬍.0001, IL-8:P⬍.0001, IFN-␥:P⬍.0001; Fig 1). The IL-10 and TGF-1 concentrations in the CSF in the acute phase were calculated to be log (0.51⫾ 0.48) pg/mL (IL-10) and log (0.43 ⫾ 0.48) pg/mL (TGF-1). Those in the recovery phase were calcu-lated to be log (1.19 ⫾ 0.61) pg/mL (IL-10) and log (1.22 ⫾ 0.45) pg/mL (TGF-1). The anti-inflamma-tory cytokine concentrations in the recovery phase were significantly higher than those in the acute phase (IL-10:P ⫽.0206, TGF-1:P⫽ .0003; Fig 2).
Correlation Among Proinflammatory Cytokines, Enteroviral Genome, and Pleocytosis in the CSF
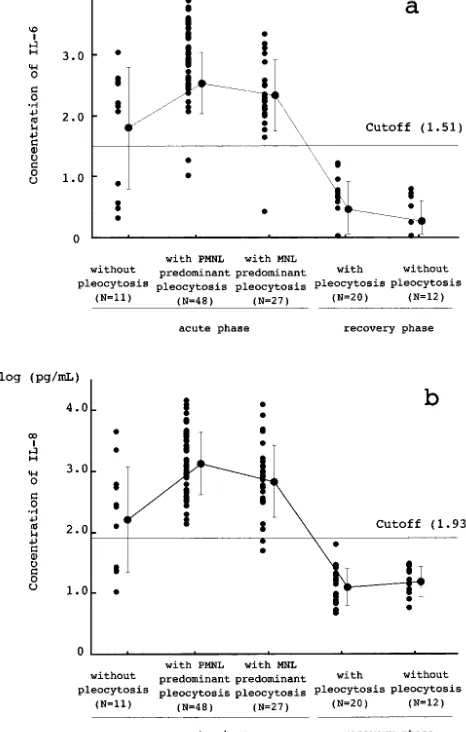
IL-6 and IL-8 concentrations in the CSF samples taken from 14 leukemia patients were calculated to be log (0.77 ⫾ 0.26) pg/mL and log (0.50 ⫾ 0.48) pg/mL, respectively. The cutoff levels of IL-6 and IL-8 were defined as log 1.51 pg/mL and log 1.93 pg/mL (mean ⫹ 3 standard deviations), respec-tively. Eighty-six patients who had a diagnosis of enteroviral meningitis in the acute phase after detect-ing enteroviral RNA in their CSF samples usdetect-ing PCR were classified into 3 groups; 48 patients with neu-trophil-predominant pleocytosis (ⱖ10 WBCs/mm3), 27 patients with lymphocyte-predominant pleocyto-sis, and 11 patients without pleocytosis (⬍10 WBCs/ mm3) in the CSF. IL-6 levels of those groups were log (2.53⫾ 0.51) pg/mL, log (2.33⫾ 0.58) pg/mL, and log (1.80 ⫾ 1.00) pg/mL, respectively, and were higher than the cutoff level in 46 (95.8%), 26 (96.3%), and 7 (63.6%) patients, respectively. The IL-8 levels were calculated to be log (3.12 ⫾ 0.51) pg/ml, log (2.83 ⫾ 0.59) pg/ml, and log (2.21 ⫾ 0.87) pg/ml, respectively, and were higher than the cutoff level in 48 (100%), 25 (92.6%), and 7 (63.6%) patients, respec-tively. In 7 of the 11 CSF samples with normal CSF cell counts, both IL-6 and IL-8 levels were above the cutoff levels (Fig 3). Thirty-two CSF samples col-lected from 21 patients in the recovery phase were classified into 2 groups; 20 samples with pleocytosis TABLE 1. Characteristics of Patients With Enteroviral Meningitis
All Patients (N⫽86)
Patients With Acute Phase Lumbar Punctures
(N⫽65)*
Patients With Repeated Lumbar Punctures
(N⫽21)*
Background
Days after onset 2.2⫾1.4 2.2⫾1.3 2.4⫾1.6
Age (y) 5.9⫾2.9 5.9⫾2.9 6.6⫾3.0
Male:female 60:26 47:18 13:8
Symptoms
Fever 100% 100% 100%
Headache 94.6% 96.0% 90.1%
Nausea 87.4% 89.3% 100%
Neck stiffness 73.4% 70.7% 81.8%
Kernig’s sign 50.2% 54.7% 36.4%
and 12 samples without. IL-6 concentrations of those samples were calculated to be log (0.46 ⫾ 0.46) pg/mL and log (0.26 ⫾ 0.35) pg/mL, respectively. The concentrations of IL-8 were calculated to be log (1.09 ⫾ 0.30) pg/mL and log (1.19 ⫾ 0.25) pg/mL, respectively. The concentrations of IL-6 and IL-8 in the CSF in the recovery phase were lower than the cutoff levels in all samples.
