Copyright 0 1988 by the Genetics Society of America
Heterochronic Mutations Affecting Shoot Development in Maize
R.
ScottPoethig
Plant Science Institute, Department of Biology, University of Pennsylvania, Philadelphia, Pennsylvania 19104-6018 Manuscript received September 14, 1987
Revised copy accepted April 13, 1988
ABSTRACT
Three semidominant, nonallelic mutations of maize, Teopod 1 ( T p l ) , Teopod 2 ( T p 2 ) and Teopod ? (Tp?), have a profound effect on both vegetative and reproductive development. Although each mutation is phenotypically distinct, they all ( 1 ) increase the number of vegetative phytomers; (2) increase the number of phytomers producing ears, tillers and prop roots; (3) increase the number of leaves bearing epidermal wax; (4) decrease the size of leaves and internodes; (5) decrease the size of both the ear and tassel; and (6) transform reproductive structures into vegetative ones. T h e analysis presented here suggests that this phenotype reflects the prolonged expression of a juvenile, vegetative developmental program which overlaps with the reproductive developmental program. T h e expres- sion of these mutations is different in each of the four inbred backgrounds used in this study. T p l
and Tp2 have similar phenotypes and are more highly expressed in the A632 and Oh51a inbred backgrounds than in W23 and Mo17. Tp? has less extreme effects than either of these mutations and has the opposite modification pattern; i.e., it is more higly expressed in W23 and Mol7 than in A632 and Oh5la. The expression of T p l and Tp2 in the presence of varying doses of their wild-type alleles indicate that both are gain-of-function mutations. T h e phenotypes of T p l and Tp2 and the nature of their response to variation in gene dose suggest that they control related, but nonidentical functions. The developmental and evolutionary implications of the heterochronic phenotype of these mutations is discussed.
A
LL organisms go through a series of morpholog- ically and physiologically distinct stages during their development. In many organisms there is a grad- ual transition from one stage to the next; other orga- nisms, such as insects and amphibians, undergo rapid and dramatic changes at particular points in their development. Either situation requires the coordinate regulation of a multitude of different processes at many different levels of organization. How this regu- lation is achieved is a central problem in develop- mental biology, and one that is still far from being solved.A great deal can be learned about the mechanism of developmental regulation from mutations that modify developmental patterns without completely destroying them. In fact, most of the recent progress in our understanding of the genetic and molecular basis of spatial patterning processes has come from the analysis of such mutations in Drosophila melano- gaster (MAHOWALD and HARDY 1985; GEHRING and HIROMI 1986; NUSSLEIN-VOLHARD, FROHNHOFER and
LEHMANN 1987) and other organisms (LOOMIS
1987, MALACINSKI and BRYANT 1984). Much less is known about the way in which temporal changes in developmental patterns are regulated. Although var- iation in the relative timing of developmental proc- esses is quite common in nature, in most cases the genetic and developmental basis for this variation is
Genetics 1 1 9 959-973 (August, 1988)
almost completely unknown. This phenomenon, which is known as heterochrony, is of considerable evolutionary interest because it is the basis for many major evolutionary trends (GOULD 1982; RAFF and KAUFMAN 1983; LORD and HILL 1987). Mutations with heterochronic phenotypes have recently been isolated in C . elegans, and are beginning to provide insight into the mechanism of temporal regulation in this species (AMBROS and HORVITZ 1984, 1987). Here I describe several mutations that may prove equally valuable for the analysis of temporal regulation in maize.
960 R. S. Poethig
its increase in size. For this reason most of the work ground are real and are not attributable to modifiers carried on the regulation of phase change in plants has been
conducted on woody species, such as English ivy, in which juvenile and adult phases of shoot growth are prolonged, stable, and exhibit numerous qualitative morphological differences (WAREING 1987; ZIMMER- MAN, HACKETT and PHARIS 1985). Unfortunately, the long life cycles of these plants makes them poor sub- jects for genetic analysis.
Although less is known about the mechanism of phase change in maize than in many other plants, maize is an excellent species in which to study this phenomenon because its developmental morphology, physiology and genetics are well characterized, and because the architecture of the shoot is based on the reiteration of a basic structural unit called the phyto- m e r (COE and NEUFFER 1976). Moreover, numerous mutations affecting shoot morphology in maize have already been identified and characterized (COE and POETHIG 1982). Several of these mutations have such profound effects on shoot morphology that it is rea- sonable to suspect that they have regulatory functions. Among these are four semidominant mutations- Corn- grass (Cg) (WHALEY a n d LEECH 1950; SINGLETON
1951; GALINAT, 1954a, b), Teopod 1 (Tpl) (LIND-
STROM 1925; WEATHERWAX 1929), and two previ- ously undescribed mutations, Tp2 and Tp3"that pro- duce striking atavistic changes in the vegetative mor- phology of the shoot and, even more remarkably, transform reproductive structures into leaves. In 1966 GALINAT proposed that this phenotype represented a defect in the transition from a juvenile to an adult phase in shoot development. The analysis of the phe- notypes of Tpl, Tp2 a n d Tp3 presented here supports this hypothesis and demonstrates that traits expressed during the juvenile phase of maize development are part of a genetically regulated developmental pro- gram.
MATERIALS AND METHODS
Genetic stocks: The mutations described in this paper originated spontaneously. Stocks of T p l and Tp2 were gen- erously provided by E. H. COE, JR., and M. G. NEUFFER and by the Maize Genetics Stock Center in Urbana, Illinois. Seed of Tp3 was obtained from J. B. BECKETT, who was the first to identify this mutation. E. H. COE and J. B. BECKETT also supplied the other genetic stocks and the inbred lines used
in this study.
Near-isogenic lines of T p l , T p 2 and Tp3 were generated by crossing these mutations six times to the inbred lines A632Ht, Mol7Ht, Oh51a and W23. A632Ht and Mol7Ht are Helminthosporium turcicum-resistant versions of A632 and Mo17. Mutant plants were used either as pollen or ear parents and no attempt was made to ensure a common cytoplasm. Parallel conversions starting with two different stocks of T p l and Tp2 were carried out in order to guard against the inadvertent selection of modifier loci. Since these parallel conversions produced stocks that were essentially identical in phenotype, it is reasonable to assume that the phenotypic differences among mutations in a common back-
along in the conversion program.
