0022-538X/80/07-0157/08$02.00/0
Control
of
Simian
Virus
40
Gene
Expression
at
the
Levels
of
RNA
Synthesis
and
Processing:
Thermally
Induced
Changes
in
the
Ratio of the
Simian
Virus 40
Early mRNA's and
Proteins
JAMES C. ALWINE'* ANDGEORGEKHOURY2
DepartmentofMicrobiology, School of Medicine, University of Pennsylvania, Philadelphia,Pennsylvania
19104,'andLaboratory ofMolecularVirology,NationalCancerInstitute,National InstitutesofHealth,
Bethesda, Maryland202052
Examination of thesimian virus40early mRNA's frominfectedAGMKor
CV-1 cells showed that the ratio of large T- tosmall t-antigen mRNA's increased
with an increased incubation temperature. In tsA58 mutant-infected cells, an
increased incubationtemperatureresulted in the overproductionofearly RNAs; however, the ratio of the early mRNA's was the same,at any temperature, in bothwild-type-andtsA58-infected cells.Thus, thethermallyinduced alteration
intheearlymRNA ratioswasapparentlynotaffected by the tsA mutationorby the overproduction of early RNA in tsA mutant-infected cells. Time course
studies atvarious temperatures showed that, although the ratio of large T- to
small t-antigen mRNA's increased with temperature,atany one temperatureit
was constantfromearlytolate times of infection. Furthernore, the ratio of the
early mRNA's adjusted intemperatureshiftexperiments. Thus, the ratio of the early mRNA's appearedtobeintrinsictothe thermodynamic environment of the cell. Thethermally induced alterationsintheearly mRNA'swerereflectedatthe protein level byparallelchanges in the ratio of largeT- tosmallt-antigens. These
datasuggest alevel ofgeneexpressioncontrol whichmayfunctionatthestageof
sphlcing.
Thesmall DNAtumorviruses haveprovided excellent modelsystemsforstudying the control ofgene expression. Inthisregard,simian virus
40 (SV40;see Fig. 1) hasbeen particularly val-uable for theanalysisoftranscriptionaland
post-transcriptional regulatory mechanisms. The temporal expression of early andlateSV40genes
appearstobe controlledatthelevel of
transcrip-tion (2, 5, 15,21). These studies showed thata
primary factor in determining the transcrip-tional activity ofthe SV40 genes isone of the early gene products, large T-antigen, which is thoughttohavearepressor-likeactivity in mod-ulating early transcription. These conclusions
are based, in part, onstudies with early SV40 temperature-sensitive mutants (tsA mutants). Atelevatedtemperaturesthesemutantsbecome
defective inlarge T-antigenfunction(la, 19, 28,
30) andoverproduceearlyviralRNA (2, 15,21).
The primary early and late transcripts are
modifiedsubsequent totheirsynthesis by
cap-ping,polyadenylation,internalmethylation, and
splicing.All of these modificationsare alsofound inthebiosynthesisofeucaryoticmRNA's. What
appears, thusfar,tobe unique forviral mRNA's
is the observation that one primary transcript
may be the precursor for different mRNA's
throughalternatesplicingevents. Forexample,
a single primary transcript of the SV40 early coding region may be the precursor for the mRNA coding for either small t- or large T-antigen,dependingonthe location of thesplicing
event(4,6). If this is thecase,thenthe selection
of the splicing eventprovidesapotential
regu-latory step inearly SV40 gene expression,and factors influencing the relative frequency of eithersplice will control the relative abundance of the two early SV40 proteins, large T- and smallt-antigens.
The study reportedhere wasinitially under-takentodeterminewhetherlarge T-antigen
reg-ulatesnotonlytherateofsynthesisofprimary early transcripts, but also the relative amounts oflarge T- and small t-antigen mRNA's. Our
results indicatedamuch more interesting
phe-nomenon;wefound inbothwild-type (WT)-and
tsA mutant-infectedcellsthat theratio oflarge
T- to small t-antigen mRNA's increased and
decreasedwith incubation temperature. These
thermally induced alterations in the early
mRNA ratios werereflected at theproteinlevel
by similaralterations in thelargeT-tosmall t-157
on November 10, 2019 by guest
http://jvi.asm.org/
158 ALWINE AND KHOURY
antigen ratio. Thus,thethermal(or thegeneral thermodynamic) environmentof thecellalters the expression of the early genes, indicating a
level ofgene
expression
controlwhich may func-tionatthe level of splicing.MATERIALS AND METHODS
Cellsand viruses. Primary African green monkey
kidneycells(AGMK) and theestablishedAGMK line CV-1 were cultivated in minimal essential medium plus 10% fetal calf serum. The virus strains used were the WTstrain VA4554 (25)anda
temperature-sensi-tive mutantderived from it, tsA58 (26, 27).