DISCUSSION
In experimentally infected animal models of pleu-ritis,6 peritonitis,4 or meningitis,5 samples without blood contamination or tissue effusion were ob-tained from the inflamed area. The cytokine concen-trations in the samples reflect the amount of cyto-kines produced by the infected tissue. In these reports, proinflammatory cytokines such as TNF-␣, IL-1, and IL-6 were detected in the initial phase (1–2 hours after infection) and reached a maximum 3 to 5 hours after infection. The proinflammatory cytokines attracted WBCs with neutrophil predominance and induced increased exudation in the pleural cavity6 or increased protein concentration in the CSF.5 Thereafter, anti-inflammatory cytokines, including IL-108,9 and TGF-1,10 which are produced shortly after the production of proinflammatory cytokines, suppressed the activities of the proinflammatory cy-tokines.
In bacterial meningitis in humans, inflammation in the acute phase is thought to be caused by the pro-duction of proinflammatory cytokines, including TNF-␣, IL-6, and IL-8, in the cerebrospinal cavity. Anti-inflammatory cytokines, including IL-10 and TGF-1, were also detected in the CSF samples in the acute phase. Those cytokines might contribute to end Fig 1. Proinflammatory cytokine concentrations in the CSF of 21
patients who received repeated lumbar punctures. ●, samples (n⫽21) with PCR-positive results collected in the acute phase of enteroviral meningitis;E, samples (n⫽32) with PCR-negative
results collected in the recovery phase of enteroviral meningitis. a, IL-6 levels in the acute phase were significantly higher than in the recovery phase (P⬍.0001). b, IL-8 levels in the acute phase were significantly higher than in the recovery phase (P ⬍ .0001). c, IFN-␥levels in the acute phase were significantly higher than in the recovery phase (P⬍.0001).
the inflammation, as the administration of dexa-methasone inhibited the production of proinflamma-tory cytokines and decreased the incidence of hear-ing impairment.11,12 In viral meningitis in humans, IL-6, IL-8, and IFN-␥were detected at relatively high concentrations.13–16 Anti-inflammatory cytokines were also detected in the CSF in the early stages of the illness.17,18 It has been inferred that those cyto-kines might play an important role in the inflamma-tory process in viral meningitis. The sequential ap-pearance of proinflammatory cytokines, WBCs, and anti-inflammatory cytokines in the CSF after viral infection, however, is not clear.
A regional epidemic of enteroviral meningitis caused by echovirus type 30 was observed in our district from June to December 1997. To investigate the role of cytokines in the inflammation of viral meningitis, we measured the concentrations of proinflammatory cytokines (IL-6, IL-8, and IFN-␥) and anti-inflammatory cytokines (IL-10 and TGF-1) in the CSF samples collected from patients with con-firmed enteroviral meningitis in the acute and recov-ery phases. The levels of proinflammatory cytokines were high in the acute phase, when the viral genome
was detected in the CSF, and decreased to normal levels in the recovery phase, when the virus disap-peared from central nervous system. Anti-inflamma-tory cytokines were produced in the recovery phase. These results indicate that the main immunologic response by the cytokine network shifts from pro-duction of proinflammatory cytokines to that of anti-inflammatory cytokines during or after the period when the virus is eliminated from the cerebrospinal cavity.
