Copyright © 2000, American Society for Microbiology. All Rights Reserved.
Brachyspira aalborgi
Infection Diagnosed by Culture and 16S
Ribosomal DNA Sequencing Using Human Colonic Biopsy Specimens
WOLFGANG KRAAZ,1BERTIL PETTERSSON,2ULF THUNBERG,3LARS ENGSTRAND,4ANDCLAES FELLSTRO¨ M5*
Department of Pathology,1Department of Oncology,3and Department of Microbiology,4University Hospital, S-751 85 Uppsala,
Department of Biotechnology, Royal Institute of Technology, S-100 44 Stockholm,2and Department of Large Animal Clinical
Sciences, Faculty of Veterinary Medicine, Swedish University of Agricultural Sciences, S-750 07 Uppsala,5Sweden
Received 17 March 2000/Returned for modification 11 June 2000/Accepted 28 July 2000
In this study we report on the isolation and characterization of the intestinal spirocheteBrachyspira aalborgi
using human mucosal biopsy specimens taken from the colon of a young adult male with intestinal spirochet-osis. A selective medium, containing 400g of spectinomycin/ml and 5g of polymyxin/ml was used for the isolation procedure. A high degree of similarity, in terms of phenotypic properties and 16S ribosomal DNA sequence, was observed between the isolated strain, named W1, and the type strain, 513A, ofB. aalborgi. A similarity of 99.7% in the nucleotide sequence was found between W1 and 513AT, based on the almost-complete
gene. A short segment of the 16S rRNA gene was amplified by PCR using genetic material enriched from paraffin-embedded biopsy specimens, which were taken from the patient on two occasions. The products showed 16S rRNA gene sequences virtually identical to that of strain 513ATin the actual region.
Immunohis-tochemistry was performed on the colonic biopsy specimens with a polyclonal antibody raised against an intestinal spirochete isolated in a previous case of human intestinal spirochetosis. The antibody reacted strongly with the spirochete on the luminal epithelium. No immune reaction was seen within or below the surface epithelium. Routine histology did not reveal signs of colitis. Electron microscopy showed spirochetes attached end-on to the colonic mucosal surface. The isolate grew poorly on a commonly used selective medium for intestinal spirochetes, which may explain previous failures to isolateB. aalborgi.
A large number of reports of intestinal spirochetosis (IS) in humans have been presented, but the clinical importance of human IS is still a controversial issue. Pathogenicity among human intestinal spirochetes has been indicated in numerous studies (2, 3, 4, 10, 12, 13, 16, 17, 20, 24, 33) and questioned in others (11, 18, 23). A constant finding, originally described by Harland and Lee in 1967 (14), is end-on attachment by the microbes to the colonic mucosal surface, but most of the re-ports do not define the spirochetes involved, and it is likely that they describe infestations of different microbial species. In addition to this bacterial infestation, most IS cases show an intact mucosal surface and no, or only minimal, nonspecific changes in the lamina propria. However, in some cases inflam-matory reactions have been recorded (1, 13, 17, 24), but the species of the spirochetes involved were unknown.
Two species of intestinal spirochetes have so far been iso-lated from humans, namely,Brachyspira(formerlySerpulina)
pilosicoliandBrachyspira aalborgi. The majority of the isolates
have been referred to asB. pilosicoli (9, 19, 20, 29, 30). B.
pilosicoliis well known as the agent of porcine IS, a common,
worldwide disease in pigs resulting in mild colitis and diarrhea (31). In addition to humans and pigs,B. pilosicoliinfects other hosts such as dogs (6) and birds (21). B. aalborgi has been isolated on only one occasion, from a human patient in Aal-borg, Denmark (15). Consequently, there is only one strain available for study (ATCC 43994). However, in a recent study, PCR amplification indicated frequent occurrences of the mi-crobe in patients with histological evidence of IS (22). Colonic
infections caused byB. aalborgiandB. pilosicolihave also been reported for nonhuman primates (5). Although somewhat smaller,B. aalborgishows a strong phenotypic similarity toB.
pilosicoli. Both organisms are weakly beta-hemolytic
anaer-obes, regularly waved, with tapered ends and four flagella inserted subterminally at each end. In 1983, Nielsen et al. claimed that B. aalborgiwas nonpathogenic to humans (23), because they were unable to relate the presence of the spiro-chetes to gastrointestinal symptoms. However, some contro-versy over the pathological and epidemiological significance of colonic colonization byB. aalborgistill exists. For example, two human immunodeficiency virus (HIV)-positive patients in Washington, D.C., with intestinal B. aalborgi infection both showed inflammatory infiltrates in the lamina propria. The infection was considered to be at least a contributing factor to intestinal symptoms experienced by one of the patients (13, 22).