Infectionof cells andpreparation of cytoplas-niicRNA. Confluent monolayers of cells in 150-cm2 Costarplasticbottles were infected at a multiplicity of
10 PFU/cell in 5 ml ofminimal essential medium
containing 2%fetal calf serum. After rocking at room temperature for 1.5h, 20 ml of medium was added, and thecells were incubated at320Cfor 40 h. At this point, cellswereharvested (32°C samples) orshifted to 41°C for5h (shifted samples) before harvesting. Othercultures wereinfected for24hat41°C before harvesting (41°C samples). Cytoplasmic RNA from infected cells was prepared as previously described (13).PolyadenylatedRNA wasselected oncolumns of
oligodeoxythymidylic acid-cellulose(3).
Preparationof52P-labeledSV40 DNA
hybrid-izationprobes.SV40 DNAwaslabeled in vivo and
purifiedaspreviouslydescribed (16). The labeled form
ISV40 DNA wasdigested with appropriate restriction endonucleases (New England BioLabs, Waltham, Mass.), using conditions indicated by the company. The reaction mixtures were phenol extracted and ethanolprecipitated,and the strands of thefragments
wereseparatedbythemethod of Hayward (10).
Sep-aratedstrands were electroeluted,self-annealed,and
chromatographed on hydroxyapatite. The resulting
purified singlestrandsweredialyzedinto10mM
Tris-hydrochloride(pH7.5)-imM EDTA.
Hybridizationofsingle-strandedDNA probes
and cytoplasmicRNA. To maintain conditionsof DNAexcess, the amountof RNAused in each hybrid-ization wasvaried according to the abundance in the
sample. DNA excessconditionswere establishedby
titration of differentsamples withseparated strand probes containing the early coding region. Samples
contained0.5to10
,g
ofpolyadenylatedRNA or5 to70jugofnonpolyadenylatedRNA. Foreach reaction,
10ng (10,000 cpm) of DNAprobe was added. The RNAandprobewereethanolprecipitatedtogether in silane-treated tubes. The pelletsweredried ina vac-uumand thendissolved in30plof 50%formamide-0.4
M NaCl-0.04 M PIPES
[piperazine-N,N'-bis(2-eth-anesulfonic acid)](pH6.5). Thesampleswere
hybrid-izedat370Cfor24to 72h. Longhybridizationtimes wereusedtoinsure that the reactionswerecomplete;
this varied with thesample and had beenpreviously
determined.
Analysis ofhybrids bynucleaseSIdigestion
and alkalinegelelectrophoresis.After hybridiza-tion, thesamplesweretreated with nucleaseS1 and
electrophoresedon 1.4 or1.8%alkaline agarosegelsas
described by Berk and Sharp (4). Thegelsweredried onDEAE paper andautoradiographedat-70°C,
us-ingKodak XR-1 X-ray film and a Du Pont Cronex Lightning-plusintensifyingscreen.
Analysis of
[3'Sjmethionine-labeled
viral pro-teins. For theanalysisof the ratio oflargeT-tosmallt-antigens,confluent monolayers of AGMK or CV-1 cells, grown in 50-mm plates (three plates per set), wereinfected with VA4554 or tsA58 at a multiplicity of 10. The infectedcells wereincubated for40h at
320C.
Atthis point, thecellswerepulse-labeledfor1 h at320C
with[nS]methionine
orshiftedto41°C for 4hbeforepulse-labelingfor1hat410C.
Athirdsetof cultures wasinfected entirely at410C
for 24 handthen pulse-labeled for 1 h. The pulse-labeling was
accomplishedby washing the cells threetimes with
appropriately warmed (32 or
410C)
methionine-freeminimalessentialmediumcontaining 2%dialyzedfetal bovine serumand then labelingeach platewith 1ml ofprewarmedmediumcontaining200giCiof [3S]me-thionine (300 Ci/mmol; Amersham Corp., Arlington
Heights,Ill.).Afterlabeling, theplateswereputonice and washed three times with cold analysis buffer
(Tis-bufferedsaline,pH8,containing1mMdithiothreitol,
300,ugofphenylmethylsulfonyl fluoride perml, and 150Agoftosylamid-2-phenylethylchloromethylketone perml).Thecellswerescrapedinto1.5ml ofanalysis
buffer,sedimentedat3,000 rpm for 10 min,suspended
inanalysisbuffercontaining0.5% NonidetP-40,
incu-bated onice for30min, andvigorouslyblended ina Vortex mixer every 5 min. The lysates were sedi-mentedat15,000 rpm for60min, and thesupernatant fractionwaswithdrawn andsaved.Samples(0.3 ml)
of the supernatants were immunoprecipitated with
heat-inactivated Staphylococcus aureus and
antise-rum (directed against the SV40 early proteins) as
described by Jay et al. (12). The serum had been titratedpreviouslytodetermineoptimalconditions for thesesamples.Both theserumand S. aureuswerethe gift ofGilbert Jay.Immunoprecipitates (in15- to 20-plvolumes)wereanalyzedbyelectrophoresison10% sodiumdodecylsulfate-polyacrylamideslabgels (11).