Phenotypic analysis: Measurements were taken on field- grown plants in the summers of 1985, 1986 and 1987. Seeds from ears segregating mutant and wild-type progeny were planted in a single row; mutations in the same genetic background were planted in adjacent rows. Measurements were taken on five to ten mutant individuals and ten or more wild-type plants-two to three wild-type plants in each segregating family. Leaves were measured as they matured since the basal leaves of the plant die relatively early in shoot growth. Leaf width was measured at the widest part of the leaf blade; leaf length was measured from the tip of the leaf blade to the ligule. A leaf was considered to have epidermal wax if wax was present anywhere on the leaf blade. On upper leaves epidermal wax was often restricted to the basal surface of the leaf tip. Axillary buds were numbered accord- ing to the number of the subtending leaf, while prop roots were numbered according to the number of the leaf arising from the node above them.
Dosage analysis: In order to study the expression of T p l
and Tp2 in the presence of varying doses of their wild-type alleles, mutant plants were pollinated by stocks carrying B- A translocations for the chromosome arms on which these mutations are located. The behavior of B-A translocations and their use in dosage analysis have been described by BECKETT (1 978) and BIRCHLER ( 1 983a, b). Because the B-A chromosome often undergoes nondisjunction at the second pollen mitosis, in addition to producing pollen grains with two identical haploid sperm B-A stocks also produce pollen grains with sperm cells that are duplicated and deficient for the B-A chromosome. Consequently, progeny from this cross have either 0, 1 or 2 copies of the genes located on the autosomal arm translocated to the B centromere.
Homozygous Tp2 g r-glTp2 g r-g plants from a stock that had been selfed three times were pollinated by plants car- rying one of the following TB-10L translocations: TB-lOLl9, TB-lOL26, TB-lOL1, TB-lOL22 and TB-1OLa. W22 stocks of the latter four translocations were kindly supplied by J. KERMICLE. Each of these translocations is broken at a differ- ent point on 1OL proximal to Tp2 (LIN 1982), and carries the R-scm allele of the R locus and Tp2' on the B-1OL
portion of the translocation. Because R-scm conditions strong pigmentation in both the aleurone layer of the en- dosperm and in the scutellum of the embryo, hypoploid, diploid and hyperploid progeny from this cross can be distinguished on the basis of the phenotype of the endo- sperm and scutellum in F, kernels. Kernels with a colored endosperm and colorless scutellum have hypoploid embryos
(Tp2 g r-g/- - -), kernels in which both the endosperm and scutellum are fully colored have diploid embryos (Tp2 g r-
g/Tp2+G R-scm), and kernels with a colorless endosperm but a colored scutellum have hyperploid embryos (Tp2 g r-gl Tp2+G R-scm/TpZ+G R-scm). Hypoploid seedlings could also be distinguished by their g phenotype because B-1OL carries the dominant allele of this locus.
Plants bearing T p l and 0 , 1 or 2 doses of the wild-type allele of this locus were generated in two ways. In one set of experiments, gl T p l /gl Tpl plants were crossed by hyper- ploid plants of the genotype 05/7-B/B-7Lb l(05 G1 Tpl +)/B-
Heterochronic Mutations in Maize 96 1
GI T p l + ) from hyperploid (gl TpllGI Tpl+/GI Tpl+) individ- uals. These genotypes were initially identified by counting
the number of chromosomes in root tip cells. Plants with 22 chromosomes were assumed to be hyperploids. Because
plants with 2 1 chromosomes can either be diploid (as a result of the normal disjunction of B-7Lb) or hyperploid (as a result of the transmission of a normal chromosome 7 and the B- 7Lb chromosome), each plant was crossed by gl and the segregation of this marker was scored in the resulting prog- eny. Plants segregating gl at a low frequency were assumed
to be hyperploid (7(Tpl gl)/7(Tplf Gl)/B-7Lb(Tpl+ GI), while plants segregating G1:gl in approximately a 1 : 1 ratio were assumed to be diploid (7(Tpl gl)/7-B/B-7L(Tpl+ GI)). Tpl/Tpl+and T p l / T p l + / T p l + individuals were also gen- erated by crossing 0 5 T p 1 / 0 5 T p l + individuals by males (L289 background) heterozygous for TB-7Lb. With the ex- ception of a few crossover progeny, kernels with an 05
endosperm have a hyperploid embryo of the genotype 05 T p l / 0 5 T p l + / 0 5 T p l + . Barring crossovers, kernels with a normal endosperm produce the following classes of seed- lings: mutant (05 T p l l - -) and wild-type (oh5 T p l + / - -)
hypoploids, mutant (05 Tplloh5 Tpl') and wild-type (oh5 Tp1+/05Tpl+) diploids, and wild-type hyperploids (oh5 T p l + / oh5 Tpl+/ 05 Tpl+). Seedlings that are hemizygous for 05 are pale green and eventually die. Thus, virtually all of the Tpl plants from kernels with a normal endosperm are diploid, while Tpl plants in the 05 endosperm class are predominantly hyperploid.
RESULTS
Identification of T p l , T p 2 and T p 3 : All three of the mutations described in this paper are semidomi- nant and are homozygous viable. T p l and Tp2 are fully penetrant in homozygous or heterozygous con- dition, but Tp3 is so susceptible to genetic modifica- tion that in many backgrounds it behaves as a recessive mutation. Because of the similarity in their pheno- types, the identity of the stocks used in this study was confirmed by linkage analysis. Consistent with their published locations (COE and NEUFFER 1976), T p l mapped 9.3 units from g l l (26/281), while Tp2 mapped 2.5 map units (22/894) from g. Tp3 was initially located on chromosome 3 by T-wx transloca- tions and subsequently mapped to the short arm of this chromosome. It is located 3 1 map units (99/32 1) distal to Lg and is very closely linked to Cg (POETHIG
1988). Tp3 is probably a weak allele of Cg, but this hypothesis is difficult to test because of the dominance of these mutations.