In this report we describe the case of a young human male with longstanding intestinal symptoms and with IS diagnosed histologically in routine colonic biopsies. The diagnosis was confirmed by isolation of spirochetes from the biopsy speci-mens. The isolate was classified as a strain ofB. aalborgi be-cause of a high 16S ribosomal DNA (rDNA) sequence simi-larity with the type strain of the species. This isolate of B.
aalborgiis the first since 1982 and the second ever reported.
MATERIALS AND METHODS
Case report.A 23-year-old male with a two-and-a-half-year history of blood and mucus in the stool was subjected to fiber endoscopy by his local medical practitioner. His medical history was unremarkable, except for the intestinal complaints and a mother with a history of colitis. Physical examination results and laboratory findings were normal. A biopsy specimen taken from the left colon showed IS but no evidence of colitis. Because of this finding, the patient was admitted to the University Hospital, Uppsala, Sweden, for a total colonos-copy, which showed normal endoscopic findings. Biopsy specimens were then
* Corresponding author. Mailing address: Department of Large An-imal Clinical Sciences, Faculty of Veterinary Medicine, Swedish Uni-versity of Agricultural Sciences, Box 7018, S-750 07 Uppsala, Sweden. Phone: 46 18 671473. Fax: 46 18 672919. E-mail: Claes.Fellstrom @kirmed.slu.se.
3555
on May 15, 2020 by guest
http://jcm.asm.org/
taken from the terminal ileum, cecum, ascending colon, transverse colon, de-scending colon, colon sigmoideum, and rectum. Corresponding biopsy specimens were used as inocula for the culture of spirochetes and taken for both routine histology and immunostaining with an antispirochete polyclonal antibody.
Histopathology.The biopsy specimens were processed and paraffin embedded for histological examination by standard techniques. The paraffin sections were stained with hematoxylin-eosin (HE) and examined at⫻400 magnification.
Immunohistochemistry.A polyclonal rabbit antiserum was produced by intra-venously immunizing rabbits with suspensions of an “uncharacterized” intestinal spirochete. The spirochete had been isolated from a colonic mucosal biopsy specimen in a previous case of human IS at the University Hospital, Uppsala. The specificity of the antibody was determined by indirect immunofluorescence (27) with the type strains of five species: humanB. aalborgiand porcineBrachyspira hyodysenteriae,Brachyspira innocens,Brachyspira murdochii, andB. pilosicoli. A strain ofEscherichia coliwas used as a negative control. The immunohistochemical examination was carried out on paraffin sections using the avidin-biotin complex (ABC) method (34).
Electron microscopy.For transmission electron microscopy (TEM), the tissue was cut from one paraffin block in small cubes measuring about 2 mm3. The
tissue was deparaffinized, postfixed in 1% osmium tetroxide (OsO4) in phosphate
buffer (pH 7.4), embedded in Epon (agar 100 resin), stained with uranyl acetate and lead citrate, and thereafter mounted on copper grids. The grids were ana-lyzed with a Philips 420 electron microscope, operating at 60 kV.