Radiolabeledproteinsweredetectedbyfluorography
(17). After location ofspecificbandsonthegels,the driedgelsegmentscontainingthemwereexcised and
rehydratedinwaterfor20minat roomtemperature.
Thegelsegmentswerethenequilibratedindimethyl
sulfoxideto removethediphenyloxazoleused in
fluo-rography(fivewashes,2ml ofdimethylsulfoxideeach,
for20minatroomtemperature).Thegelsliceswere
equilibrated inwater and thentreated with 1 ml of 0.8% ammonium bicarbonatecontaining60
pLg
of tryp-sinfor12hat370C.
Themixturewasthencountedin 10ml ofAquasol(NewEnglandNuclearCorp., Bos-ton,Mass.).RESULTS
Experimental
approach.Totalcytoplasmicor polyadenylated cytoplasmic RNA (both of
which gave identical results in the following
experiments) was isolated from SV40-infected monkeykidney cells under avariety of experi-mentalconditions. TheseRNAswereannealed
with 3P-labeled, separated, single-stranded
DNAprobes containingtheentireknownearly coding regions oftheSV40genome.Thehybrid
on November 10, 2019 by guest
http://jvi.asm.org/
moleculeswereassayed by theSi nuclease
tech-niqueofBerk and Sharp (4), followed by alkaline
agarose gel electrophoresis and
autoradiogra-phy. For the quantitation of the amounts of
mRNAspeciesfrom autoradiographic band
in-tensities, hybridization reactions were
per-forned in DNA excess, and incubation times
wereprolongedtoinsure that reactions hadgone tocompletion.
Synthesis of large T- and small t-antigen mRNA's. Previous experiments showed that earlySV40 RNA is overproduced in the absence ofafunctional large T-antigen (2, 15, 21). We wanted todetermine whether thelarge
T-anti-gen wasinvolved(i) only in the control of
initi-ation ofanyRNAsynthesis from the early cod-ing region, presumably by operatcod-ingonthe
pro-moter, or(ii) inregulation of theamountof large
T-antigen mRNA relative to small t-antigen mRNA. Parallel cultures of AGMK cells were
infected withanearlySV40
temperature-sensi-tivemutant(tsA58)atthe permissive
tempera-ture (32°0) for40h. At that time, one culture
washeldat32°C (320Csamples), and the other
was shifted to the nonpermissive temperature
(410C) for 5 h (shifted samples). In a control experiment, similar RNAsampleswereprepared
from parallel cultures infected with WT SV40. Samples of the RNAs purified from these
cul-tures were annealed with a 3P-labeled SV40
DNAproberepresentingthe earlystrand ofan HpaII-BamHIfragment (0.14to0.72SV40 map unit
[m.u.]
[Fig. 1]) which contains the early coding region (input amounts of RNA varied due to overproduction in the tsA-shifted sam-ples; see legendto Fig. 2). The analysisof the RNAsfrom these cultures is shown inFig.2.Ineach track, there are three prominent bands (2,000,580,and320nucleotides
[n])
whichrep-resentthe common early RNA body (2,000 n)
and theleaders for smallt-antigen mRNA (580 n) and largeT-antigen mRNA (320 n) (4; Fig. 1).In addition, a 1,900-n bandwas oftenseen,
which arises dueto the 3'overlapofearlyand lateSV40mRNA's whichcausesprobe
displace-ment (1). UnderDNA probe excess hybridiza-tion conditions, the relative band intensities of
the 580- and 320-n segments corresponded to
EcoRI
0.14 BamHI
0.17
Hpa110.73-P
Orep
Q.67BglI
TaqI
FIG. 1. Map of the SV40 genome. The heavy outer circle represents the viral DNA with the restriction endonucleasecleavage sites indicated by arrows.
O,,p
denotes the origin ofDNA replication, the site at which large T-antigenbinds(20, 31) and the presumedsiteofitsregulatory control on early RNA synthesis (2, 15, 21). Thetriangles mark the positions of RNA endpoints (0.15 and 0.17m.u)and splice junctions(0.59,0.54,0.76,and 0.94m.u.).The counterclockwise arrows indicate the map locations of the two major polyadenylated
cytoplasmic mRNA's found early in viral infection, i.e., before the onset of viral DNA synthesis. The early RNA coding for large T-antigen has a leader which extends from 0.660 to0.600 m.u., spliced to a "body" extendingfrom0.533to 0.154 m.u. The other early RNA, coding forsmall t-antigen, consists of a larger leader
(0.660to0.546m.u.),spliced to the same body as the largeT-antigen mRNA. The clockwise arrows indicate
themap locationsof the additional polyadenylated cytoplasmic mRNA 's found late ininfection(i.e., after the onsetof viral DNAreplication), the 19S and168RNAs, whichcode for the viral structural proteins. Map positionsarederivedfrom references 4, 7, 9,16, 18, and 19.
on November 10, 2019 by guest
http://jvi.asm.org/
[image:3.508.128.374.329.546.2]cv-1
A W-T A58 A58 WT M 32 Shift 32 Shift 32
AGMK
WT A58 A58 Shift 32 Shift 2145
1867
1171 1087 828 528
447X
[image:4.508.270.458.509.567.2]367 .LX .i
FIG. 2. Nuclease SI analysis of SV40 cytoplasmic
earlymRNA. TotalcytoplasmicRNA isolated from CV-1orAGMK cellsinfectedwith WTVA4554(WT)
ortsA58 virus washybridizedto theearly (minus) strand ofthe larger fragment of 32P-labeled SV40 DNAgeneratedby digestionwith therestriction
en-donucleases HpaIIand BamHI (0.14 to 0.73 m.u.