Morphology of wild-type maize: T h e morphology of maize has been described by a number of authors (KIESSELBACH 1949; BONNETT 1953; POETHIG 1982). For the purpose of this study, it is appropriate to describe the shoot as being organized into a succession of mirror image segments, or phytomers. Each phy- tomer has four components. During the vegetative phase of shoot development these components take the form of a leaf, an internode (the section of the stem between leaves), a bud located in the axil of the leaf, and a leaf-like structure called the prophyll located between the bud and the internode (GALINAT 1959,
1963). T h e character of these components varies con- siderably within the shoot. T h e basal-most phytomers of the shoot, which are produced during the juvenile phase of vegetative growth, have short, root-bearing internodes, narrow, wax-coated leaves devoid of epi- dermal hairs, a diminutive prophyll, and an axillary shoot that, in appropriate genetic backgrounds, gives rise to branches (tillers). T h e architecture of a tiller is identical to that of the primary axis of the plant. From basal phytomers to the ear node, internodes become progressively larger, adventitious roots are sup- pressed, leaf size increases, epidermal wax is replaced by epidermal hairs on the leaf blade, prophylls in- crease in size, and axillary buds become progressively feminized and ear-like. Between the ear node and the tassel, leaves and internodes progressively decrease in size and the prophyll and axillary bud are completely suppressed (STEPHENS 1948; KIESSELBACH 1949; GREYSON, WALDEN and SMITH 1982; A. BASSIRI, per- sonal communication).
T h e morphology of phytomers during the repro- ductive phase of shoot development is dramatically different from that during the vegetative phase (GAL-
INAT 1959, 1963; BONNETT 1953). In both the male
(tassel) and female (ear) inflorescences, the structures homologous to leaves, prophylls and internodes are rudimentary in size and may bear little resemblance to the vegetative counterpart of these structures. Along the primary axis of the ear and the tassel, for example, internodes are so tightly condensed that they cannot be readily assigned to single phytomers. In the tassel the prophyll may be replaced by lateral ridges of tissue called the rachis flaps or be reduced to a pulvinus in the axil of a tassel branch. In the ear the prophyll is represented by cupule wings associated with each pair of female spikelets. Homologs of the leaf are often invisible in the ear and tassel, but occasionally take the form of a small outgrowth called the glume cushion (BONNETT 1953; GALINAT 1959). Axillary shoots from the primary axis of the tassel give rise either to a pair of flower-bearing branches called spikelets, or to long secondary branches from which pairs of spikelets arise. Buds on the primary axis of the ear give rise solely to spikelet pairs. In both the ear and the tassel, each spikelet possesses two leaf- like structures called glumes (which are not associated with an axillary bud or prophyll) and a pair of flower buds. Each flower bud is subtended by a lemma (a structure homologous to a leaf) and is enclosed in the palea (a structure homologous to the prophyll) (Figure
1 a).
Morphology of T p l and T p 2 : T p l and Tp2 have such striking effects on both vegetative and reproduc-
962 R. S. Poc
q g-in
lo/
gc
a
FIGURE 1 .-The morphology of wild-type (a) and mutant (b and c) tassel spikelets. a and b are longitudinal representations, c is a
cross-section of the structures in b. Note that mutant spikelets are
unbranched, lack glumes and lemmas, and have enlarged, foliose
lodicules and a prophyll-like palea. Glume (gl), lemma (le), palea
(pa), stamens (st), lodicules (lo), spathe (sp), glume cushion (gc), internode (in).
the number of phytomers, (2) increase the number of phytomers bearing adventitious roots, (3) increase the number of leaves with epidermal wax, (4) increase the number of tillers and ears, ( 5 ) elevate ears to higher nodes, (6) decrease leaf size, and (7) decrease the length and circumference of internodes (Table 1 ;
Figure 3). Two aspects of this phenotype are particu- larly noteworthy: first, that the number of additional phytomers in mutant plants generally matches the number of additional phytomers expressing such ju- venile traits as prop roots and epidermal wax. In an A632 background, for example, T p l / T p l + plants have four additional phytomers, four additional leaves with epidermal wax and five additional nodes with prop roots; in Mol
7 ,
T p l / T p l + plants have four additional phytomers, five additional leaves with epi- dermal wax and three additional nodes with prop roots (Table 1). In the case of Tp2/Tp2+, the number of additional phytomers is approximately the same as the number of additional nodes bearing prop roots, but epidermal wax is much more extensive. Second,it is important to note that T p l and Tp2 do not alter the position of the ear relative to the tassel. In both mutant and normal plants, the topmost ear is located about five or six nodes from the tassel (Table 1 ; Figure 3). Thus, all the additional phytomers in mutant plants are located below the ear and are juvenile in character. This feature, as well as the diminutive size of the leaves and internodes in mutant plants support GALI-
NAT'S (1966) hypothesis that the phenotype of T p l
and Tp2 reflects the prolonged expression of a juve- nile developmental program.
Tpl and Tp2 do not simply delay the transition from juvenile to adult growth. If this were true, then the
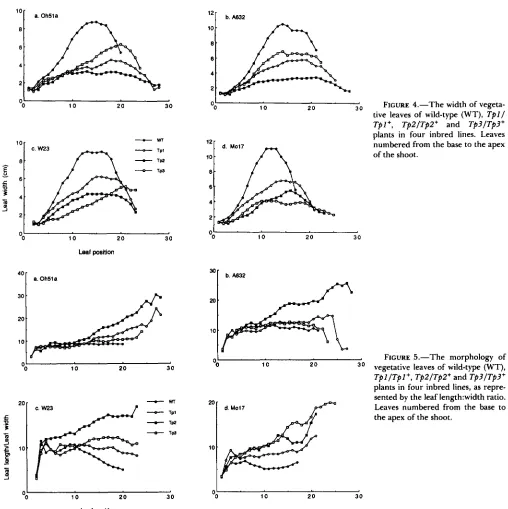
first few phytomers of mutant plants should be iden- tical to those of wild-type plants and the shoot should eventually adopt an adult vegative pattern, but at an abnormally high position. Instead, mutant leaves are significantly narrower than normal by the third or fourth phytomer and in some instances as early as the first or second (Figure 4). In addition, Tpl and Tp2
:thig
stimulate the production of tillers by basal phytomers even in genetic stocks that do not normally form tillers (Table 1; Figures 2 and 3). Thus, although the early development of mutant plants is similar to that of wild-type plants, it is not completely normal. Later stages of shoot growth are abnormal as well. Although the 1ength:width ratio ( L I W ) of the first few leaves of Tpl and Tp2 plants is similar to that of wild-type leaves, this ratio generally increases toward the tip of the shoot in mutant plants, but remains constant or decreases in wild-type individuals (Figure 5). Further- more, in the case of Tp2 epidermal wax is present on almost every leaf. Thus, Tpl and Tp2 modify all phases of vegetative growth, rather than simply delay- ing the transition from one phase to the next.