Isolation and culture of spirochetes.Isolation was attempted on seven speci-mens of biopsy material obtained during colonoscopy from sites covering the ileum-colon-rectum of the patient. The biopsy specimens were streaked onto agar plates within 1 h after sampling. A selective medium was used, consisting of tryptose soy agar (TSA) to which was added 10% bovine blood, 400g of spectinomycin per ml, and 5g of polymyxin per ml (15). The same medium, but without antibiotics, was used to determine the intensity of beta-hemolysis and for maintenance of the isolates. Spirochetes were also cultured on FA agar (Fastid-ious Anaerobe agar; Lab M code LAB 90 BaktDia; National Veterinary Insti-tute, Uppsala, Sweden) and in two liquid media, brain heart infusion (BHI) broth with 5% calf blood and aBorreliamedium, BSK-H (Sigma). Finally, an attempt was made to culture the spirochetes onSerpulinaagar plates (blood agar base
number 2 [Oxoid code CM 271], supplemented with 5% citrated sheep blood, 1% sodium ribonucleat, 25g of vancomycin HCl solution, 25g of colistin sulfate, and 800 g of spectinomycin per ml). All plates were incubated in anaerobic jars at 37°C for 5 to 28 days under an atmosphere of 90 to 95% H2and
5 to 10% CO2. Plates were also incubated in a cabinet (model 1024/1028; Forma
Scientific) under an atmosphere of N, CO, and H (80:10:10). Growth of spiro-chetes was confirmed, and the organisms were studied, by phase-contrast mi-croscopy (1,000⫻). After examination and biochemical testing, cultures were frozen and stored in liquid nitrogen (⫺196°C). The storage medium used was beef broth with 10% horse serum and 15% glycerol. Plates containing the spirochetes were also stored anaerobically at room temperature for as long as 3 months.
Type strains of six species of intestinal spirochetes,B. hyodysenteriaeB78,
Brachyspira intermediaPWS/A,B. innocensB256,B. murdochii155-20,B. pilosi-coliP43, andB. aalborgi513A (NCTC 11,492), originating from a strain collec-tion at the Nacollec-tional Veterinary Institute, were cultured as previously described (7).
Biochemical testing.The enzymatic reactions of isolated human spirochetes and the type strains were tested with the API-ZYM system, as described by the manufacturer (API, Marcy-I’Etoile, France). A spot test (28) was used to deter-mine indole production by smearing the growth from a culture onto a filter paper saturated with the indole reagent (1%p-dimethylaminocinnamaldehyde in 10% hydrochloric acid). The method of Ru¨bsamen and Ru¨bsamen (26) was used to test for hippurate hydrolysis.
PCR amplification, sequencing, and sequence analysis of the 16S rDNA gene.
A pair of primers to specifically detect the presence of the 16S rDNA gene of spirochetes of the genusBrachyspira(Serpulina) was designed from sequences obtained from the GenBank sequence database. Primers 5⬘-GTCTTAAGCAT GCAAGTC and 3⬘-AACAGGCTAATAGGCCG, generating a 207-bp frag-ment, were used. The PCR assay was carried out on two biopsy specimens taken during colonoscopy on two subsequent occasions from the patient in this study. The PCR amplicons were used directly for sequencing (U. Thunberg, unpub-lished data).
[image:2.612.91.514.71.399.2]The virtually complete 16S rDNA sequence of the isolated spirochete was determined by direct solid-phase DNA sequencing with primers and protocols as FIG. 1. HE-stained section shows a hematoxyphilic fringe on the brush border of the colonic surface epithelium. The fringe consists of microorganisms attached
end-on to the epithelial surface.
on May 15, 2020 by guest
http://jcm.asm.org/
described previously (25). This sequence and the partial 16S rDNA sequences were aligned with that ofB. aalborgistrain 513AT(accession number Z22781). Nucleotide sequence accession number.The virtually complete 16S rDNA sequence of the isolated spirochete was deposited in GenBank under accession number AF200693.
RESULTS
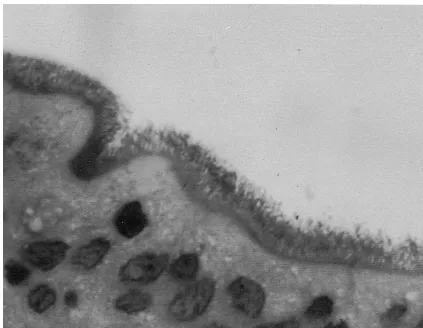
Light microscopy. IS was diagnosed histologically in HE-stained sections by the presence of a hematoxyphilic “fuzzy coat” on the brush border of the surface epithelium. Spiro-chete attachment to epithelial cells was present in all sections of the large intestine, with decreasing intensity, however, from the cecum to the rectum. No bacteria were seen in the crypts. At⫻400 magnification this coat seemed to consist of a forest of thin sinusoidal microorganisms attached end-on to the cell membrane (Fig. 1). The mucosal crypts showed normal archi-tecture and undamaged goblet cells. There was no evidence of colitis.