In viral meningitis, WBCs infiltrate the cerebrospi-nal cavity with neutrophil predominance at an early stage of the illness; thereafter, lymphocytes become the predominant cells. A few reports demonstrated enteroviral meningitis without pleocytosis.19,20 This was considered the initial stage of the illness when WBCs had not infiltrated the cerebrospinal cavity.19 We suggest that the acute phase of enteroviral men-ingitis may be divided into 3 time periods: the period without pleocytosis, the period with neutrophil-pre-dominant pleocytosis, and the period with lympho-cyte-predominant pleocytosis. Seven (63.6%) of 11 samples collected from patients without pleocytosis in the CSF in the acute phase had high concentrations of both IL-6 and IL-8, as did 75 samples collected from patients with pleocytosis in the acute phase. Those 7 patients could be considered to be in the initial stage of illness when virus is present and when WBCs have not yet infiltrated the cerebrospi-nal cavity. The other 4 patients with enteroviral ge-nome and normal CSF cytokine level might be con-sidered to be at slightly earlier stage immediately after viral infection.
The results observed in this study suggest a pos-sible sequence of events. Immediately after viral in-fection, proinflammatory cytokines such as IL-6, IL-8, and IFN-␥ are produced by endothelial cells, arachnoid cells, and/or monocytes.21 These proin-flammatory cytokines attract WBCs into the cerebro-spinal cavity. After elimination of virus, the proin-flammatory period is terminated by the production of anti-inflammatory cytokines produced by acti-vated T cells and/or monocytes.21This sequence of events that we have observed in viral meningitis in humans is comparable to that observed in animal models.4 – 6Our observations suggest that the inflam-matory process in viral meningitis is regulated by the production of cytokines produced in a sequence.
REFERENCES
1. Curfs JHA, Meis JFGM, Hoogkamp-Korstanje JAA. A primer on cytokines: sources, receptors, effects, and inducers.Clin Microbiol Rev.
1997;10:742–780
2. Hirano T, Kishimato T. Interleukin-6. In: Sporn MB, Roberts AB, eds.
Handbook of Experimental Pharmacology: Peptide Growth Factors and Their Receptors. Heidelberg, Germany: Springer-Verlag; 1990:633– 665 3. Smith WB, Gamble JR, Vadas MA. The role of granulocyte-macrophage
and granulocyte colony-stimulating factors in neutrophil transendothe-lial migration with interleukin-8.Exp Hematol. 1994;22:329 –334 4. Goto K, Nakamura S, Goto F, Yoshinaga M. Generation of an
interleu-kin-1-like lymphocyte-stimulating factor at inflammatory sites: correla-tion with the infiltracorrela-tion of polymorphonuclear leukocytes.Br J Exp Pathol.1984;65:521–532
5. Mustafa MM, Ramilo O, Olsen KD, et al. Tumor necrosis factor in mediating experimentalHaemophilus influenzaetype B meningitis.J Clin Invest.1989;84:1253–1259
6. Utsunomiya I, Nagai S, Oh-ishi S. Sequential appearance of IL-1 and IL-6 activities in rat carrageenin-induced pleurisy.J Immunol.1991;147: 1803–1809
7. Hosoya M, Honzumi K, Suzuki H. Detection of enterovirus by poly-merase chain reaction and culture in cerebrospinal fluid of children with transient neurologic complications associated with acute febrile illness.J Infect Dis.1997;175:700 –703
8. Gallo P, Sivieri S, Rinaldi L, et al. Intrathecal synthesis of interleukin-10 (IL-10) in viral and inflammatory diseases of the central nervous sys-tem.J Neurol Sci.1994;126:49 –53
9. Paris MM, Hickey SM, Trujillo M, Ahmed A, Olsen K, McCracken GH Jr. The effect of interleukin-10 on meningeal inflammation in experi-mental bacterial meningitis.J Infect Dis.1997;176:1239 –1246
10. Ossege LM, Voss B, Wiethege T, Sindern E, Malin JP. Detection of transforming growth factor beta 1 mRNA in cerebrospinal fluid cells of patients with meningitis by nonradioactive in situ hybridization.J Neu-rol.1994;242:14 –19
11. Waage A, Halstensen A, Shalaby R, Brandzaeg P, Kierulf P, Espevik T. Local production of tumor necrosis factor-␣, interleukin 1, and inter-leukin 6 in meningococcal meningitis.J Exp Med.1989;170:1859 –1867 12. Odio CM, Faingezicht I, Paris M, et al. The bacterial effects of early