[Fig. 1]). Samples were hybridized, nuclease SI treated, electrophoresed, and autoradiographed as
described in the text. The 32°Csamples (32) were
harvested after40 hofinfectionat32'C. Theshifted samples(shift) wereharvestedaftera5-hshift from 32to41'C.TheinputamountsofRNAvariedamong samplestomaintainSV40 DNAexcesshybridization
conditions.InputRNAamountswere asfollows: 64
and 54pg,respectively, for WT-infectedCV-1 cellsat
32'Candshift;22 and 5 pg,respectively, for
tsA58-infected CV-1 cellsat32°Candshift; 14and 9pg,
respectively, for WT-infectedAGMKcells at 32°C andshift;and 7 and2.5pgfortsA58-infectedAGMK cellsat32'C andshift.LaneM containsSV40DNA fragmentmarkers. Similar resultswereobtained with
polyadenylated cytoplasmic RNAs. Itwill be noted that insomeofthesamples, the2,000-nband isnot as intense as would be expectedfrom comparison with the 580- and3(0-n bands. Thisoccurs insome samplesduetothelonghybridizationtimesnecessary
to obtain complete hybridization reactions. This causes some breakdown ofthe RNA, having the greatest effect on the detection oflargersegments.
This didnotoccurin most ofthe samplesused to
determine the ratio oflarge T- to small t-antigen
mRNA's. The data abovewerechosenfor reproduc-tion in thispaperbecausethey clearlyshow allofthe
results discussedon onegel.
the relativeamountsofthesmallt-andlarge
T-antigenmRNA's,respectively. Inspectionof the
relative intensitiesofthesebands inFig.2
sug-gested thatafterashift to410C,the amount of
large T-antigenmRNA relative tosmall
t-anti-genmRNA increasedsignificantly, both in cells
infected by tsA58and in cells infected by WT
SV40.A similar shift towardproductionoflarge
T-antigenmRNA at thehigher temperaturewas
seen inCV-1 cellsinfected eitherbyWT SV40
orbytsA58(Fig. 2). For thequantitation of the
relative amounts of the early SV40mRNA's,
autoradiogramssimilarto those shown in Fig.2
wereanalyzed bymicrodensitometry (Table 1). Although therewas two to three times asmuch large T-antigen mRNA as small t-antigen
mRNA at320C, this ratioincreasedto between
five andseven timesduring shift-upconditions
forbothWT- andtsA58-infectedcultures. When
infected AGMK cells were incubated
continu-ouslyat410C (41°C samples),theratio of large
T-to smallt-antigenmRNA's was even greater
(8-to11-fold).
The experiments aboveshow that there was asubstantial increase in theratio oflargeT- to
smallt-antigen mRNA's withanincreasein the
temperatureofincubation. Itcan be argued that,
during the course of a lytic infection at any
temperature, the ratio oftheearly mRNA's may change with time. Thus, in the shift from 32to
410Cthe lytic process may have been
acceler-ated to a later phase with ahigher ratio of large
T- tosmall t-antigen mRNA's. Likewise,the
24-hinfectionat410Cmayrepresent an even later
phase of infection. To determine whether this
wasthesourceof the ratio changes,AGMK cells
wereinfected withWT SV40 for various times
at 32, 37, and 410C; the ratios of cytoplasmic early mRNA's were then determined as de-scribed above. Table 2 showsthat the specific early mRNA ratio for anyof thetemperatures
remained the same from early tolate times of infection atthat temperature. Inaddition, the ratio ofearly mRNA's adjustedtothe expected values in temperature shift-up and shift-down experiments (datanotshown). Thus, the ratio oflargeT- tosmallt-antigenmRNA's appeared
to be an intrinsic property determined by the
thermal environment of theinfectedcell. Since
the temperature shift experiments (Table 1)
TABLE 1. Ratiooflarge T-tosmall t-antigen
mRNA'sin WT- andtsA58-infectedcells under various temperatureconditionsa
Infecting AGMKcells CV-1cells
virus 320°Cb Shiftc 41oCd 32oCb Shifte 41 oCd
VA4554 2.1 5.7 8.8 2.4 5.2 ND'
(WT)
tsA58 2.7 6.6 10.7 2.8 6.3 ND
aRatiosweredeterminedbymicrodensitometertracingof the 580- and 320-n bandsofautoradiographssuchasthose shown inFig.2.The valuesshownarethe average of at least twodeterminations andaremultiplied by1.72 tocorrectfor the difference in sizesof the leadersegmentsforlargeT-and
smallt-antigenmRNA'sdetermined from nucleotide sequence
data(18, 19).Allnumbers haveastandarderrorof±0.20.
bInfectionat32'C for40h.
