ARTICLE
Axial and Radial Diffusivity in Preterm Infants Who
Have Diffuse White Matter Changes on Magnetic
Resonance Imaging at Term-Equivalent Age
Serena J. Counsell, PhDa, Yuji Shen, PhDa, James P. Boardman, MRCPCHb, David J. Larkman, PhDa, Olga Kapellou, MRCPCHb, Philip Ward, BScb,
Joanna M. Allsop, DCRa, Frances M. Cowan, PhDb, Joseph V. Hajnal, PhDa, A. David Edwards, F Med Scia,b, Mary A. Rutherford, FRCR MDa,b
aRobert Steiner MR Unit, Imaging Sciences Department, MRC Clinical Sciences Centre, andbDivision of Paediatrics, Obstetrics and Gynaecology, Imperial College London, Hammersmith Hospital, London, United Kingdom
The authors have indicated they have no financial relationships relevant to this article to disclose.
ABSTRACT
OBJECTIVE.Diffuse excessive high signal intensity (DEHSI) is observed in the majority of preterm infants at term-equivalent age on conventional MRI, and diffusion-weighted imaging has shown that apparent diffusion coefficient values are ele-vated in the white matter (WM) in DEHSI. Our aim was to obtain diffusion tensor imaging on preterm infants at term-equivalent age and term control infants to test the hypothesis that radial diffusivity was significantly different in the WM in preterm infants with DEHSI compared with both preterm infants with normal-appearing WM on conventional MRI and term control infants.
METHODS.Diffusion tensor imaging was obtained on 38 preterm infants at term-equivalent age and 8 term control infants. Values for axial (1) and radial [(2⫹
3)/2] diffusivity were calculated in regions of interest positioned in the central WM at the level of the centrum semiovale, frontal WM, posterior periventricular WM, occipital WM, anterior and posterior portions of the posterior limb of the internal capsule, and the genu and splenium of the corpus callosum.
RESULTS.Radial diffusivity was elevated significantly in the posterior portion of the posterior limb of the internal capsule and the splenium of the corpus callosum, and both axial and radial diffusivity were elevated significantly in the WM at the level of the centrum semiovale, the frontal WM, the periventricular WM, and the occipital WM in preterm infants with DEHSI compared with preterm infants with normal-appearing WM and term control infants. There was no significant differ-ence between term control infants and preterm infants with normal-appearing WM in any region studied.
CONCLUSIONS.These findings suggest that DEHSI represents an oligodendrocyte and/or axonal abnormality that is widespread throughout the cerebral WM.
www.pediatrics.org/cgi/doi/10.1542/ peds.2005-0820
doi:10.1542/peds.2005-0820
This work was presented in part at the annual conference of the Pediatric Academic Societies; May 1– 4, 2004; San Francisco, CA.
Key Words
preterm, brain, diffusion tensor imaging, magnetic resonance imaging
Abbreviations GA— gestational age
DEHSI— diffuse excessive high signal intensity
DWI— diffusion-weighted imaging ADC—apparent diffusion coefficient WM—white matter
DTI— diffusion tensor imaging PLIC—posterior limb of the internal capsule
PMA—postmenstrual age FOV—field of view ROI—region of interest
Accepted for publication May 31, 2005 Address correspondence to Mary A. Rutherford, FRCR MD, Robert Steiner MR Unit, Imaging Sciences Department, MRC Clinical Sciences Centre, Imperial College London, Hammersmith Campus, DuCane Rd, London W12 0HS, United Kingdom. E-mail: m.rutherford@imperial.ac.uk
T
HE DEVELOPING BRAIN is vulnerable to injury from many causes, resulting in significant mortality and morbidity. At 30 months of age, impairment can be identified in one half of all infants who are born at 25weeks’ gestational age (GA) or less.1Even those with no
identifiable disability at this age may experience learning difficulties when they enter mainstream school or have
behavioral problems in adolescence.2–4Although cranial
ultrasound and MRI studies have demonstrated a rela-tionship between periventricular hemorrhagic infarction and cystic periventricular leukomalacia and the devel-opment of cerebral palsy,5–9the pathologic correlates for the spectrum of neurocognitive impairments seen in the child who was born preterm remain incompletely de-fined.
Qualitative MRI studies have demonstrated a number of differences in the preterm brain at term-equivalent age compared with term-born control infants, including
ventricular dilation, enlarged extracerebral space,10,11
and diffuse excessive high signal intensity (DEHSI) on T2
weighted MRI.12 In addition, quantitative volumetric
MRI studies in preterm infants at term-equivalent age and in later childhood have identified structural abnor-malities associated with preterm birth.13–17
Diffusion-weighted imaging (DWI) demonstrated that infants with DEHSI had apparent diffusion coeffi-cient (ADC) values (the apparent diffusivity of tissue water determined by applying a standard free diffusion model to diffusion-sensitized MR data) in the white matter (WM) that were similar to preterm infants with major focal lesions, suggesting that DEHSI was a
neuro-imaging correlate of diffuse WM abnormality.18
How-ever, this DWI study was not able to assess further the mechanisms that underlie this abnormal WM. One in-vestigational approach would be to assess diffusion an-isotropy, the directional dependence of water diffusion, in the developing neonatal brain.
