Removal of Cu from aqueous solution by
using adsorbent prepared from wood
apple shell
Animesh Agarwal and Nitin Kumar Agrawal
Moradabad Institute of Technology,
Ramganga Vihar Phase – 2, Moradabad – 244001, INDIA
Email: ani25dec@yahoo.co.in
ABSTRACT
The adsorption efficiency of activated carbon developed from wood apple shell for the removal of Cu from its aqueous solution was studied. The research is a batch scale experiment using different amount of adsorbent in solution shows that prepared adsorbent was very effective for the removal of Cu. The study also indicates that several factors affected the adsorption efficiency of adsorbent like contact time, initial concentration, temperature and pH. The verification of Langmuir isotherm indicates that wood apple shell activated carbon is a good adsorbent for the removal of Cu. The adsorbent is prepared from waste material and conveniently used for the treatment of industrial waste water containing Cu.
Key words: Wood apple shell, Adsorption, Copper, batch experiment, Activated charcoal.
INTRODUCTION
The water bodies in the world are facing a serious problem continuously mixing industrial and domestic wastes which make these water bodies non suitable for agricultural and other uses. The industrialization and urbanization activities contaminate water bodies by introducing excess amounts of toxic heavy metals into the aquatic system [1, 2]. The use decorative items made by metals are also increased now days in modern high class societies. These wastes are continuously mixing in rivers, lakes, ponds and thus very severely contaminated the aquatic system [3]. Heavy metals even in low concentrations are highly toxic therefore have adverse effects on animals and human beings
These toxic heavy metals adversely affect our nerves system, bones, kidney, lungs, liver and also some cases affect the important enzymes of the body. Various industrial processes and other mankind activities are the main sources of heavy metals in surface water [4]. Many manufacturing Industries of chemical, steel and iron, brass, paint etc. are discharging effluents which contain heavy metals like Cr, Ni, Cu, Pb, Cd, etc.. Ion-exchange, Chromatography, chemical precipitation, adsorption is the various techniques used to remove heavy metal ions from river water [5, 6]. Adsorption process is found to low cost and most effective process among all these process for the removal of heavy metal from waste water. Various authors reported that the adsorption of heavy metals on activated carbon is an effective and versatile method [7-10]. Activated carbon has high porosity and high surface area therefore is effectively used for the removal of Cu metal from waste water. Wood apple fruit is a seasonal fruit which is mainly fertile in summer in India and people widely used it directly or in the form juice and shell of these fruits are thrown as waste. In the present study activated carbon is prepared from wood apple waste hard shell and used to remove Cu from its aqueous solution.
In the present study, a good quality activated carbon has been developed economically by using Wood Apple shell (Limonia acidissima) and used as adsorbent for copper removal.
MATERIAL AND METHOD
Preparation of activated carbon
Wood apple fruit was purchased from local market and then fruit shells were first washed properly with distilled water and then with deionized water and kept for sun dries. After 48 hrs sun and air drying the wood apple shells has been crushed and again washed with distilled water and put in an oven at 1200 C for further
drying for one day. Now broken pieces were treated with 6N sulphuric acid for 24 hrs. Now the treated wood apple shell pieces again washed with distilled water and put in muffle furnace at 3100C for one hrs preparation
of activated carbon. The prepared activated carbon after sieving to a fine powder of 200 µm particle size was used in this study.
Adsorbate solution
Stock solution of copper was prepared (1000mg/L) by desired quantity of Cu(NO3)2.3H2O. Standard base of
0.1M NaOH and acid of 0.1M HCl solutions were used for pH adjustment. All chemicals used in this study were of analytical grade.
Adsorption studies
Adsorption experiment were carried out to establish the effect of contact time on the adsorption process , effect of initial concentration of adsorbate ,effect of pH, effect of temperature and effect of adsorbent dose on adsorption of Cu. Isotherms were obtained by adsorbing different concentrations of metal ion.
Experimental methodology
All the experiments were done in triplicate and mean concentration was calculated by averaging them. The general experimental procedure is as follows:-
An individual solution of Cu metal with known concentration was prepared. The pH is adjusted at a desired value. The temperature of the solution is maintained at a desired value. A given amount of Adsorbent is added in to the solution. Shake the solution on a magnetic stirrer at 150 rpm for a certain time. After prescribed contact time the solution was filtered and the concentrations of metal ion were determined by atomic spectrometry.
Results and Discussion
Effect of contact time on Adsorption process
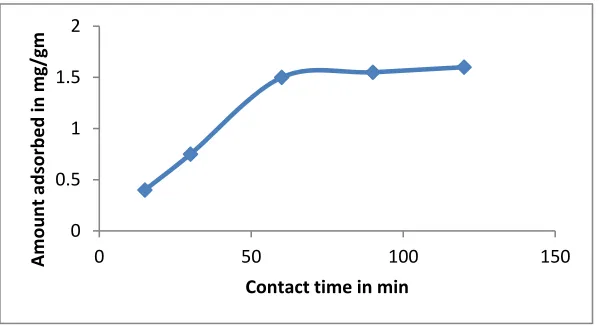
The initial concentration of Cu was taken as 16 mg /L .The metal solution is added with a fixed amount of adsorbent dose of 10 gm/L and allowed for a contact time of 15, 30, 60, 90,120 minutes respectively. The adsorption data for the uptake of Cu on different contact times for a fixed adsorbent dose is shown in figure 1.