T h e most striking and characteristic feature of the phenotype of T p l and Tp2 is their effect on the morphology of the ear and the tassel. Both mutations reduce the degree of proliferation by these inflores- cences, cause the development of a leaf at the base of spikelet branches, and increase the size and complex- ity of the bracts (glumes, lemmas and paleas) in the spikelet (Figures 1 b and 6). T h e extent to which these traits are expressed varies considerably with genetic background, but there is a clear hierarchy to their appearance. In backgrounds that strongly suppress the expression of T p l and Tp2, these mutations usu- ally only reduce or eliminate lateral tassel branches without modifying any other reproductive traits. In moderately suppressive backgrounds, these mutations reduce the number of lateral tassel branches, suppress the pedicillate member of the spikelet pair and, in addition, stimulate the production of leaves at the base of some spikelets, particularly those near the base of the inflorescence (Figure 6b). These reproductive leaves (spathes) arise from the primary axis of the inflorescence and are often indistinguishable from vegetative leaves except for their small size. These spathes are clearly developmentally related to vege- tative leaves both because of their positional homology and because mutations which eliminate the ligule from vegetative leaves (Igl and Lg3) produce liguleless spathes in combination with T p l and Tp2.
In their most extreme expression, T p l and Tp2 reduce tassel branching by a further order and dra- matically change the phyllotaxis of the tassel as well. Virtually all the spikelets in such tassels are subtended by enlarged spathes and consist solely of a 1-2-cm long prophyll,' 1-2 foliose lodicules, and 1-2 stamens or a well developed pistil (Figure lb). This complex of structures is almost identical to the vegetative form
modified tassels are generally single, bi-keeled structures, in many cases they
I It is interesting to note that although the prophylls in these highly
Heterochronic Mutations in Maize 963
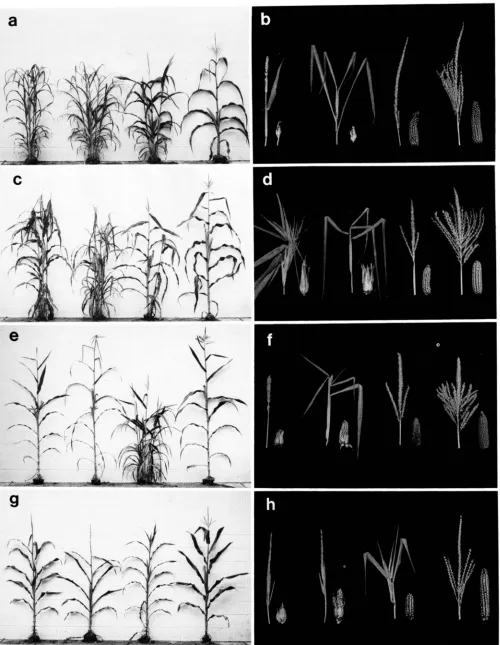
a
I
C
~ "I"
FIGURE 2.-The phenotypes of Tpl/Tpl+, Tp2/Tp2+ and Tp3/Tp3+ in four inbred lines. The left column of photographs illustrates the vegetative morphology of mature plants, the right column of photographs illustrates mature ears and tassels. In each photograph the order
964 R. S. Poethig
TABLE 1
Morphological parameters of T p I , Tp2 and Tp3 in four inbred lines
Oh51a A632 W23 Mol7
Parameters WT" Tpl Tp2 Tp3 W T Tpl Tp2 Tp3 W T Tpl Tp2 Tp3 W T Tpl Tp2 Tp.7
No. of vegetative 20 25 27 23 21 25 28 22 19 21 22 22 17 21 22 25
Ear nodeb 14 16 23 18 15 19 24 16 14 17 17 18 12 16 17 21
No. of nodes with 8 14 13 14 8 13 14 11 6 10 11 13 7 10 12 15
No. of leaves 9 14 27 16 8 14 28 11 5 7 10 14 6 11 22 20
phytomers
roots
with wax
No. of tillers 0 4 4 4 0 3 4 1 0 0 0 7 0 0 0 0
' Wild type.
'
Measured from the base of the plant.a. Oh51a
,
WT
c. W23
WT
b.
A632d. Mol7
FIGURE 3.-Schematic diagram of the morphology of wild-type (WT), Tpl/Tpl+, Tp2/Tp2+ and Tp3/Tp3+ plants in four inbred lines. Although internodes are illustrated here as being equal in length, basal internodes are actually shorter than apical internodes and the
internodes of mutant plants are shorter than those of wild-type plants. stippled leaves = epidermal wax; stippled internodes = prop roots,
?
= tiller terminating in a pistillate inflorescence; i$ = tiller terminating in a mixed staminate and pistillate inflorescence; 4 = ear;
Heterochronic Mutations in Maize 965
"1
8.Oh51a''1
b.A6321 0 2 0 30
-wr
"e Tpl
-
Tp2Leaf position
401
a. Oh51810 20 3 0
c. w23 ?
I
OO 2 0 1 0 3 0
Leaf podtion
0: 1 0 2 0
io
FIGURE 4.-The width of vegeta- tive leaves of wild-type (WT), T p l l TpI+, TpZ/TpZ+ and Tp3/Tp3+plants in four inbred lines. Leaves numbered from the base to the apex of the shoot.
6 .
4 '
2 '
10 2 0 30
"I
b.A83210 '
FIGURE 5.-The morphology of
TpI/Tpl+, TpZ/Tp2+ and Tp3/Tp3+
plants in four inbred lines, as repre- sented by the leaf 1ength:width ratio.