Diffusion tensor imaging (DTI) is a sensitive, non-invasive tool for assessing WM development. It assesses the random Brownian motion of water molecules within tissue. This motion is hindered by structures within tis-sue, such as cell membranes, macromolecules, and WM
fibers.19 In cerebral WM, water diffuses preferentially
along the direction of axons and is restricted perpendic-ular to axons.20This directional dependence of diffusion in tissue is evident before myelination.21Indeed, animal studies have shown that the geometric alignment of fibers and axonal membranes results in hindered
diffu-sion perpendicular to fibers in the absence of myelin.22
The development of the myelin membrane, however, does cause additional increases in measured
anisotro-py.23DTI has previously been used to examine the
pre-term brain,24–28 and this technique may provide
addi-tional insight into the nature of diffuse WM disease in this patient group.
In addition to calculating mean diffusivity and diffu-sion anisotropy, investigating the nature of diffudiffu-sion parallel (axial diffusion) and perpendicular (radial diffu-sion) to WM tracts may offer additional information regarding brain tissue microstructure in preterm infants. Changes in anisotropy with development are predomi-nantly attributable to decreases in radial diffusion,28,29 reflecting myelination and premyelination events, such
as increased axonal calibre,30 decreased membrane
per-meability,31 and the development of functioning ionic
channels.21Furthermore, in animal studies of
dysmyeli-nation, radial diffusion is increased whereas axial
diffu-sion remains unchanged.32
The aim of this study was to obtain DTI on preterm infants at term-equivalent age and term control infants to test the hypothesis that radial diffusivity was signifi-cantly different throughout the WM in preterm infants with DEHSI compared with both preterm infants with normal-appearing WM on conventional MRI and term control infants. To this end, DTI data were used to cal-culate axial and radial diffusivity in the central WM at the level of the centrum semiovale, the frontal WM, the posterior periventricular WM, the occipital WM, the genu and splenium of the corpus callosum, and the anterior and posterior portions of the posterior limb of the internal capsule (PLIC).
METHODS
Ethical permission for this study was granted by the Hammersmith Hospital Research Ethics Committee. Written, informed parental consent was obtained for each infant before scanning.
Patients
Infants with overt WM lesions on conventional MRI, for example periventricular leukomalacia or periventricular hemorrhagic infarction, were excluded from the study group. DTI was obtained on 38 preterm infants (20 male and 18 female) at term-equivalent age. The infants were recruited at random from the NICU as part of an ongoing MRI project to examine brain development in preterm infants. The median (range) GA of the infants at birth was 30 weeks (25.14 –34 weeks), and the median birth weight was 1348 g (610 –2226 g). The median postmen-strual age (PMA) at the time of imaging was 40.43 weeks (38.86 – 43.86 weeks). The median weight and head cir-cumference at the time of imaging were 3105 g (2536 – 3900 g) and 34.7 cm (32.4 –37.1 cm), respectively.
MRI
MRI was performed on a 1.5 Tesla Eclipse system (Phil-ips Medical Systems, Best, The Netherlands) with max-imum gradient strength of 27 mT/m and slew rate of 72 mT/m per ms on each axis using a dedicated pediatric head coil (diameter 20 cm). The preterm infants were sedated for scanning with oral chloral hydrate (20 –50 mg/kg). Term-born control infants were imaged during natural sleep and were not sedated for imaging. Ear protection was used for each infant. This comprised both individually molded earplugs made from a silicone-based putty (President putty; Coltene/Whaledent, Mah-wah, NJ) placed in the external ear and commercially available neonatal ear muffs (Natus MiniMuffs; Natus Medical Inc, San Carlos, CA) placed over the ear. The infant’s head was immobilized using a pillow filled with polystyrene beads, from which the air was evacuated. Pulse oximetry and electrocardiograph were monitored, and a neonatologist who was trained in MRI procedures was in attendance throughout the examination. Trans-verse T1-weighted conventional spin echo (repeat time [TR] 500 ms, echo time [TE] 15 ms, slice thickness 5
mm, field of view [FOV] 240 mm, 192⫻256 matrix),
transverse T2-weighted fast-spin echo (TR 4100 ms, TE
208 ms, slice thickness 5 mm, FOV 240 mm, 192⫻256
matrix) and sagittally acquired 3D radio frequency spoiled gradient echo (TR 30 ms, TE 4.5 ms, flip angle 30
degrees, voxel size 0.96⫻0.96⫻1.6 mm3) images were
obtained before the DTI sequence.