'Infectionat32'C for 40 h witha5-hshift to 41'C.
dInfection doneentirelyat41°Cfor 24h.
'ND,Not determined. Bzses
J. VIROL.
ffT
on November 10, 2019 by guest
http://jvi.asm.org/
showed that theearly mRNA ratios varied with
temperature identically in WT- and
tsA58-in-fected cells,wealsoconclude thatlarge
T-anti-gen (atleastthe portion affected bythe tsA58
mutation) and overproduction ofearlymRNA have no significant effect on the earlymRNA
ratio.
Effect ofthermally induced alterationsof early mRNA ratiosonthesynthesisoflarge T- and small t-antigens. We nextwanted to
determine whether the temperature-dependent alterations in the early mRNA ratio were
re-flected atthe proteinlevel. To determine this, AGMKorCV-1 cellswereinfected with WTor
tsA58 at (i) 320C for 40 h; (ii) 320C for40 h, followedbya5-hshiftto410C;and(iii)410Cfor
24 h. During the last hour of each infection period, the cells were pulse-labeled with [3S]-methionine. After extraction and immunopre-cipitationwithanti-T-serum,theearly proteins
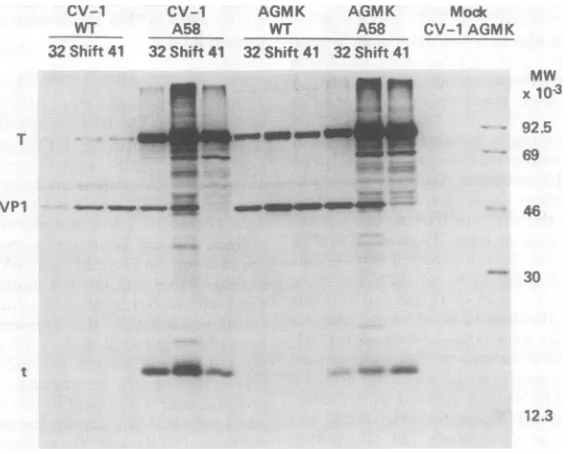
were analyzed by sodiumdodecyl sulfate-poly-acrylamide gel electrophoresis (Fig. 3). As
ex-pected from a previous study (26, 29), tsA58-infected cells synthesized morelarge T-antigen after ashiftto
410C.
Small t-antigen synthesiswasalso increased in these cells. In theexposure
of the gel shown in Fig. 3, the small t-antigen
bandswere notvisible in the WT-infected CV-1 cellsamples; however, theywereeasily detected
uponlongerexposures (datanotshown). Fora
determination of theratio oflargeT- tosmall
t-antigens inthesesamples, the bands containing
the pulse-labeled polypeptides were excised from the gel and counted as described above. Table 3shows theratio of large T- to small
t-antigens determined from thecountsin the gel bands; these ratios have been corrected forthe
difference in the number of methionines in the
twoproteinsasdetermined from thenucleotide
sequence(9, 19).Theratios of theearly proteins
[image:5.508.53.244.510.640.2]atthe variousincubation temperatures variedin a fashion similartothe ratio of their mRNA's.
TABLE 2. Ratios of large T- tosmallt-antigen mRNA's inWT-infectedAGMK cellsfromearly to
latetimesofinfection at 32, 37, and41C°Ca
320C 370C 410C
Time Time Time
postin- Rto postin- Rto
postin-Rai
fection
ftfection
fection(h) (h) (h)
12 2.1 8 4.3 6 8.8
24 2.4 21 4.4 12 8.5
48 2.3 40 4.2 24 8.1
aRatiosweredetermined asdescribed in Table 1, footnotea, and thetext. AGMK cellswere infected withWTVA4554 at 32, 37, and 41°C at 10PFU/cell. Atthe notedtimespostinfection,thetotalcytoplasmic RNA washarvestedandanalyzed.
Thus,theresults show that thethermaleffects ontheearlymRNA ratios were reflected at the
proteinlevel.
DISCUSSION
The absoluteamountsofSV40 earlymRNA's
in WT- andtsA58 mutant-infected cells at
dif-ferent temperaturesarenot thesame(2, 15,21); however, the relative ratio oflarge T-tosmall
t-antigen mRNA'svariedsimilarlywith
tempera-ture in both WT- and tsA58 mutant-infected
cells (Table 1). Thus, it appears that large
T-antigen doesnotinfluence the determinationof
the ratio of thetwoearly mRNA's.Although it
isconceivable that the tsA mutantlarge T-an-tigen maybetemperature insensitive for sucha
regulatory function, this appearsunlikely. For example,Tegtmeyeretal. (29) have shown that
the tsA large T-antigen is rapidly degraded at
the nonpermissive temperature. It seems im-probable thatafragment of themutantprotein
could maintain WTactivityforthis function.In
addition, Handa and Sharp (personal
commu-nication) have shown that the ratio of thetwo
early SV40 mRNA's at 370C isnot altered by
the presenceofinhibitors such as emitine and
cycloheximidewhichblock the synthesis of the earlyproteins. Weconclude, therefore,thatlarge T-antigen controlsearlygeneexpression primar-ily by generalregulation of allearly RNA
tran-scription,presumably byfunctioningonthe
pro-moter. The relativeproportions oflargeT-and
small t-antigen mRNA's appear to be
deter-mined by otherfactors whichareunaffectedby thetsA mutationortheoverproduction of early
RNAin tsAmutantinfections.