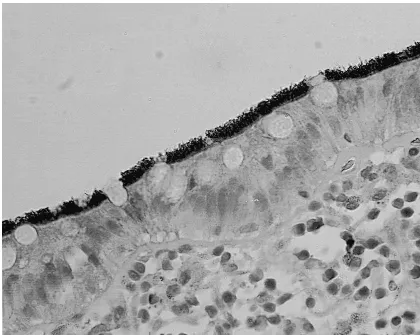
Immunohistochemistry. The antiserum reacted strongly with the spirochetal antigen in the paraffin sections, producing a marked contrast of the fuzzy coat against the epithelial sur-face with a preserved spiral shape of the microbes (Fig. 2). No further antigen deposits were detected below the surface epi-thelium, either in the crypts or in the lamina propria. The polyclonal antibody reacted with all the tested type strains of the intestinal spirochetes, but not with theE. colistrain, in the indirect immunofluorescence test.
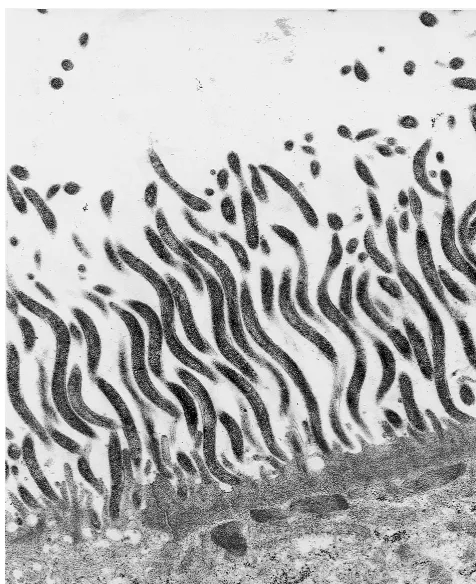
Electron microscopy.Numerous spiral-shaped microorgan-isms attached to the luminal surface of the epithelium were observed (Fig. 3). Except for effacement of the microvilli, the epithelial cells appeared unaffected. The spirochetes were al-ways attached end-on to the cell membrane, between and par-allel to the microvilli. Condensation of uncharacterized elec-tron-dense material was observed along the apical cytoplasm of colonized epithelial cells.
[image:3.612.92.512.72.407.2]Culture of human spirochetes.After 1 to 2 weeks of incu-bation, a thin haze of small, pinpoint-like colonies of bacterial growth in a mixed flora could be seen on all seven agar plates inoculated with biopsy material. Phase-contrast microscopy re-vealed spirochetes and unclassified cocci. Spirochetes were routinely transferred to fresh TSA and FA agar plates every 2 weeks to obtain pure cultures for further analyses. With the exception of theSerpulinaplates, where only poor growth was achieved, the types of solid medium used did not appear to affect either the number of organisms or the type of growth. The spirochetes grew well in both of the liquid media tested, BSK-H (Sigma) and BHI broth with 5% calf blood. Viable spirochetes could be recovered from plates which had been kept in an anaerobic jar for more than 3 months at room temperature. Enhanced microbial growth was achieved after subculturing. The first appearance of bacterial growth was then seen after 5 to 7 days of incubation as pinpoint-like, transpar-ent colonies with weak hemolytic activity. After 2 to 4 weeks, the spirochete colonies fused, forming opaque, grayish carpets
FIG. 2. Upon immunostaining the microorganisms are seen to react with the spirochete antiserum, producing a marked contrast with the fringe detected over the colonic mucosal surface. Antigen deposits cannot be detected in the lamina propria.
on May 15, 2020 by guest
http://jcm.asm.org/
at the sites of inoculation. Spirochetes from one plate were selected for further studies. This isolate was designated W1.
Biochemical testing. The enzymatic and biochemical reac-tions of the isolated human spirochete W1 and the six type strains of intestinal spirochetes are presented in Table 1. The reaction pattern of W1 was similar to that of the type strain of
B. aalborgi, 513A. Minor differences were noted with regard to
the alkaline and esterase activities. Furthermore, a resem-blance between the biochemical reaction pattern of W1 and that of theB. pilosicolitype strain, P43, was noted. W1 shared
a negative-glucosidase reaction with P43. A negative -glu-cosidase reaction of intestinal spirochetes has been described only forB. aalborgi(15) andB. pilosicoli(8). Strain W1 differed from P43T by showing negative ␣-galactosidase activity.