Single-shot echo planar DTI was acquired in 6 non-colinear directions. For correction of for eddy current distortions, the DT images in the 6 reverse directions were also acquired. This allows the data to be analyzed using an in-house developed program, which corrects
for both low- and high-order eddy current distortions,33
and has the additional benefit of increasing signal-to-noise ratio. The pulse sequence parameters used were as
follows: TR 6000 ms; TE 100 ms; FOV ⫽ 24 cm; slice
thickness⫽5 mm; matrix⫽100⫻100; NSA⫽1; and
b ⫽ 710 seconds/mm2. The scanning time for the DTI
sequence was 1 minute 55 seconds.
Phantom Tests
The DTI sequence and the stability of the MR system over time were tested using a spherical phantom that contained distilled water at 20°C.
Image Analysis
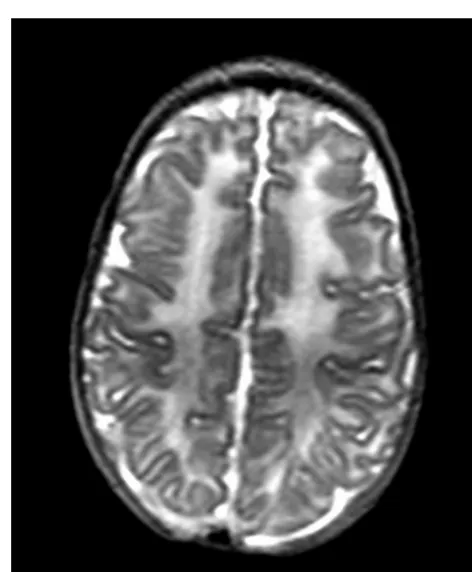
The conventional MR images were reviewed by an ex-perienced neonatal neuroradiologist (M.A.R.), who was unaware of the infants’ clinical course, and the preterm infants were divided into 2 groups on the basis of their conventional MRI results: group 1, preterm infants with normal-appearing WM on conventional MRI (Fig 1); and group 2, preterm infants with evidence of DEHSI (Fig 2).
FIGURE 1
T2-weighted fast-spin echo images at the level of the centrum semiovale in a preterm infant with normal-appearing WM at term-equivalent age.
FIGURE 2
DTI analysis was performed off-line on a DEC Alpha workstation using an in-house developed program writ-ten in IDL 5.6 (Research Systems, Boulder, CO) on a
Linux operating system.33 For each slice, 6
distortion-corrected images were produced, and these were pro-cessed together with the reference image to create DT maps. The DT maps were calculated on a pixel-by-pixel
basis,34 and eigenvalues and eigenvectors were
deter-mined. ADC was calculated by using equation 1, and fractional anisotropy (FA) was calculated by using equa-tion 2.
D ⫽ 1⫹2⫹3
3 (1)
FA ⫽
冑
32
冑
共1⫺D兲2 ⫹ 共2 ⫺ D兲2 ⫹ 共3 ⫺ D兲2冑
12 ⫹ 22 ⫹ 32 (2)where 1, 2, and 3 are eigenvalues of the
dif-fusion tensor with 1 ⬎ 2 ⬎ 3, and D is mean
diffusivity.
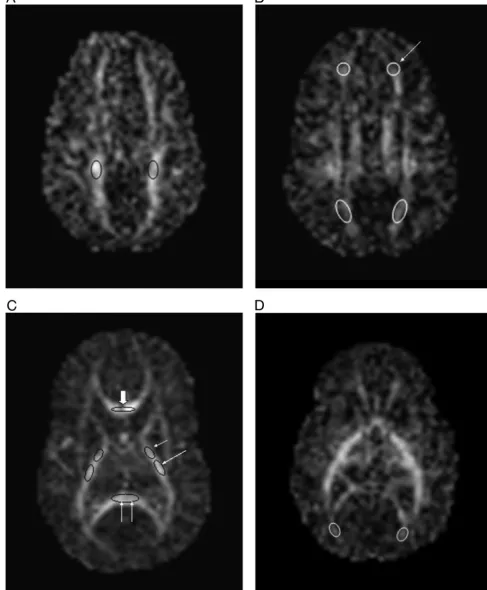
The values for the 3 eigenvalues were obtained from regions of interest (ROIs) positioned in the central WM
of the centrum semiovale (area⫽21.9⫾2.5 mm2), the
frontal WM (area ⫽ 27.4 ⫾ 2.8 mm2), the posterior
periventricular WM (area ⫽33.7⫾ 3.2 mm2), the
oc-cipital WM (area⫽19.54⫾2.9 mm2), and the anterior
and posterior portions of the PLIC (anterior portion area
⫽11.96⫾1.9 mm2, posterior portion area⫽16.7⫾2.1
mm2) bilaterally and in the genu (area ⫽ 15.3 ⫾ 3.5
mm2) and splenium (area ⫽ 28.6 ⫾ 5.1 mm2) of the
corpus callosum. Consistency of positioning was ensured by having all ROIs positioned by a single investigator (S.J.C.), who was unaware of the conventional MRI findings and clinical history of the infants. As WM tracts could be identified more clearly on the FA maps, these were used for ROI localization. Once ROIs were defined, these were transferred directly to the other DTI-derived images. Figure 3 demonstrates the positioning of the ROIs on the FA maps.