Thethermally induced variationsintheearly mRNA (andprotein) ratiosmay occur atseveral levels. Recent sequencing studies (18) have showntwodifferent5'terminifor theearly SV40 mRNA's(within7 nof eachother).Althoughno
evidencesuggeststhat either terminusisspecific for one of the early mRNA's, these data may indicate that there are two early promoters.
Thus, thermal factors may alter the early
mRNAratiobyinfluencingpromoterselection. Anotherpossible mechanism foraltering the ratio of theearly mRNA's derives from the fact thatbothearly mRNA's share thesamegenomic
sequences,differing onlyintheirsplice positions.
Thus, the thermally induced alterationsin the
mRNA ratios mayinvolve the relatively small
sequencedifferences whichdetermine large
T-and small t-antigen mRNA's (in the 0.534 to
0.60-m.u. region [Fig. 1]).Forexample, thermal
effects could alter theactivityof anuclease(s),
thus differentially affecting the early mRNA
stabilities, or alter the transport mechanism
such that the rate oftransport of specific mRNA
on November 10, 2019 by guest
http://jvi.asm.org/
CV-1 WST 32Shift 41
CV-1
A58 32 Shift 41
AGMK WT 32Shift41
AGMK A58 32Shift 41
T
VP1 __ _ FW
Mock CV-1 AGMK
MW X10)-3 92.5 69
46
30
t _,_ _;_
12.3
FIG. 3. Examinationofpulse-labeledSV40earlyproteins. Cultures of CV-1 or AGMK cells were infected with WTVA4554 (WT)ortsA58virus. The infection conditions were as follows: (i) 32°C for 40 h (32);(ii) 32°C for40h,followed by a 5-h shift to 41 °C (shift); (iii) 41 °C for 24 h (41). During the last hour of infection at the various temperatures, the proteins were pulse-labeled with [35SJmethionine asdescribed in the text. The labeledproteinswereextracted, immunoprecipitated and analyzed on a sodium dodecyl sulfate-polyacryl-amide gel (see text) with appropriate markers. Thepositionsof large T-antigen (T) andsmall t-antigen (t) are indicated. Inaddition, the serum used in this experiment precipitated the viral coat proteins (predominantly VP1). Note that the 41 °C samples from cells infected with tsA58 contain little or no VP1, as expected for a tsA mutantinfection.
TABLE 3. Ratiosof large T- tosmall t-antigen proteins in WT- andtsA58-infectedcells atvarious
incubationtemperaturesa
Infecting AGMK cells CV-1cells
Virus 320C Shift 410C 32°C Shift 410C
VA4554 2.5 4.6 5.5 1.8 3.5 4.9 (WT)
tsA58 2.1 8.6 10.6 1.8 4.7 6.0
aRatiosweredetermined fromcountsinpulse-labeled im-munoprecipitated large T- and small t-antigens after
separa-tionon asodiumdodecylsulfate-polyacrylamide gel (Fig.3). Theratiosarecorrected fordifferences innumbers of methi-onines between the two proteins asdetermined from the nucleotidesequence(9, 19).
species changes. It seems likely that the two
early mRNA'sare derived froma common
pri-mary transcript of the early coding region by
different splicing events. With this
considera-tion, the thermal effects on the early mRNA
ratiosmayresult fromanalterationofsequence
recognition by a splicing enzyme(s) such that
therelativefrequency ofsplicingeventschanges
orfromanalteration ofthestability ofsecondary
structuresintheprimary RNA transcript which
arerequiredforthesplicingevents,thus
chang-ing theequilibriumofsplicereactions.
Theselattertwopossibilitiesaresupported by previous studies (2, 21) whichindicate that the total amount of early RNA (or precursor) is
relatively equal in the cytoplasm and nuclei of WT- infected cellsat320C and aftera shift to 410C.Thissuggeststhattheratiochangesoccur
by qualitative variation within a fixedamount
ofprecursor. Inaddition, examination of deletion
mutants in the 0.535- to 0.60-m.u. region, the
unique codingregionforsmallt-antigen(Fig. 1), hasyielded information aboutpossiblesequence
requirements for thesplicing ofearly mRNA's
(14; G. Khoury,J. C.Alwine,R.Dhar,P.Gruss, C.-J. Lai, S. Segal, and I. Seif, Cold Spring
Harbor Symp. Quant. Biol., in press). None of
theseviable deletionmutantsaffects theability
to produce the large T-antigen mRNA splice.
