Extraordinary disease free survival in a rare malignant extrarenal rhabdoid tumor: a case report and review of the literature
Full text
Figure
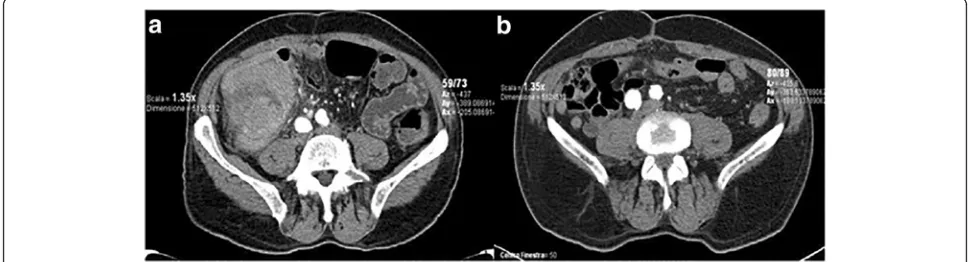

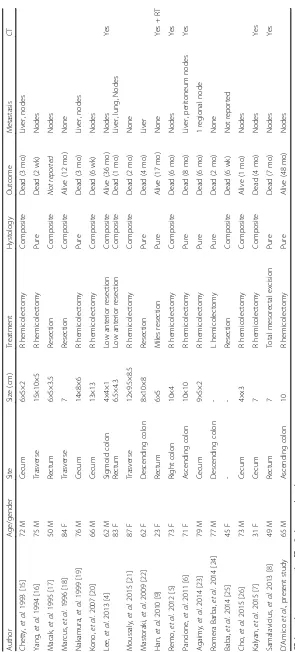

Related documents
adult males in this age group prefer a testosterone treat- ment gel dispensed in small units allowing accurate dosage measurement, a shorter waiting time after the gel applica-
This paper puts forward an in- line detection method based on inertial navigation, which can be employed to perform the all-round detection of flexural strain
In order to achieve an authentic simulation effect in garment simulation and to avoid the occurrence of over-stretching (super stretch), it’s necessary to make a
A variety of particle sizes, shapes, and densities were obtained through the use of polystyrene, nylon, polyester, styrene acrylonitrile, and glass particles, used in configu-
embryonic stem cell research, only 15 states have legislation relating to human cloning, and, within these laws what is covered and prohibited varies (The National Conference of State
The INDDEP study is a naturalistic study, which aims at the description of symptom courses in patients with de- pression receiving inpatient or day hospital treatment in
A random sample of 260 children (11%) who were classified as “negative for asthma” with the screening procedure, ie, classified as having no asthma with the symptom questionnaire ( n
Control practices completed a modified version of the same ques- tionnaire (without specific intervention questions) immediately after enrolling in the study. We were