Statistical Considerations
Statistical analyses were performed using SPSS 12 (SPSS Inc, Chicago, IL). The data were tested for normality using a Shapiro Wilks test. Statistical analysis of axial and radial diffusivity in each region was performed using multivariate analysis of variance, using infant group as a fixed factor and PMA at the time of scanning as a co-variate, with a full factorial model, simple contrast, and type III sum of squares. As ADC and FA values are calculated from the eigenvalues, statistical analysis of these measurements was not performed. Test-retest
con-sistency for ROI analysis was assessed by calculating the coefficient of variation.
RESULTS
Phantom Studies
Variability of the measured eigenvalues over time was
⬍1.5%. The mean (⫾SD) ADC of the distilled water was
2.09⫾0.03⫻10⫺3mm2/seconds, and the mean FA was
0.045⫾0.01. These values are comparable to published
data.35–37
MRI Findings
Nine preterm infants (4 male and 5 female) had normal-appearing WM on conventional MRI, and 29 (16 male and 13 female) had evidence of DEHSI. There was no significant difference in the GA at birth (P⫽.43), birth
weight (P⫽ .43), or head circumference at birth (P ⫽
.45) between the 2 groups of preterm infants. There was
no significant difference in the PMA at scanning (P ⫽
.89) among the 3 groups of infants. Table 1 shows the clinical characteristics of the preterm infants.
Eigenvalue Analysis
Unpaired t tests showed no significant difference
be-tween the right and left hemispheres (P⬎.05), so the
mean value was calculated from the bilateral measure-ments to give values of 1, 2, and 3for each region studied.
There were no significant differences in axial diffusiv-ity or radial diffusivdiffusiv-ity between the term control infants and the preterm infants with normal-appearing WM in any region studied. Axial and radial diffusivity were significantly elevated in the central WM at the level of the centrum semiovale, in the frontal WM, in the pos-terior periventricular WM, and in the occipital WM in preterm infants with DEHSI compared with term control infants and preterm infants with normal-appearing WM. In the splenium of the corpus callosum and the posterior portion of the PLIC, radial diffusivity was elevated in the infants with DEHSI compared with the term control infants and the preterm infants with normal-appearing WM. However, there were no significant differences in axial diffusivity in these regions between the 3 groups of infants. There were no significant differences in axial or radial diffusivity among the 3 groups of infants in the genu of the corpus callosum or the anterior portion of the PLIC. Table 2 shows the results for axial and radial diffusivity for the term control infants, preterm infants with normal-appearing WM, and preterm infants with DEHSI.
The coefficient of variation for the measurement of
eigenvalues was ⬍4.5% for the genu and splenium of
the corpus callosum, ⬍4% for the PLIC,⬍3% for the
central WM at the level of the centrum semiovale, and
FIGURE 3
DISCUSSION
This study shows that DEHSI is extremely common in preterm infants at term-equivalent age and is associated with significantly elevated axial and radial diffusion in the central WM of the centrum semiovale, the frontal WM, the posterior periventricular WM, and the occipital WM compared with term control infants and preterm infants with normal-appearing WM on conventional MRI. In addition, radial diffusivity was elevated signifi-cantly in the posterior portion of the PLIC and splenium of the corpus callosum in infants with DEHSI, with no change in axial diffusivity. However, there were no sig-nificant differences in diffusion measures obtained in the genu of the corpus callosum or the anterior portion of the PLIC among the 3 groups of infants. Both axial and radial diffusivity were similar in the preterm infants with normal-appearing WM and the term control infants in all regions.
The incidence of cystic periventricular leukomalacia38 has declined in recent years, and noncystic diffuse WM abnormality is now the most prevalent form of WM injury observed on MRI studies of the preterm brain.10,12 The cause of preterm diffuse WM abnormality is not fully understood; however, oligodendrocyte precursors are known to be particularly vulnerable to infection,39–41 hypoxia-ischemia,42and glutamate toxicity.43,44 Further-more, animal studies have shown that suboptimal nu-trition, in particular, deficiencies in essential fatty acids,
have a deleterious effect on WM development.45,46
Al-though the median GA at birth and birth weight were lower, the median number of days on continuous posi-tive airways pressure were higher, and the number of infants who experienced prolonged rupture of mem-branes and culture-positive postnatal sepsis and required ionotropes were greater in the group of infants with DEHSI compared with those with normal-appearing WM, none of these differences reached significance in isolation (Table 1). We therefore were unable to identify any single clinical factor that was associated with DEHSI,
perhaps because there were too few infants in this study to demonstrate significant differences; however, it is en-tirely possible that the cause of diffuse WM abnormality in preterm infants is multifactorial.