Since these mutants include deletions which
removethe smallt-antigenmRNAsplicedonor
sequenceat0.546 m.u.(18,24),itisunlikelythat
thesmallt-antigenmRNAspliceisa
prerequi-site forlargeT-antigenmRNAsynthesis.Onthe
otherhand, specific deletionswithinthisregion
which do not involvesuspected splicejunctions
willgreatlydecrease the amount ofstable small
J. VIROL.
I.i,, 1: I
PI
ammm
41A R4:%:::--:..,'
on November 10, 2019 by guest
http://jvi.asm.org/
[image:6.508.122.406.80.307.2] [image:6.508.64.257.415.494.2]t-antigen mRNA produced. This indicates that specificsequences,well removed from thesplice
junctions, maybeimportantfor this splice (14;
Khoury et al., in press). It has been suggested
fromthese data that these deletions mayalter conformations inprimarytranscripts whichare
required for the splice. If this is the case, it is possible that thermal (or thermodynamic) ef-fects whichalter the stabilities of these confor-mationschange theratesofsplicngeventsand, hence, the ratios oftheearly mRNA's. Another indicationof theimportance ofsequence in
splic-ing isnoted in theobservationthatmanyRNA's
are spliced identically in severaldifferent cells (Khoury et al., in press). Our examination of early mRNA's in SV40 (VA4554)-transformed hamstercells demonstrates thatnotonlyarethe splices thesame, but alsothe ratios of theearly mRNA'svarywithtemperatureinthesame way as in lytically infected, monkey cells (data not
shown).
Although these data indicate thatinformation within the sequence may be involved in the
determination oftheearly mRNA ratio,we
can-not rule out a high degree of conservation of
splicing factors between species. In this regard, therequirement for factors other than primary
RNAsequenceisimplied by the demonstration
ofcomplete unspliced early transcripts in SV40-infectedmouseteratocarcinomacellsbefore
dif-ferentiation; after the induction ofcell differen-tiation by retinoic acid, correctly spliced early
mRNA's are found (22, 23). This implies that
factors may be induced during differentiation which directly or indirectly affect splicing of
RNAmolecules.
Thus, there are several possible levels at
whichtheenvironment ofthecellmay influence
the early mRNA ratios. Whatever the
mecha-nism, these changesinearlymRNA ratios
indi-cate apotentially importantlevel forcontrol of
geneexpression.In ageneral model,this control may allow aDNA sequence which encodes
in-formation formore than oneproductto
differ-entially express the products by altering the
processing which produces the final mRNA's.
Thisdifferentialexpression may be determined
bytemperature or otherthermodynamic
varia-bles(pHandionicstrength) of the environment ofthecell.
LITERATURE CITED
1. Alwine, J. C., R. Dhar, and G. R. Khoury. 1980.A
small RNA induced lateinSV40lytic infection can associate with early viral mRNAs. Proc. Natl. Acad. Sci. U.S.A. 77:1379-1383.
la.Alwine, J. C., S. I. Reed, J. Ferguson, and G. R.
Stark. 1975.Propertiesof Tantigens inducedby wild typeSV40 andtsAmutants in lytic infection.Cell6: 529-533.
2. Alwine, J. C., S. L. Reed, and G. R. Stark. 1977. Characterization of the autoregulation of simian virus 40gene A. J.Virol. 24:22-27.
3. Aviv, H.,and P.Leder. 1972. Purification of biologically active globinmessenger RNA by chromatography on oligothymidylic-acid-cellulose. Proc. Natl. Acad. Sci. U.S.A. 69:1408-1412.
4. Berk, A.J., and P. A. Sharp. 1978. Spliced early mRNAs of simian virus 40. Proc. Natl. Acad. Sci. U.S.A. 75: 1274-1278.
5. Cowan, K., P. Tegtmeyer, and P. P.Antony. 1973. Relationship of replication and transcription of simian virus 40 DNA. Proc. Natl. Acad.Sci. U.S.A. 70:1927-1930.
6. Crawford, L. V., C. N. Cole, A. E. Smith, E.Paucha, P.Tegtmeyer, K. Rundell, and P. Berg. 1978. Or-ganization and ezpression ofearly genes of simian virus 40.Proc.Natl. Acad. Sci. U.S.A. 75:117-121. 7. Dhar, R., K. N. Subramanian, J. Pan, and S. M.
Weissman. 1977. Nucleotide sequence of afragment of SV40 DNA that contains the origin of DNA replication andspecifies the 5'-ends ofearly and late viral RNA. J. Biol.Chem. 252:368-376.
8. Ferdinand, F.-J., M. Brown,and G. Khoury. 1977. Synthesis and characterization of late lytic simian virus 40RNA from transcriptionalcomplexes. Virology 78: 150-161.