Figure 1: Effect of contact time on Adsorption process 0
0.5 1 1.5 2
0 50 100 150
Amount
adsorbed
in
mg/gm
Figure 1 shows that the curve is linear up to 50 min for the Cu metal, after then it saturates. It means at starting metal ion adsorption is increasing rapidly and there is no appreciable change in the metal ion concentration after 50 min. This represents the equilibrium time at which an equilibrium metal ion concentration have been attained.
Effect of initial concentration of adsorbate on the adsorption process
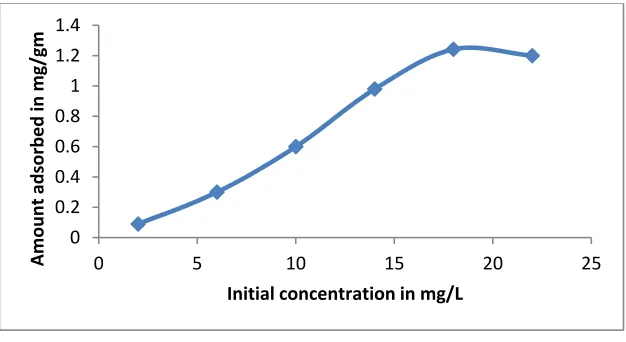
Initial concentration of Cu is increased gradually at the fixed amount of adsorbent dose for a constant contact time in each case and the results obtained are shown in figure 2.
Figure 2: Effect of initial concentration of adsorbate
Figure 2 shows that the adsorption is increased sharply in the starting and its rate becomes slow towards the end of the run. A close observation of the curve shows that the adsorption of Cu is increased from 1 to 18 mg/L and then became almost constant. It indicates that the maximum adsorption occurs at 18 mg/L for Cu.
Effect of pH on the adsorption process:
The initial concentration of Cu is taken as 16 mg /L. The pH of the solution is varied for a fixed dose of 10 gm/L of adsorbent .The results obtained are shown in figure 3. It shows the effect of pH on the adsorption of Cu metal ion. As pH is increasing the adsorption of Cu ion is also increasing and it is maximum at pH 6 and if pH is further increased adsorption is decreasing.
At this pH, there may be two species of Cu present in the solution-Cu (II) in large quantity and Cu(OH)2 in
small quantity. The maximum adsorption at pH 6 indicates that Cu (II) ions are predominantly adsorbed [since Cu (II) ions are present in large quantity in this pH range] either by ion exchange or by hydrogen bonding. The adsorption increases slowly with increasing pH and decreases above pH6 due to precipitation of Cu (II) as Cu (OH) 2 [11].
0 0.2 0.4 0.6 0.8 1 1.2 1.4
0 5 10 15 20 25
Amount
adsorbed
in
mg/gm
Figure3: Effect of pH
Effect of adsorbent dose on adsorption of Cu
The initial concentration of Cu taken as 16 mg /L and the amount of adsorbent is increased gradually from 5 gm/L to 25 gm/L for a constant contact time. The results obtained are represented in figure 4. The copper adsorption increases from 0.8 mg/gm to 1.5 mg/gm with increase in the adsorbent dose of 5 to 10 gm/L and then become almost constant. The result indicates that the dose of 10 gm/L is optimum for Cu removal.
Figure 4: Effect of adsorbent dose
Effect of temperature on the adsorption process
Initial concentration of Cu is increased gradually from 2 to 22 mg/L and the fixed amount of adsorbent (10 gm/L) is added for a constant contact time at different temperature of 300C, 400C and 500C and the results are
shown in figure 5which clearly indicate that the adsorption is increasing while increasing the concentration of adsorbates and it is also increasing with the increase in temperature. The adsorption of Cu ion follows the order 300C>400C >500C [12].