1 0 2 0 30 vegetative leaves of wild-type (WT),
2"1
d.MO17 pk" Leaves numbered from the base toI
the apex of the shoot.10 .
1 0 2 0 30
of the phytomer. In fact, highly transformed phyto- mers are so similar to vegetative phytomers that it is often difficult to determine the boundary between the tassel and the vegetative axis. At the base of the tassel, for example, phytomers are arranged distichously, as they are in the vegetative axis, rather than in their normal spiral pattern. Furthermore, both the spathes and the internodes in these highly modified tassels are similar in size to their normal counterparts, although they may be compressed in a zig-zag fashion, as though their expansion was constrained by the enclosing leaf sheaths.
The effect of Tpl and Tp2 on the morphology of the ear shoot is as dramatic as their effect on the tassel
966 R. S . Poethig
pairs, or solitary spikelets from one cupule, are usually enclosed in a spathe.
Both Tpl and Tp2 are more strongly expressed in the basal region of the ear and the tassel than at the tip of these inflorescences. Spathes, for example, are longer and more complex at the base of the ear or tassel than at the tip of these inflorescences, and are often confined to this basal region. T h e gradient in spikelet morphology may also be quite dramatic. In some cases, the range may extend from the type of spikelets shown in Figure l a to spikelets of the type shown in Figure lb.
Several other features of the phenotype of Tpl and
Tp2 are worth noting. Although in general the sever-
ity of the reproductive phenotype of these mutations parallels their effect on vegetative morphology, this is not true in the case of tiller number. In some back- grounds, such as W23 (Figure 2, e and f ) and Mol7 (Figure 2, g and h), Tpl and Tp2 plants have few or no tillers but possess ears and tassels that are almost as aberrant as those of the highly tillered Oh51a versions of these mutations (Figure 2b). It is also the case that although Tpl has the same range of effects as Tp2, they do not have identical phenotypes. In almost all respects the phenotype of Tp2 is more severe than that of T p l . Perhaps the most distinctive difference between these mutations is their effect on tassel morphology. Plants heterozygous for Tpl gen- erally have a well-developed, albeit highly modified, tassel containing staminate spikelets (Figure 2, b, d, f and h). Plants heterozygous for Tp2, on the other hand, usually have an extremely reduced tassel and often completely lack normal spikelets (Figure 2, b, d, f and h). Tp2 also has a much more significant effect on epidermal wax than T p l . Almost all of the leaves in Tp2 plants are partially covered by a waxy "bloom"; epidermal wax on Tpl plants is limited to basal leaves
FIGURE 6.-The effect of Tp2 on
floral morphology in the tassel. a, Normal spikelet pair. The glumes and lemmas have been removed from the sessile spikelet on the right, ex- posing a pair of male florets. Each floret contains three stamens and two lodicules and is enclosed in a palea. b, Tp2 spikelet with the subtending
spathe removed. Note that the spi- kelet is unpaired and bears a single floret. The palea has been replaced by 2 large structures which probably represent the components of the pro- phyll. These structures enclose a fo- liose lodicule and a pair of stamens. c, Tp2 (left) and normal (right) lodi-
cules. d, A Tp2 spikelet, with the
subtending spathe removed, consist- ing of a lodicule and a single, partially foliose stamen.
and is less extensive than on comparable leaves of Tp2
plants. Tp2 also has a more significant effect on node number and the number of nodes bearing prop roots than T p l . Given this general pattern, it is interesting that Tp2 has a less severe effect on ear morphology than Tpl. Tp2 ears are almost always larger than those of Tpl and are often not as highly modified (Figure 2, b, d, f and h).
Morphology of T p 3 : Tp3 has a more subtle phe- notype than Tpl and Tp2 (Figure 2). In backgrounds in which Tp3 is highly expressed, its effect on vegeta- tive and reproductive development is similar to that of Tpl and Tp2, although not as severe. Like these mutations, Tp3 increases the total number of phyto- mers and the number of phytomers with juvenile characteristics, and causes the tassel and ear to become vegetatively transformed (Table 1 ; Figures 2 and 3). This reproductive phenotype is somewhat uncom- mon, however, and in many backgrounds Tp3 is so
weakly expressed that it is difficult to distinguish Tp3/ Tp3+ plants from normal. Tp3 plants usually have somewhat smaller ears and less highly branched tassels than normal, but these quantitative differences are usually not significant enough to permit mutant plants
to be reliably identified. One of the most reliable features of Tp3 is its effect on tiller development. In addition to promoting the formation of tillers in non- tillering backgrounds, Tp3 characteristically produces tillers that terminate in an unbranched inflorescence with a pistillate, ear-like, basal section and a terminal staminate section. In contrast to Tpl and Tp2, the reproductive morphology of even very highly tillered
Tp3 plants is often quite normal.
Mutant phenotypes in inbred backgrounds: In order to define the primary effects of T p l , Tp2 and
Heterochronic Mutations in Maize 967
tations was quite uniform in each of the inbred back- grounds examined. With a few exceptions the range in the total number of nodes, and the location of tillers, buds, prop roots and epidermal wax, was about two nodes.
Oh51a: T p l and Tp2 are more severely expressed in Oh51a than in all other backgrounds examined (Figure 2, a and b; Figure 3a; Table 1). Although the phenotypes of these mutations are superficially iden- tical, Tp2 has a more significant effect than T p l on almost every parameter measured. This difference is most obvious in their effect on epidermal wax and on the morphology of the tassel. On average, only the bottom 14 leaf blades of T p l / T p l + plants have epi- dermal leaf wax; in contrast, all leaves on Tp2/Tp2+
plants have at least some epidermal wax. While the terminal section of T p l / T p l + tassels is frequently normal, Tp2/Tp2+ “tassels” are completely vegetative and terminate in a thin, sterile spike lacking both leaves and spikelets. Both mutations produce dimin- utive, leafy ears usually bearing around 10 kernels. In many cases, however, ears are so highly modified that they are completely sterile.