The diffusion findings in the WM of the centrum semiovale in this study are consistent with our earlier DWI study, which demonstrated elevated ADC values in the WM of the centrum semiovale in preterm infants who had DEHSI compared with preterm infants with
normal-appearing WM.18The slightly higher ADC values
in the centrum semiovale in this study compared with those that we reported previously are probably
attribut-able to the lowerbvalue (measure of diffusion
sensiti-zation) used in the present study, as ADC values decline with increasingbvalue.47,48
Changes in radial diffusivity, with no change in axial diffusivity, were observed in preterm infants with DEHSI in highly anisotropic regions, where the WM tracts are arranged as highly organized fiber bundles (the posterior portion of the PLIC and the splenium of the corpus callosum). In contrast, both axial and radial diffusion were elevated in this group of infants in the less aniso-tropic regions. Similar findings have been reported in adult patients with relapsing, remitting multiple
sclero-sis.49In WM regions of low to moderate anisotropy, the
imaging voxels contain crossing fibers or less coherently organized tracts, so an increase in diffusion radial to fibers that are running in different directions could result in an apparent increase in all eigenvalues within a voxel.49
Previous studies have observed reduced anisotropy in the PLIC in preterm infants at term compared with term
control infants,24and in older children who have
atten-tion-deficit/hyperactivity disorder and were born pre-term, FA values were diminished in the anterior portion
of the PLIC.50 In this study, radial diffusivity in the
myelinated posterior PLIC was significantly higher in the infants with DEHSI compared with preterm infants with normal-appearing WM and term control infants (Table
TABLE 1 Characteristics of the Preterm Infants
Preterm Infants With Normal-Appearing WM (n⫽9)
Preterm Infants With DEHSI (n⫽29)
P
GA, median (range), wk 32.29 (26.71–33.71) 30 (25.14–34.43) .43
Birth weight, median (range), g 1558 (766–2186) 1450 (610–2226) .43
Head circumference at birth, median (range), cm 29.25 (24.2–31.2) 28.05 (23.0–32.5) .45
Male gender,n(%) 4 (44) 16 (55) .59
PROM⬎48 h,n(%) 2 (22) 9 (31) .62
Postnatal sepsis,n(%) 2 (22) 13 (49) .23
Antenatal steroids,n(%) 9 (100) 26 (90) .95
Days ventilated, median (range) 0 (0–1) 0 (0–23) .53
Days of CPAP, median (range) 1 (0–44) 3 (0–90) .20
Infants who required ionotropes,n(%) 0 3 (10) .32
Intraventricular hemorrhage on ultrasound,n(%) 0 4 (14) .25
2); however, no differences were detected in the unmy-elinated anterior portion of the PLIC in preterm infants compared with term control infants.
Increased radial diffusivity, with no change in axial diffusivity, has been observed in mature animal models
of dysmyelination,32 so, in regions that are myelinating
at this age, such as the posterior portion of the PLIC and the central WM at the level of the centrum semiovale, these findings may be indicative of delayed myelination in preterm infants with DEHSI; however, this study also showed elevated radial diffusivity in regions that are not myelinated at term-equivalent age, including the frontal WM, posterior periventricular WM, occipital WM, and splenium of the corpus callosum. In these regions, the increase in radial diffusivity cannot be attributed to def-icits in myelin per se. It is possible that these findings are attributable to delayed or deficient wrapping of the oli-godendrocyte around the axon before myelination, which may result in increased membrane permeability
and a decreased axonal diameter.24
This study was not able to detect diffusion differences in the genu of the corpus callosum among the 3 groups of infants. This may be because the genu of the corpus callosum is relatively spared, or it may be that the image resolution was not sufficient to detect changes in this region. Studies using high-resolution DTI at higher field strengths may identify abnormalities in this brain region that this study was not able to detect. As structural MRI studies have identified thinning of the body of the
cor-pus callosum in adolescents who were born preterm51,52
and callosal volume has been associated with motor
performance in children who were born preterm,53this
region warrants additional investigation in future work. This area was not always delineated clearly on the dif-fusion images in this study but may be more clearly delineated on color FA maps or diffusion images ob-tained in the sagittal plane.
We did not attempt to grade the imaging findings into moderate or severe DEHSI as the assessment of DEHSI by visual analysis is subjective. Furthermore, although the diffusion characteristics of numerous WM regions differed significantly between preterm infants with nor-mal-appearing WM and those with DEHSI, there was some overlap in the values for axial and radial diffusivity between these 2 groups. Quantitative MR techniques, such as DTI, therefore may prove to be more useful than visual assessment in differentiating preterm infants with diffuse WM abnormality. In addition, as recent studies have demonstrated the feasibility of performing func-tional MRI (fMRI) studies in infants,54future studies that combine DTI and fMRI may provide insights into the structure-function relationship in these infants.