Fi-nally, W1 showed weaker hippurate hydrolysis and alkaline phosphatase, esterase, and esterase-lipase reactions than P43. A strong hippurate cleavage reaction is a feature commonly used for identification ofB. pilosicoli.
Morphology of spirochetes in phase-contrast microscopy. On primary plates, the appearance of the organisms was very similar to the original description of B. aalborgi (15). The spirochetes were short and thin (Fig. 1). Some cells were com-ma-shaped, and others were helical with one or two complete turns. Notably, and in agreement with the original description, many of the microbes were attached to the cover glass by one end, around which they rapidly gyrated.
After several freeze-thaw steps, microbes of strain W1 looked larger, more motile, and similar to intestinal spiro-chetes of other species, e.g.,B. hyodysenteriaeandB. innocens. PCR amplification and sequence analysis of the 16S rDNA gene.PCR products of the expected size, ⬃207 bp, were ob-tained from both of the biopsy specimens, investigated. The partial 16S rRNA sequences were identical with the corre-sponding sequence of the type strain of B. aalborgi, 513A. Nucleotide sequence comparison using almost-complete pri-mary structures from strain W1 andB. aalborgi513ATrevealed
a nucleotide similarity of 99.7%. Differences in nucleotide composition were as follows (positions given according toE. colinumbering). Strain W1 was found to have guanosine res-idues in positions 38, 1089, 1094, and 1388, whereB. aalborgi
513ATlacks nucleotide information. Furthermore, strain W1
had G, G, C, and G in positions 630, 1099, 1246, and 1475, whileB. aalborgi513AThas A, C, T, and T, respectively.
Both its phenotypic properties and its high 16S rDNA sim-ilarity toB. aalborgi513ATjustify the classification of strain W1
as belonging to the speciesB. aalborgi.
DISCUSSION
This is the first reported isolation ofB. aalborgiby culture since 1982 and the second ever reported. We found thatB.
aalborgi grew poorly on a selective medium which included
three antibiotics; vancomycin, colistin, and spectinomycin. Similar media are commonly used in routine diagnostics of intestinal spirochetes. In the original description ofB. aalborgi
[image:4.612.55.293.71.363.2](15), a selective medium which included only two antibiotics,
FIG. 3. Transmission electron micrograph of organisms attached end-on to the colonic mucosa, showing a “false brush border.” The photomicrograph shows effacement of the microvilli and condensation of uncharacterized electron-dense material along the apical cytoplasm of colonized epithelial cells. Magnification, ⫻11,000.
TABLE 1. Biochemical reactions ofB. aalborgistrain W1 and sixBrachyspiratype strains
Strain Reaction
awith enzymeb:
Hc Id Hee 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20
B. aalborgiW1 0 0 1 1 0 0 0 0 0 0 2 2 0 5 0 0 0 0 0 0 w 0 w
B. aalborgi513AT 0 1 0 1 0 0 0 0 0 0 2 2 0 5 0 0 0 0 0 0 w 0 w
B. hyodysenteriaeB78T 0 3 1 1 0 0 0 0 0 0 3 2 0 5 0 3 4 0 0 0 0 ⫹ s
B. intermediaPWS/AT 0 2 1 1 0 0 0 0 0 0 2 2 0 5 0 2 3 0 0 0 0 ⫹ w
B. innocensB256T 0 3 2 1 0 0 0 0 0 0 4 2 5 5 1 3 5 0 0 0 0 0 w
B. murdochii155-20T 0 3 3 1 0 0 0 0 0 0 3 2 0 5 0 3 4 0 0 0 0 0 w
B. pilosicoliP43T 0 3 3 2 0 0 0 0 0 0 2 2 2 5 0 0 0 0 0 0 s 0 w
aNumerals indicate levels of reaction; 0, negative reaction; 1, weak positive reaction; 5, strong positive reaction.