Tp3/Tp3+ plants share many traits with T p l or Tp2,
but have tassels and ears that differ from normal only in being somewhat reduced in size (Figure 2, a and b; Figure 3a; Table 1). Like T p l and Tp2, Tp3 increases the number of phytomers and the number of phyto- mers with juvenile characteristics, but to a much smaller extent.
A632: In most respects T p l and Tp2 have the same effects in A632 as they do in Oh51a (Figure 2, c and d; Figure 3b; Table 1). In A632, however, T p l pro- duces an extremely leafy tassel with essentially no normal spikelets, while in O h 5 l a it reduces the size of the tassel but does not completely suppress normal spikelet development. Tp2 has the same effect on tassel development in both backgrounds in that it completely, or nearly completely, suppresses spikelet formation. T h e ears of T p l / T p l + and Tp2/Tp2+
plants are small and every spikelet or spikelet pair is covered by a spathe borne on the rachis. Although
Tp2 ears tend to be slightly larger than those of T p l ,
they cannot be reliably distinguished from one an- other. Both mutations also greatly enhance the pro- duction of tillers, prop roots and epidermal wax in this background.
T h e phenotype of Tp3 in A632 is subtle, and not unlike its phenotype in Oh51a (Figure 2, c and d; Figure 3b; Table 1). Mutant plants differ from normal
in that they are shorter, have a reduced number of tassel branches, narrow leaves, and one or two tillers. Mutant ears are somewhat smaller than normal, but like the tassel, do not possess spathes.
W23: T p l and Tp2 have a less extreme phenotype in W23 than in either A632 or Oh51a (Figure
2,
eand f; Figure 3c; Table 1). In this background T p l l T p l + and Tp2/Tp2+ usually lack tillers or, at most, have only one or two. T h e tassels of T p l / T p l + plants are reduced to a single spike and are about half as long as the central spike of wild-type plants, but pos- sess spikelets that differ from normal only in that they are often unpaired and have slightly enlarged glumes. When present, spathes are short and are confined to a few spikelets at the base of the tassel. Tp2/Tp2+
tassels consist of a thin, barren rachis that terminates in one or a few spikelets. As in the case of T p l , these spikelets are usually normal but are frequently borne singly rather than in pairs. T h e ears of mutant plants are about half the size of wild-type ears and in both cases have short spathes limited to the base of the ear. Although Tp2 has a stronger effect on phytomer number, leaf wax, leaf width, and tassel development than T p l , it has a weaker effect on ear number, ear morphology and tiller number.
Tp3 has essentially the same effect on tassel and ear morphology, node number, and the distribution of roots and epidermal wax in W23 as in Oh51a or A632, but has a much more striking effect on tiller production in this background (Figure 2, e and f;
Figure 3c; Table 1). In W23, Tp3/Tp3+ plants gen- erally have between five and seven tillers, all of which terminate in a mixed inflorescence with a basal pistil- late section and a terminal staminate section.
Mo17: Although T p l and Tp2 do not have a strong effect on vegetative morphology in Mo17, they have a dramatic effect on reproductive development (Fig- ure 2, g and h; Figure 3d; Table 1). Every spikelet in the tassel of these mutants is subtended by a short spathe, and consists solely of a prophyll, a single foliose lodicule, and one or two stamens. T p l / T p l + and Tp2/ Tp2+ ears are substantially smaller than wild type, and in both cases every kernel is enclosed in a spathe. T p 2 / Tp2+ ears are about twice the size of T p l / T p l + ears.
T h e phenotype of Tp3 in Mol7 is significantly different from its phenotype in the other three inbreds (Figure 2, g and h; Figure 3d; Table 1). Although
Tp3/Tp3+ plants do not develop tillers, the vegetative and reproductive morphology of these plants are more highly modified than in any other background. In fact, in this background Tp3 has a more significant effect on tassel morphology, node number, epidermal wax, prop roots, and leaf width than either Tpl or
Tp2. T h e effect of Tp3 on tassel morphology is partic- ularly striking, given the relatively normal morphol- ogy of Tp3 tassels in the other three backgrounds. In Mol 7, Tp3/Tp3+ tassels exhibit all the features typical of extreme forms of T p l and Tp2 (Figure 1). Given this extreme phenotype, it is somewhat surprising that
968 R. S . Poethig
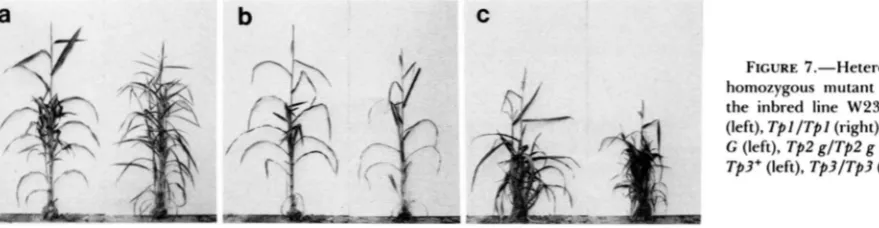
FIGURE 7.-Heterozygous and homozygous mutant phenotypes in the inbred line W23. a, TpI/TpI+
(left), TpI/TpI (right); b, Tp2g/Tp2+ G (left), Tp2 g/Tp2 g (right); c, Tp3/ Tp3+ (left), Tp3/Tp3 (right).
not usually possess spathes or any other unusual fea- tures.
Genetic interactions: Genetic analysis of Tp 1, Tp2
and Tp3 was facilitated by the modified phenotype of these mutations in W23. In this background, hetero- zygous forms of all three mutations are male fertile and produce a relatively large ear. This fortunate situation made it possible to compare the homozygous phenotypes of these mutations and their interactions in pairwise combinations. Since each of these muta- tions has a uniform and characteristic phenotype in this background, the genotype of the progeny in a segregating family could be reliably predicted on the basis of their phenotype and segregation frequency. These predictions were confirmed by progeny testing selected individuals. In the case of Tp2, homozygous individuals were identified by virtue of the expression of the closely linked marker,
g.