Recent work examining neurodevelopmental assess-ment scores in preterm infants at 2 years’ corrected age has shown that infants who had evidence of DEHSI at term-equivalent age score less well than their peers who had
normal-appearing WM on conventional MRI.55
Neurode-velopmental studies of older children who had DEHSI at term-equivalent age are essential in confirming that DEHSI is associated with developmental delays. Nevertheless, this early neurodevelopmental study, in combination with the diffusion findings presented here, support the hypothesis that DEHSI is a neuroimaging correlate of clinically signif-icant WM disease of prematurity.
CONCLUSIONS
In the absence of histologic correlation, the neuropatho-logic correlates of DEHSI are not known; however, our findings of elevated radial diffusivity in the posterior por-tion of the PLIC and splenium of the corpus callosum and increased axial and radial diffusivity in the WM of the centrum semiovale, the frontal WM, the posterior periven-tricular WM, and the occipital WM in infants with DEHSI compared with term control infants and preterm infants with normal-appearing WM are consistent with wide-spread axonal and oligodendrocyte abnormalities in this group of preterm infants. Axial and radial diffusivity values were similar in preterm infants with normal-appearing WM and term control infants in every region that we examined, indicating that preterm birth in itself is not necessarily associated with abnormal WM development.
ACKNOWLEDGMENTS
This study was funded by the Medical Research Council (United Kingdom), the Health Foundation, the Academy of Medical Sciences, and Philips Medical Systems.
REFERENCES
1. Wood NS, Marlow N, Costeloe K, Gibson AT, Wilkinson AR. Neurologic and developmental disability after extremely
pre-term birth. EPICure Study Group. N Engl J Med.2000;343:
378 –384
2. Botting N, Powls A, Cooke RW, Marlow N. Cognitive and educational outcome of very-low-birthweight children in early
adolescence.Dev Med Child Neurol.1998;40:652– 660
3. Botting N, Powls A, Cooke RW, Marlow N. Attention deficit hyperactivity disorders and other psychiatric outcomes in very
low birthweight children at 12 years.J Child Psychol Psychiatry.
1997;38:931–941
4. Hoekstra RE, Ferrara TB, Couser RJ, Payne NR, Connett JE. Survival and long-term neurodevelopmental outcome of ex-tremely premature infants born at 23–26 weeks’ gestational
age at a tertiary center. Pediatrics.2004;113(1). Available at:
www.pediatrics.org/cgi/content/full/113/1/e1
5. de Vries LS, Dubowitz LM, Dubowitz V, et al. Predictive value
of cranial ultrasound in the newborn baby: a reappraisal.
Lan-cet.1985;2:137–140
6. de Vries LS, Dubowitz LM, Pennock JM, Bydder GM. Extensive cystic leucomalacia: correlation of cranial ultrasound, magnetic resonance imaging and clinical findings in sequential studies. Clin Radiol.1989;40:158 –166
7. de Vries LS, Eken P, Groenendaal F, van Haastert IC, Meiners LC. Correlation between the degree of periventricular leu-komalacia diagnosed using cranial ultrasound and MRI later in
infancy in children with cerebral palsy.Neuropediatrics.1993;
8. de Vries LS, Van Haastert IL, Rademaker KJ, Koopman C, Groenendaal F. Ultrasound abnormalities preceding cerebral
palsy in high-risk preterm infants.J Pediatr.2004;144:815– 820
9. Roelants-van Rijn AM, Groenendaal F, Beek FJ, Eken P, van Haastert IC, de Vries LS. Parenchymal brain injury in the preterm infant: comparison of cranial ultrasound, MRI and
neurodevelopmental outcome.Neuropediatrics.2001;32:80 – 89
10. Inder TE, Wells SJ, Mogridge NB, Spencer C, Volpe JJ. Defining the nature of the cerebral abnormalities in the premature
infant: a qualitative magnetic resonance imaging study.J
Pedi-atr.2003;143:171–179
11. Inder TE, Anderson NJ, Spencer C, Wells S, Volpe JJ. White matter injury in the premature infant: a comparison between
serial cranial sonographic and MR findings at term.AJNR Am J
Neuroradiol.2003;24:805– 809
12. Maalouf EF, Duggan PJ, Rutherford MA, et al. Magnetic reso-nance imaging of the brain in a cohort of extremely preterm
infants.J Pediatr.1999;135:351–357
13. Isaacs EB, Edmonds CJ, Lucas A, Gadian DG. Calculation dif-ficulties in children of very low birthweight: a neural correlate. Brain.2001;124(pt 9):1701–1707
14. Isaacs EB, Edmonds CJ, Chong WK, Lucas A, Gadian DG. Cortical anomalies associated with visuospatial processing
def-icits.Ann Neurol.2003;53:768 –773
15. Peterson BS, Anderson AW, Ehrenkranz R, et al. Regional brain volumes and their later neurodevelopmental correlates
in term and preterm infants.Pediatrics.2003;111:939 –948
16. Inder TE, Warfield SK, Wang H, Huppi PS, Volpe JJ. Abnormal cerebral structure is present at term in premature infants. Pediatrics.2005;115:286 –294