0 0.2 0.4 0.6 0.8 1 1.2 1.4 1.6
0 2 4 6 8 10 12
Amount
adsorbed
in
mg/gm
pH
0 0.2 0.4 0.6 0.8 1 1.2 1.4 1.6 1.8
0 5 10 15 20 25 30
Amount
adsorbed
in
mg/gm
Figure 5: Effect of temperature on adsorption of cu
Adsorption isotherms
The results obtained from the adsorption of Cu were analysed by the Langmuir isotherm. The linear form of Langmuir isotherm is given by the following equation:
Where qe is the amount of Cu adsorbed in mg/gm and Ce is the equilibrium concentration of Cu in mg/gm while Q0 and b are the Langmuir constant which are related to adsorption capacity and apparent heat change
respectively at different temperatures .The values of Q0 and b are determined from the slopes and intercepts of
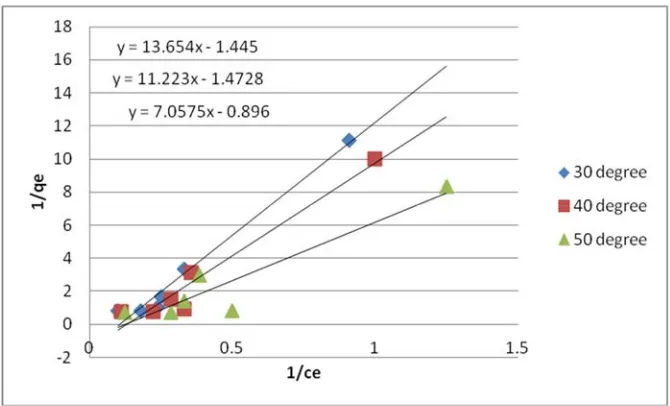
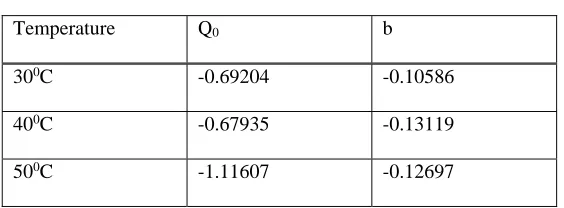
linear plots of 1/qe versus 1/Ce. The adsorption isotherms are drawn for copper at different temperatures and are shown in figure 6 . Table 1 summarizes the values of langmuir constants which favors the Cu adsorption.
Figure 6: Langmuir curve for Cu for different temperatures
Table 1: Langmuir constants
Temperature Q0 b
300C -0.69204 -0.10586
400C -0.67935 -0.13119
500C -1.11607 -0.12697
Conclusion
The above study clearly shows that the wood apple shell activated charcoal is a good adsorbent for the removal of Cu. The study also indicates that several factors affected the adsorption efficiency of adsorbent like contact time, initial concentration, temperature and pH. The verification of Langmuir isotherm indicates that wood apple shell activated charcoal is a good adsorbent for the removal of Cu The raw material used for preparing the adsorbent is widely available and inexpensive. The study is helpful for removing metal ions from waste water and can solve the problem of toxic effects of waste water on living organism.
REFERENCE
1. F. Gholami, A. H.Mahvi, Gh. A.Omrani, S.Nazmara, A. Ghasri, Removal of chromium (VI) from aqueous solution by ulmus leaves, Iran. J. Environ. Health. Sci. Eng. 3 97-102,2006.
2. Agarwal A and Saxena M,.Assessment of toxic effect of Brass and Steel industries waste on labeo Rohita in nearby river, International Journal of Environmental Engineering and Management, 2(1) 107 110, 2011.
3. K. O. Olayinka, B. I. Alo, T. Adu, Sorption of heavy metals from electroplating effluents by low cost adsorbents II: Use of waste tea, coconut shell and coconut husk, J. Appl. Sci. 7 2307-2313,2007.
4. A. Saravanan, V. Brindha, R. Manimekalai, S. Krishnan, An evaluation of chromium and zinc biosorption by a sea weed (Sargassum sp.) under optimized conditions, Ind. J. Sci. Technol. 2 53-56,2009.
5. I. Bhatti, K. Qureshi, R.A. Kazi, A.K. Ansari, Preparation and characterization of chemically activated almond shells by optimization of adsorption parameters for removal of chromium VI from aqueous solutions. World Acad. Sci. Eng. Technol. 34 199-204, 2007. 6. V. Sarin, K.K. Pant, Removal of chromium from industrial waste by using eucalyptus bark. Bioresource Technol. 97 15-20.,2006. 7. Z. Hu, L. Lei, Li. Yijiu, Y. Ni, Chromium adsorption on high performance activated carbons from aqueous solution, Sep .Purif.
Technol. 31 13-18,2003.
8. V. K. Gupta, A.Rastogi, A. Nayak, Adsorption studies on the removal of hexavalent chromium from aqueous solution using a low cost fertilizer industry waste material, J. Colloid Interf. Sci. 342 135- 141, 2010.
9. L. Monser, N. Adhoum, Modified activated carbon for the removal of copper, zinc, chromium and cyanide from wastewater, Sep. Purif. Technol. 26 137-146,2002.
10. Y. Jiang, Y. Wu, J. Liu, X. Xia, D. Wang, Ammonium pyrrolidinedithiocarbamate modified activated carbon micro-column extraction for the determination of As(III) in water by graphite furnace atomic absorption spectrometry, Microchim Acta 161 137-142,2008.
11. Vinay K. Singh et.al., “Removal of malathion from aqueous solutions and waste water using fly ash” J. Water Resource and Protection,2010,2,322-330.