bEnzyme numbers 1 to 20 refer to the following enzymes: 1, control; 2, alkaline phosphatase; 3, esterase; 4, esterase-lipase; 5, lipase; 6, leucine arylamidase; 7, valine arylamidase; 8, cysteine arylamidase; 9, trypsin; 10, chymotrypsin; 11, acid phosphatase; 12, naphthol-AS-BI-phosphohydrolase; 13,␣-galactosidase; 14,-galactosidase; 15,-glucuronidase; 16,␣-glucosidase; 17,-glucosidase; 18,N-acetyl--glucosidase; 19,␣-mannosidase; 20,␣-fucosidase.
cH, hippurate cleavage reaction; w, weak reaction; s, strong reaction. dI, indole reaction;⫹, positive reaction.
eHe, hemolysis.
on May 15, 2020 by guest
http://jcm.asm.org/
[image:4.612.51.552.571.671.2]spectinomycin and polymyxin, was used. A similar medium was used in this study for the isolation procedure. As the antibac-terial resistance pattern ofB. aalborgiis unknown, the inclu-sion of antibacterials different from spectinomycin and poly-myxin in selective media may very well explain previous failures to isolateB. aalborgi. Another reason for the rarity of
B. aalborgiisolates may be the slow growth in vitro compared
to other known species of intestinal spirochetes (15).B.
aal-borgiresembles the frequently isolated intestinal spirocheteB.
pilosicoliin many respects, e.g., morphology and the end-on
attachment to the epithelial surface. Furthermore, our results indicate that commonly used biochemical tests cannot provide reliable differentiation between the two species. Although some differences were noted between the B. aalborgistrains and the type strain of B. pilosicoli (e.g., B. aalborgi strains showed a negative ␣-galactosidase reaction and no evident hippurate cleavage capacity), B. pilosicoliisolates with diver-gent biochemical reactions have been reported (8). The slower growth rate ofB. aalborgi after primary isolation (⬎1 week) compared to B. pilosicoli(2 to 4 days), as well as the lack of
␣-galactosidase and evident hippurate cleavage capacity, may be used to indicate the presence ofB. aalborgi. However, more strains have to be isolated and tested. A more reliable diagno-sis might be achieved by using aB. aalborgi-specific PCR assay. Such assays, based on 16S rDNA sequences, have been de-scribed previously (5, 22). A problem with the PCR assays is that, due to the lack of availableB. aalborgistrains, the primer pairs used have been designed without knowledge of the in-traspecies 16S rRNA nucleotide variation. Therefore, it is not known if the primer pairs designed detect all strains of B.
aalborgi. B. aalborgi has been identified only in humans and
nonhuman primates. It is not known if other hosts exist that could act as sources of infection for humans. We found that the microbes could survive for more than 3 months if they were stored anaerobically at room temperature. Therefore it is likely that fecal environmental contamination constitutes a long-standing potential risk of infection withB. aalborgi.
In this study we described a patient whose symptoms were characterized by blood and mucus in the stool, but with normal endoscopic and laboratory findings. The only abnormal feature was the presence of large numbers of B. aalborgiorganisms attached to the colonic surface epithelium. No histological signs of microbial invasion and/or inflammatory reactions were found. A literature survey did not reveal whether infection by
B. aalborgiis always harmless, or whether the spirochetes may
be pathogenic under certain conditions. The blockage of pas-sive absorption by large numbers of spirochetes on the colonic epithelium has been suggested as a possible pathogenic mech-anism in IS (10). Such a blockage could probably result in diarrhea, but this was not a prominent symptom in this patient. Furthermore, the spirochetes might irritate the mucin-secret-ing cells of the mucosa, thus leadmucin-secret-ing to increased mucin pro-duction. The pathogenicity of the organism may therefore be dependent on the extent and degree of infestation (10). A third explanation for the intestinal complaints may be the organism’s interference with neural transmission, causing altered colonic motility. In the latter case, the symptoms may persist even after the resolution of the infection (24). However, in conclusion, the capacity of B. aalborgi to cause disease in humans still requires further assessment.
ACKNOWLEDGMENTS
This work was supported by grants from the Ivar and Elsa Sandbergs Foundation.
We acknowledge the skillful technical assistance of Mia Thorse´lius, Zhongmin Guo, Tapio Nikkila¨, and Ulla Zimmerman.