T p l , Tp2 and Tp3 have unique homozygous phe- notypes that are more severe than their heterozygous phenotypes. In the case of T p l , this difference is quite dramatic (Figure 7a). Plants homozygous for Tpl have highly reduced tassels nearly devoid of male spikelets, diminutive ears bearing less than 10 kernels, and significantly narrower leaves and more tillers than heterozygotes. In the case of Tp2, the difference be- tween homozygous and heterozygous forms is less pronounced (Figure 7b). Tp2 homozygotes are shorter and have smaller ears and tassels than heterozygotes. Although they sometimes possess several tillers, till- ering is not a typical feature of their phenotype. Tp3
homozygotes are typically dwarfed and very highly tillered, bear very small, waxy leaves, and have dimin- utive ears and a reduced tassel (Figure 7c). Tp3 homo- zygotes are also characterized by the presence of spathes in the ear and the tassel, traits which are not expressed by Tp3 heterozygotes in this background.
Tp3ITp3 plants undergo anthesis several days later than Tp3/Tp3+ plants and produce very little pollen.
The phenotype of Tpl/Tpl+; Tp2/Tp2+ double mutants is distinctly more extreme than that of T p l l T p l + or Tp2/Tp2+ (Figure 8a). Double mutants lack a tassel and are shorter, have smaller ears and leaves, more prop roots, and significantly more tillers than their T p l / T p l + or Tp2/Tp2+ siblings. In these re-
spects, the phenotype of Tpl /Tpl+; Tp2/Tp2+ plants is similar to that of Tpl homozygotes. Tpl /Tpl+; Tp3/ Tp3+ and Tp2/Tp2+; Tp3/Tp3+ plants also have a phenotype that is unlike that of the solitary mutations (Figure 8, b and c). The tassel and ear morphology of
Tpl/Tpl+; Tp3/Tp3+ individuals is identical to that of T p l / T p l + , while the number of tillers in such double mutants is similar to that of Tp3/Tp3+. In one experiment, T p l / T p l + segregants had an average of 2 tillers (n = 12; SD = 1.5), Tp3/Tp3+ plants averaged 6 tillers (n = 8; SD = 2.2) and Tpl/Tpl+; Tp3/Tp3+
plants had an average of 7 tillers (n = 10; SD = 1.9). Although other morphological features of T p l / T p l + ; Tp3/Tp3+ plants have not been carefully measured, the phenotype of these double mutants appears to be a composite of Tpl and Tp3 traits. The phenotype of plants heterozygous for Tp2 and Tp3 is more difficult to interpret. Such plants have a more severe pheno- type than Tpl /Tpl+; Tp3/Tp3+ plants, but are strik- ingly similar to Tpl/Tpl+; Tp2/Tp2+. Although plants heterozygous for Tp2 and Tp3 have the tassel morphology of Tp2/Tp2+ and about the same number of tillers as Tp3/Tp3+, other traits such as leaf and ear morphology are more severely affected in double mutants than in either Tp2/Tp2+ or Tp3/Tp3+ (Fig- ure 8c). Whether this interaction is additive or syner- gistic is not clear.
Dosage analysis: In order to understand the ge- netic basis of the phenotypes described above, it is crucial to determine the nature of these dominant mutations. While recessive mutations are generally assumed to represent a loss-of-function, dominant mu- tations can represent a number of different types of change. Loss-of-function mutations will have a domi- nant phenotype if the product of the locus is required in more than one dose. More commonly, dominant mutations represent a gain-of-function, such as the overproduction of a gene product (hypermorphic mu- tations), the production of a product with an antago- nistic function (antimorphic mutations), or the pro- duction of a product with an entirely new function or pattern of expression (neomorphic mutations)
Heterochronic Mutations in Maize 969
a
/b
2
double mutants in the inbred line FIGURE 8.-The phenotypes of W23. a, TpI/TpI+ (left); T p I / T p I + , Tp2/Tp2+ (center); Tp2/Tp2+ (right); b, TpI/TpI+ (left); TpI/TpI+, Tp3/ Tp3+ (center); Tp3/Tp3+ (right); c. Tp2/Tp2+ (left); Tp2/Tp2+, Tp3/ Tp3+ (center); Tp3/Tp3+ (right).TABLE 2
Effect of variation in gene dose on the expression of TpI and TP2
Genotype" Phenotypeb Genotype' Phenotype
TPIITPI X X X X Tp2/Tp2 xxx
Tp 11- X X X Tp2/- X
TP I / + xx TP2/+ xx
TPll+l+ X TP2/+l+ xx
+/-,
+/+,
+/+I+ Wild type +I-,+/+,
+I+/+ Wild typea Absence or duplication of the
+
symbol represents a deficiency*
X represents the severity of the Tpl or Tp2 phenotype.' Absence or duplication of the
+
symbol represents a deficiency or duplication of B-7Lb.or duplication of B-IOLa, B-IOLI, B-10L19. B-IOL22 or B-IOL26.
of its wild-type allele. Plants carrying Tpl or Tp2 and
0, 1 or 2 copies of the wild-type alleles of these loci were generated using B-A translocations, as described in the MATERIALS AND METHODS. T h e results described below and summarized in Table
2
are based on an examination of at least five plants in each dose class in two or more families.Plants hypoploid for Tp l (T p l l - ) have a much more severe mutant phenotype than those carrying one
( T p l / T p l + ) or two copies ( T p l / T p l + / T p l + ) of the wild-type allele of this locus. Although the nature of their phenotype varies in different backgrounds, hy- poploid individuals are usually about half as tall and have more highly transformed tassels and ears than their diploid and hyperploid sibs. T h e reduced height of these plants is probably unrelated to the expression of Tpl because wild-type hypoploids also exhibit this trait. Although the vegetative phenotypes of T p l l T p l + and T p l / T p l + / T p l + plants are virtually identi- cal in the suppressive background used in this study, the reproductive morphology of T p l / T p l + / T p l + is considerably more normal than that of T p l / T p l + . In this particular background, T p l / T p l + / T p l + tassels differ from normal only in being reduced to a single main spike, whereas T p l / T p l + tassels are highly mod- ified.