17. Boardman JP, Counsell SJ, Bhatia K, et al. Computational morphometry detects white matter abnormality in association with deep grey nuclear volume reduction in preterm infants at
term equivalent [abstract].Pediatr Res.2004;55:2334
18. Counsell SJ, Allsop JM, Harrison MC, et al. Diffusion-weighted imaging of the brain in preterm infants with focal and diffuse
white matter abnormality.Pediatrics.2003;112(pt 1):1–7
19. Beaulieu C. The basis of anisotropic water diffusion in the
nervous system: a technical review. NMR Biomed. 2002;15:
435– 455
20. Moseley ME, Cohen Y, Kucharczyk J, et al. Diffusion-weighted MR imaging of anisotropic water diffusion in cat central
ner-vous system.Radiology.1990;176:439 – 445
21. Wimberger DM, Roberts TP, Barkovich AJ, Prayer LM, Moseley ME, Kucharczyk J. Identification of “premyelination” by
diffu-sion-weighted MRI.J Comput Assist Tomogr.1995;19:28 –33
22. Beaulieu C, Allen PS. Determinants of anisotropic water
diffu-sion in nerves.Magn Reson Med.1994;31:394 – 400
23. Gulani V, Webb AG, Duncan ID, Lauterbur PC. Apparent dif-fusion tensor measurements in myelin-deficient rat spinal
cords.Magn Reson Med.2001;45:191–195
24. Huppi PS, Maier SE, Peled S, et al. Microstructural develop-ment of human newborn cerebral white matter assessed in
vivo by diffusion tensor magnetic resonance imaging.Pediatr
Res.1998;44:584 –590
25. Huppi PS, Murphy B, Maier SE, et al. Microstructural brain development after perinatal cerebral white matter injury
as-sessed by diffusion tensor magnetic resonance imaging.
Pediat-rics.2001;107:455– 460
26. Miller SP, Vigneron DB, Henry RG, et al. Serial quantitative diffusion tensor MRI of the premature brain: development in
newborns with and without injury. J Magn Reson Imaging.
2002;16:621– 632
27. Neil JJ, Shiran SI, McKinstry RC, et al. Normal brain in human newborns: apparent diffusion coefficient and diffusion
anisot-ropy measured by using diffusion tensor MR imaging.
Radiol-ogy.1998;209:57– 66
28. Partridge SC, Mukherjee P, Henry RG, et al. Diffusion tensor imaging: serial quantitation of white matter tract maturity in
premature newborns.Neuroimage.2004;22:1302–1314
29. Suzuki Y, Matsuzawa H, Kwee IL, Nakada T. Absolute eigen-value diffusion tensor analysis for human brain maturation. NMR Biomed.2003;16:257–260
30. Hildebrand C, Waxman SG. Postnatal differentiation of rat optic nerve fibers: electron microscopic observations on the
development of nodes of Ranvier and axoglial relations.J Comp
Neurol.1984;224:25–37
31. Fields RD, Waxman SG. Regional membrane heterogeneity in premyelinated CNS axons: factors influencing the binding of
sterol-specific probes.Brain Res.1988;443:231–242
32. Song SK, Sun SW, Ramsbottom MJ, Chang C, Russell J, Cross AH. Dysmyelination revealed through MRI as increased radial
(but unchanged axial) diffusion of water.Neuroimage.2002;17:
1429 –1436
33. Shen Y, Larkman DJ, Counsell S, Pu IM, Edwards D, Hajnal JV. Correction of high-order eddy current induced geometric
dis-tortion in diffusion-weighted echo-planar images.Magn Reson
Med.2004;52:1184 –1189
34. Basser PJ, Pierpaoli C. A simplified method to measure the
diffusion tensor from seven MR images.Magn Reson Med.1998;
39:928 –934
35. Skare S, Li T, Nordell B, Ingvar M. Noise considerations in the
determination of diffusion tensor anisotropy.Magn Reson
Im-aging.2000;18:659 – 669
36. Toft PB, Leth H, Peitersen B, Lou HC, Thomsen C. The apparent diffusion coefficient of water in gray and white matter of the
infant brain.J Comput Assist Tomogr.1996;20:1006 –1011
37. Tanner SF, Ramenghi LA, Ridgway JP, et al. Quantitative comparison of intrabrain diffusion in adults and preterm and
term neonates and infants. AJR Am J Roentgenol.2000;174:
1643–1649
38. Hamrick SE, Miller SP, Leonard C, et al. Trends in severe brain injury and neurodevelopmental outcome in premature new-born infants: the role of cystic periventricular leukomalacia. J Pediatr.2004;145:593–599
39. Hagberg H, Peebles D, Mallard C. Models of white matter injury: comparison of infectious, hypoxic-ischemic, and
exci-totoxic insults.Ment Retard Dev Disabil Res Rev.2002;8:30 –38
40. Duggan PJ, Maalouf EF, Watts TL, et al. Intrauterine T-cell activation and increased proinflammatory cytokine
concentra-tions in preterm infants with cerebral lesions.Lancet.2001;358:
1699 –1700
41. Dammann O, Kuban KC, Leviton A. Perinatal infection, fetal inflammatory response, white matter damage, and cognitive
limitations in children born preterm.Ment Retard Dev Disabil Res
Rev.2002;8:46 –50
42. Back SA, Han BH, Luo NL, et al. Selective vulnerability of late
oligodendrocyte precursors to hypoxia-ischemia. J Neurosci.