REFERENCES
1.Antonakopoulos, G., J. Newman, and M. Wilkinson.1981. Intestinal spiro-chaetosis: an electron microscopic study of an unusual case. Histopathology
6:477–488.
2.Cotton, D. W. K., N. Kirkham, and D. A. Hicks.1984. Rectal spirochaetosis. Br. J. Vener. Dis.60:106–109.
3.Crusioli, V., and A. Busuttil.1981. Human intestinal spirochaetosis. Scand. J. Infect. Dis.16:177–179.
4.Douglas, J. G., and V. Crusioli.1981. Spirochaetosis: a remediable cause of diarrhoea and rectal bleeding. Br. Med. J.283:1362.
5.Duhamel, G. E., R. O. Elder, N. Muniappa, M. R. Mathiesen, W. J. Wong, and R. P. Tarara.1997. Colonic spirochetal infections in nonhuman primates that were associated withBrachyspira aalborgi,Serpulina pilosicoliand un-classified flagellated bacteria. Clin. Infect. Dis.25:186–188.
6.Duhamel, G. E., D. J. Trott, M. R. Muniappa, M. R. Mathiesen, K. Tarasuik, J. I. Lee, and D. J. Hampson.1998. Canine intestinal spirochetes consist of
Serpulina pilosicoliand a newly identified group provisionally designated “Serpulina canis” sp. nov. J. Clin. Microbiol.36:2264–2270.
7.Fellstro¨m, C., and A. Gunnarsson.1995. Phenotypical characterisation of intestinal spirochetes isolated from swine. Res. Vet. Sci.59:1–4.
8.Fellstro¨m, C., B. Pettersson, J. Thomson, A. Gunnarsson, M. Persson, and K.-E. Johansson.1997. Identification ofSerpulinaspecies associated with porcine colitis by biochemical analysis and PCR. J. Clin. Microbiol.35:462– 467.
9.Fournie´-Amazouz, E., G. Baranton, J. P. Carlier, G. Chambreuil, F. Coha-don, P. Collin, A. G. Jolivet, I. Hermes, C. Lemarie, and I. Saint Girons.
1995. Isolations of intestinal spirochetes from the blood of human patients. J. Hosp. Infect.30:160–162.
10. Gad, A., R. Wille´n, K. Furugård, B. Fors, and M. Hradsky.1977. Intestinal spirochaetosis as a cause of long-standing diarrhoea. Uppsala J. Med. Sci.
82:49–54.
11. Gear, E. V., and W. O. Dobbins.1968. Rectal biopsy. Gastroenterology
55:522–544.
12. Gebbers, J. O., D. J. P. Ferguson, C. Mason, P. Kelly, and D. P. Jewell.1987. Spirochaetosis of the human rectum associated with an intraepithelial mast cell and IgE plasma cell response. Gut28:588–593.
13. Guccion, J. G., D. A. Benator, J. Zeller, B. Termanini, and N. Saini.1995. Intestinal spirochetosis and acquired immunodeficiency syndrome: ultra-structural studies of two cases. Ultrastruct. Pathol.19:15–22.
14. Harland, W. A., and F. D. Lee.1967. Intestinal spirochaetosis. Br. Med. J.
3:718–719.
15. Hovind-Hougen, K., A. Birch-Andersen, R. Henrik-Nielsen, M. Orholm, J. O. Pedersen, P. S. Teglbjærg, and E. H. Thayson.1982. Intestinal spiro-chetosis: morphological characterization of the spirocheteBrachyspira aal-borgigen. nov., sp. nov. J. Clin. Microbiol.16:1127–1136.
16. Kaplan, L. R., and A. Takeuchi.1979. Purulent rectal discharge associated with a nontreponemal spirochete. JAMA241:52–53.
17. Kostman, J. R., M. Patel, E. Catalano, J. Camacho, J. Hoffpauir, and M. J. DiNubile.1995. Invasive colitis and hepatitis due to previously uncharacter-ized spirochetes in patients with advanced human immunodeficiency virus infection. Clin. Infect. Dis.21:1159–1165.
18. Lee, F. D., A. Kraszevski, J. Gordon, J. G. R. Howie, D. McSeveney, and W. A. Harland.1971. Intestinal spirochaetosis. Gut12:126–133.