9. Fiers, W., R.Contreras, G. Haegeman, R. Rogiers, A. Vande Voorde, H. VanHeuverawyn,J. Van
Her-reweghe, G. Volckaert, and M. Ysebaert. 1978. Complete nucleotide sequence of SV40 DNA. Nature (London) 273:113-120.
10. Hayward,G. 1972. Gel electrophoretic separation of the complementary strands of bacteriophage DNA. Virol-ogy 49:342-347.
11.Jay, G.,F. T.Jay,R. M.Friedman,andA. S. Levine. 1977. Simian virus 40-specific ribosome-binding pro-teins induced by a nondefective adenovirus2-simian virus 40hybrid. J. Virol. 23:692-699.
12. Jay,G.,R. P.C.Shiu,F. T.Jay, A. J.Levine, and I. Pastan. 1978.Identification of a transformed specific protein induced byaRous sarcoma virus.Cell 13:527-534.
13. Khoury,G.,B. J.Carter,F.-J.Ferdinand,P. M. How-ley, M. Brown, and M. A. Martin. 1976. Genome localization of simian virus 40 RNA species. J. Virol. 17:832-40.
14. Khoury, G.,P.Gruss,R.Dhar,and C.-J. Lai. 1979. Processing andexpressionofSV40mRNA: theeffect of
earlydeletions.Cell18:85-92.
15.Khoury,G.,and E.May.1977.Regulationofearlyand late simian virus40 transcription: overproduction of
earlyviral RNA in the absence ofafunctional T-anti-gen. J.Virol. 23:167-176.
16.Lai, C.-J., R.Dhar,andG.Khoury.1978.Mappingthe
splicedandunsplicedlatelytic SV40RNAs.Cell 14: 971-982.
17.Laskey,R.A.,and A. D.Mills.1975.Quantitativefilm detection of 3H and"4Cinpolyacrylamidegelsby fluo-rography. Eur. J. Biochem. 56:335-341.
18.Reddy,V.B.,P.K.Ghosh,P.Lebowitz,M.Piatak,
and S. M. Weissman. 1979. Simian virus40 early
mRNA's.I.Genomiclocalization of 3' and 5'terminiof two majorsplices in mRNA from transformed and lytically infectedcells. J. Virol. 30:279-296.
19.Reddy,V.B.,B.Thimmappaya,R.Dhar,K. N. Sub-ramanian, B. S.Zain,J.Pan,P. K.Ghosh,M.L. Celma,andS.M.Weissman. 1978.Thegenomeof simian virus 40.Science206:494-502.
20. Reed,S.I.,J.Ferguson,R.Davis,andG. R. Stark. 1975.T-antigen bindstosimianvirus 40 DNAatthe originofreplication. Proc. Natl. Acad. Sci. U.S.A. 72: 1605-1609.
on November 10, 2019 by guest
http://jvi.asm.org/
21. Reed, S. I., G. R. Stark, and J. C. Alwine. 1976. Autoregulation of simian virus40geneA byT-antigen.
Proc. Natl. Acad. Sci. U.S.A. 73:3083-3087.
22. Segal, S., and G. Khoury. 1979. Differentiation as a
requirement forSV40geneexpression in F-9 embryonal
carcinomacells. Proc. Natl. Acad.Sci. U.S.A. 76:1746-1751.
23. Segal, S., A. J. Levine, and G. Khoury.1979.Evidence fornon-spliced SV40 RNA in undifferentiated murine teratocarcinomastemcells.Nature(London) 280:335-338.
24. Seif, I.,G. Khoury, and R. Dhar. 1979. BKVsplice
sequencesbased on analysis of preferred donor and
acceptorsites. Nucleic Acids Res. 6:3387-3398. 25.Tegtmeyer, P. 1972. Simian virus40 deoxyribonucleic
acidsynthesis: the viral replicon. J. Virol. 10:591-598. 26.Tegtmeyer, P. 1974. Alteredpatternsofprotein synthesis
in infections by SV40mutants. Cold Spring Harbor
Symp. Quant. Biol. 39:9-16.
27. Tegtmeyer, P., C. Dohan, and C. Reznikoff. 1970. Inactivating and mutagenic effects of nitrosoguanidine
onsimian virus40.Proc.Natl. Acad. Sci. U.S.A.66:
745-752.
28. Tegtmeyer, P., K.Rundell, and J. K. Collins. 1977. Modification of simian virus40proteinA.J. Virol.21: 647-657.
29. Tegtmeyer, P., M. Schwartz, J. K. Collins, and K. Rundell.1975.Regulation oftumorantigen synthesis of simian virus40geneA. J. Virol. 16:168-178. 30. Tenen, D. G., P. Baygel, and J. Livingston. 1975.
Thermolabile T (tumor) antigen from cells transformed byatemperaturesensitivemutantof simian virus40. Proc. Natl. Acad. Sci. U.S.A.72:43514355.
31.ITian,R. 1978. Thebinding siteonSV40 DNA foraT antigen-related protein. Cell 13:165-179.
on November 10, 2019 by guest
http://jvi.asm.org/