2002;22:455– 463
43. Yoshioka A, Bacskai B, Pleasure D. Pathophysiology of
oligo-dendroglial excitotoxicity.J Neurosci Res.1996;46:427– 437
44. Oka A, Belliveau MJ, Rosenberg PA, Volpe JJ. Vulnerability of oligodendroglia to glutamate: pharmacology, mechanisms, and
prevention.J Neurosci.1993;13:1441–1453
45. Wiggins RC, Bissell AC, Durham L, Samorajski T. The corpus callosum during postnatal undernourishment and recovery: a
morphometric analysis of myelin and axon relationships.Brain
Res.1985;328:51–57
46. Wiggins RC. Myelin development and nutritional
insuffi-ciency.Brain Res.1982;257:151–175
47. Mulkern RV, Vajapeyam S, Robertson RL, Caruso PA, Rivkin MJ, Maier SE. Biexponential apparent diffusion coefficient
parametrization in adult vs newborn brain.Magn Reson
48. Clark CA, Le Bihan D. Water diffusion compartmentation and
anisotropy at high b values in the human brain.Magn Reson Med.
2000;44:852– 859
49. Henry RG, Oh J, Nelson SJ, Pelletier D. Directional diffusion in relapsing-remitting multiple sclerosis: a possible in vivo
signa-ture of Wallerian degeneration.J Magn Reson Imaging.2003;18:
420 – 426
50. Nagy Z, Westerberg H, Skare S, et al. Preterm children have disturbances of white matter at 11 years of age as shown by
diffusion tensor imaging.Pediatr Res.2003;54:672– 679
51. Stewart AL, Rifkin L, Amess PN, et al. Brain structure and neurocognitive and behavioural function in
adoles-cents who were born very preterm. Lancet. 1999;353:
1653–1657
52. Cooke RW, Abernethy LJ. Cranial magnetic resonance imaging
and school performance in very low birth weight infants in
adolescence. Arch Dis Child Fetal Neonatal Ed. 1999;81:
F116 –F121
53. Rademaker KJ, Lam JN, van Haastert IC, et al. Larger corpus callosum size with better motor performance in prematurely
born children.Semin Perinatol.2004;28:279 –287
54. Seghier ML, Lazeyras F, Zimine S, et al. Combination of event-related fMRI and diffusion tensor imaging in an infant with
perinatal stroke.Neuroimage.2004;21:463– 472
DOI: 10.1542/peds.2005-0820
2006;117;376
Pediatrics
Edwards and Mary A. Rutherford
Philip Ward, Joanna M. Allsop, Frances M. Cowan, Joseph V. Hajnal, A. David
Serena J. Counsell, Yuji Shen, James P. Boardman, David J. Larkman, Olga Kapellou,
Changes on Magnetic Resonance Imaging at Term-Equivalent Age
Axial and Radial Diffusivity in Preterm Infants Who Have Diffuse White Matter
Services
Updated Information &
http://pediatrics.aappublications.org/content/117/2/376 including high resolution figures, can be found at:
References
http://pediatrics.aappublications.org/content/117/2/376#BIBL This article cites 51 articles, 7 of which you can access for free at:
Subspecialty Collections
http://www.aappublications.org/cgi/collection/radiology_sub
Radiology
sub
http://www.aappublications.org/cgi/collection/fetus:newborn_infant_
Fetus/Newborn Infant
following collection(s):
This article, along with others on similar topics, appears in the
Permissions & Licensing
http://www.aappublications.org/site/misc/Permissions.xhtml in its entirety can be found online at:
Information about reproducing this article in parts (figures, tables) or
Reprints
DOI: 10.1542/peds.2005-0820
2006;117;376
Pediatrics
Edwards and Mary A. Rutherford
Philip Ward, Joanna M. Allsop, Frances M. Cowan, Joseph V. Hajnal, A. David
Serena J. Counsell, Yuji Shen, James P. Boardman, David J. Larkman, Olga Kapellou,
Changes on Magnetic Resonance Imaging at Term-Equivalent Age
Axial and Radial Diffusivity in Preterm Infants Who Have Diffuse White Matter
http://pediatrics.aappublications.org/content/117/2/376
located on the World Wide Web at:
The online version of this article, along with updated information and services, is
by the American Academy of Pediatrics. All rights reserved. Print ISSN: 1073-0397.