19. Lee, J. I., and D. J. Hampson.1992. Intestinal spirochetes colonizing Ab-origines from communities in the remote north of Western Australia. Epi-demiol. Infect.109:133–141.
20. Lee, J. I., and D. J. Hampson.1994. Genetic characterisation of intestinal spirochetes and their association with disease. J. Med. Microbiol.40:365– 371.
21. McLaren, A. J., D. J. Trott, D. E. Swayne, S. L. Oxberry, and D. J. Hampson.
1997. Genetic and phenotypic characterization of intestinal spirochetes col-onizing chickens and allocation of known pathogenic isolates to three dis-tinct genetic groups. J. Clin. Microbiol.35:412–417.
22. Mikosza, A. S. J., T. La, J. Brooke, C. Lindboe, P. B. Ward, R. G. Heine, J. G. Guccion, W. Bastiaan de Boer, and D. J. Hampson.1999. PCR amplification from fixed tissue indicates frequent involvement ofBrachyspira aalborgiin human intestinal spirochetosis. J. Clin. Microbiol.37:2093–2098. 23. Nielsen, R. H., M. Orholm, J. O. Pedersen, K. Hovind-Hougen, P. S.
Tegl-bjærg, and E. H. Thaysen.1983. Colorectal spirochaetosis: clinical signifi-cance of the disease. Gastroenterology85:62–67.
24. Padmanabhan, V., J. Dahlstrom, L. Maxwell, G. Kaye, A. Clarke, and P. J. Barrett.1996. Invasive intestinal spirochetosis: a report of three cases. Pa-thology28:283–286.
25. Pettersson, B., C. Fellstro¨m, A. Andersson, M. Uhle´n, A. Gunnarsson, and K.-E. Johansson.1996. The phylogeny of intestinal porcine spirochetes ( Ser-pulinaspecies), based on sequence analysis of the 16S rRNA gene. J. Bac-teriol.178:4189–4199.
26. Ru¨bsamen, S., and S. Ru¨bsamen.1986. Hippurat-hydrolyse: ein Schnelltest zur Unterscheidung vonTreponema hyodysenteriaeundTreponema innocens. Tiera¨rztl. Umschau.41:673–677.
27. Saunders, C. N., and D. Hunter.1974. A fluorescent antibody staining technique for the diagnosis of swine dysentery. Vet. Rec.94:491–492.
on May 15, 2020 by guest
http://jcm.asm.org/
28.Sutter, V. L., and W. T. Carter.1972. Evaluation of media and reagents for indole-spot tests in anaerobic bacteriology. Am. J. Clin. Pathol.58:335–338. 29. Trivett-Moore, N. L., G. L. Gilbert, C. L. H. Law, D. J. Trott, and D. J. Hampson.1998. Isolation ofSerpulina pilosicolifrom rectal biopsy specimens showing evidence of intestinal spirochetosis. J. Clin. Microbiol.36:261–265. 30.Trott, D. J., C. R. Huxtable, and D. J. Hampson.1996. Experimental infec-tion of newly weaned pigs with human and porcine strains ofSerpulina pilosicoli. Infect. Immun.64:4648–4654.
31.Trott, D. J., T. B. Stanton, N. S. Jensen, G. E. Duhamel, J. L. Johnson, and D. J. Hampson.1996.Serpulina pilosicolisp. nov.: the agent of porcine intestinal spirochetosis. Int. J. Syst. Bacteriol.46:206–215.
32.Trott, D. J., B. G. Combs, A. S. J. Mikosza, S. L. Oxberry, I. D. Robertson, M. Passey, J. Taime, R. Sehuko, M. P. Alpers, and D. J. Hampson.1997. The prevalence ofSerpulina pilosicoliin humans and domestic animals living in the Eastern Highlands of Papua New Guinea. Epidemiol. Infect.119:369– 379.
33.Wille´n, R., B. Carle´n, J. Cronstedt, and H. Wille´n.1985. Intestinal spiro-chaetosis of the colon diagnosed with colono-ileoscopy and multiple biop-sies. Endoscopy17:86–88.
34.Wood, G. S., and R. Warnke.1981. Suppression of endogenous avidin-binding activity in tissues and its relevance to biotin-avidin detection systems. J. Histochem. Cytochem.29:1196–1204.