dexamethasone administration in infants and children with bacterial meningitis.N Engl J Med.1991;324:1525–1531
13. Dulkerian SJ, Kilpatrick L, Costarino AT Jr, et al. Cytokine elevations in infants with bacterial and aseptic meningitis.J Pediatr.1995;126:872– 876
14. Ramilo O, Mustafa MM, Poter J, et al. Detection of interleukin 1 beta but not tumor necrosis factor-␣ in cerebrospinal fluid of children with aseptic meningitis.Am J Dis Child.1990;144:349 –352
15. Ishiguro A, Suzuki Y, Inaba Y, et al. The production of IL-8 in cerebro-spinal fluid in aseptic meningitis of children.Clin Exp Immunol.1997; 109:426 – 430
16. Ohga S, Aoki T, Okada K, et al. Cerebrospinal fluid concentrations of interleukin-1, and tumor necrosis factor alpha, interferon gamma in bacterial meningitis.Arch Dis Child.1994;70:123–125
17. Ishiguro A, Suzuki Y, Inaba Y, Komiyama A, Koeffler HP, Shimbo T. Production of interleukin-10 in the cerebrospinal fluid in aseptic men-ingitis of children.Pediatr Res.1996;40:610 – 614
18. Ossege LM, Sindern E, Voss B, Malin JP. Expression of tumor necrosis factor-␣and transforming growth factor-beta-1 in cerebrospinal fluid cells in meningitis.J Neurol Sci.1996;144:1–13
19. Sawyer MH, Holland D, Aintablian N, Connor JD, Keyser EF, Waecker NJ Jr. Diagnosis of enteroviral central nervous system infection by polymerase chain reaction during a large community outbreak.Pediatr Infect Dis J.1994;13:177–182
20. Wildin S, Chonmaitree T. The importance of the virology laboratory in the diagnosis and management of viral meningitis. Am J Dis Child.
1987;141:454 – 457
21. Ta¨uber MG, Moser B. Cytokines and chemokines in meningeal inflammation: biology and clinical implications.Clin Infect Dis.1999:28: 1–11
McFARLANE’S INSIGHT
“ . . . Since only 20 of 55 (36%) new fellows of the Academy of Medical Sciences elected in 2003 work in the four institutions mentioned, mid-career research productivity does not seem to be over-represented in those who receive the greatest funding and who are associated with the greatest supposed critical mass. . . . McFarlane’s law states that ‘when conflicting theories co-exist, any point on which they all agree is the one most likely to be wrong.’ An apparently widespread agreement by policy makers that further concentration of clinical research would both improve research productivity and not damage quality in the NHS seems to breach this law. The dependence or non-dependence of productivity on so-called critical mass needs more sophisticated analysis than it has so far received.”
Boyd R. McFarlane’s law, critical mass, and clinical research.Lancet. June 7, 2003
DOI: 10.1542/peds.112.5.1103
2003;112;1103
Pediatrics
Shiro Shigeta and Hitoshi Suzuki
Masatoki Sato, Mitsuaki Hosoya, Ken Honzumi, Mikako Watanabe, Norio Ninomiya,
Cytokine and Cellular Inflammatory Sequence in Enteroviral Meningitis
Services
Updated Information &
http://pediatrics.aappublications.org/content/112/5/1103
including high resolution figures, can be found at:
References
http://pediatrics.aappublications.org/content/112/5/1103#BIBL
This article cites 19 articles, 4 of which you can access for free at:
Subspecialty Collections
b
http://www.aappublications.org/cgi/collection/infectious_diseases_su
Infectious Disease following collection(s):
This article, along with others on similar topics, appears in the
Permissions & Licensing
http://www.aappublications.org/site/misc/Permissions.xhtml
in its entirety can be found online at:
Information about reproducing this article in parts (figures, tables) or
Reprints
http://www.aappublications.org/site/misc/reprints.xhtml
DOI: 10.1542/peds.112.5.1103
2003;112;1103
Pediatrics
Shiro Shigeta and Hitoshi Suzuki
Masatoki Sato, Mitsuaki Hosoya, Ken Honzumi, Mikako Watanabe, Norio Ninomiya,
Cytokine and Cellular Inflammatory Sequence in Enteroviral Meningitis
http://pediatrics.aappublications.org/content/112/5/1103
located on the World Wide Web at:
The online version of this article, along with updated information and services, is
by the American Academy of Pediatrics. All rights reserved. Print ISSN: 1073-0397.