Several Tpl hypoploids were selfed in order to generate Tpl /Tpl homozygotes (progeny obtained by
self-pollinating a T p l l - plant are expected to be
homozygous for Tpl because chromosomes with large terminal deficiencies, such as 7L-B, are not pollen or
egg transmissible). T h e following season, the pheno- type of these
FP
T p l /T p l progeny was compared with that of hypoploids from the original F1 ear. T h e phenotype of T p l l T p l plants was significantly more severe than that of T p l / - plants in the parental family. Because these plants have closely related ge- netic backgrounds, this result strongly suggests thatTp1 is more strongly expressed in homozygous con- dition than in hemizygous condition.
T h e fact that Tpl is expressed in hyperploid plants demonstrates that this mutation involves a gain-of- function since a null or hypomorphic mutation would have been suppressed by a duplication of the wild- type allele. This conclusion is supported by the obser- vation that the phenotype of T p l / Tp l is more severe than the phenotype of this mutation over a deficiency. If Tpl involved a loss-of-function, the expression of this mutation in hemizygous condition should either
be identical to or more severe than its homozygous phenotype. Tpl is unlikely to be an overproducer because wild-type plants hyperploid for B-7Lb do not have a teopod phenotype, and because its expression is suppressed rather than enhanced by increasing the number of wild-type alleles of this locus. This latter observation suggests that Tpl encodes a product that in some way antagonizes normal gene activity. T h e possibility that this result is due to the presence of an unrelated suppressor locus on B-7Lb cannot be elimi- nated, however.
In contrast to the results obtained with T p l , varia- tion in wild-type gene dose has almost no effect on the expression of Tp2. Although plants hemizygous for Tp2 (Tp2/-) are slightly but consistently more normal than diploid (Tp2/Tp2+) or hyperploid (Tp2/ Tp2+/Tp2+) individuals, all of these dose classes have essentially the same mutant phenotype. This result was obtained with five different TB-10L translocations with breakpoints proximal to Tp2 in the region from the centromere (TB-lOL19) to 0.35 (TB-1OLa) on 1OL.
970 R. S. Poethig
loci are present in the region distal to 0.35 on 1OL is unknown. T p 2 / T p 2 plants, obtained by selfing T p 2 /
-
hypoploids, had a more extreme phenotype than any Tp2 plant in the parental family.T h e fact that Tp2 is expressed in the presence of two doses of the wild-type allele of this locus indicates that, like T p l , T p 2 is a gain-of-function mutation. It is also clear that Tp2 is not a simple over-producer because wild-type plants hyperploid for IOL do not have a teopod phenotype, and because its expression is not significantly normalized by hemizygosity or enhanced by a duplication of the wild type allele. T h e insensitivity of Tp2 to wild type gene dose suggests that this mutation encodes a neomorphic function. T h e possibilities include a product with a completely new function or, more likely, a normal function that is expressed at an inappropriate time or place. This latter interpretation is suggested by the observation that the expression of Antp73b, a dominant mutation of the Antennapedia locus which causes a wild-type gene product to be expressed in an inappropriate location (FRISCHER, HAGEN and GARBER 1986), is unaffected by an increase in the dose of the wild-type allele of this locus (T. KAUFMAN, personal communi- cation).
DISCUSSION
Many of the complex morphological differences between species can be attributed to positive or neg- ative changes in three temporal parameters: the time at which a process begins, the time at which it ends and the rate at which it occurs (ALBERCH et al. 1979; GOULD 1982). In plants, such changes can also cause morphological variation within an organism because of the prolonged, polar nature of shoot growth. All plants undergo significant changes in morphology during shoot growth. Leaves produced early in shoot development, for example, are smaller and often more simple in shape than leaves produced later. Similarly, in some species, there are significant morphological differences between flowers produced at different times in shoot development. This phenomenon is termed heteroblasty, and can often be attributed to the temporal parameters listed above (LORD and HILL 1987; POETHIG 1984).
T h e genetic basis of this variation in shoot mor- phology is poorly understood. In the case of plants that possess stable, morphologically well defined ju- venile and adult phases, there is good reason to believe that these phases are governed by some sort of epi- genetic autoregulatory system (MEINS and BINNS
1979; WAREING 1987). By contrast, in plants in which the morphology of the shoot changes gradually, it is difficult to distinguish morphological changes that result from quantitative changes in the size or physi-
ology of the plant (ALLSOPP 1963, 1965) from those that are genetically regulated.
T h e mutations described in this paper are signifi- cant, therefore, both because they reveal the existence of a genetically regulated juvenile developmental pro- gram in maize, and because they define the compo- nents of this program. T h e conclusion that these mutations represent genes that regulate juvenile growth is based on the observations that (1) each mutation causes several traits which are normally ex- pressed in the basal part of the maize plant to be expressed at higher nodes, (2) all three mutations affect the same set of traits, (3) none of these muta- tions has any obvious deleterious effects on cell growth and (4) T p l and T p 2 do not affect the overall duration of shoot growth or the rate of leaf initiation, but do prolong the vegetative phase of shoot growth at the expense of reproductive development (A. BASSIRI, E. IRISH, J. NADEAU and S. POETHIG, unpublished data). If the phenotype of these mutations resulted from nonspecific changes in cell behavior (e.g., a general change in growth rate or the orientation of cell divi- sion) one would expect these mutations to have a less predictable phenotype and more deleterious effects
o n growth rate or plant morphology.
Because T p l , T p 2 and T p 3 cause structures located at various positions along the shoot to resemble struc- tures normally found elsewhere they were initially assumed to be analogous to homeotic mutations in
Drosophila (POETHIG 1985). T h e more careful analysis of their phenotype presented here indicates that they differ from homeotic mutations in D. melanogaster in that they do not cause a one-for-one replacement of one structure with a structure of a different type (OUWENEEL 1976). Instead of replacing distal phyto- mers with basal phytomers, they produce a variety of complex changes which include both the addition of phytomers to the vegetative section of the plant, and the suppression of phytomers in reproductive branches. Moreover, mutant phytomers are similar, but not identical to normal juvenile phytomers. These observations suggest that the morphology of mutant phytomers reflects the imposition of juvenile traits on an otherwise normal pattern of shoot growth rather than the replacement of one type of structure with a normal structure of a different type. This interpreta- tion implies, of course, that the morphology of the shoot is determined by overlapping developmental programs which interact in an additive or synergistic fashion. This conclusion is most strongly supported by the highly vegetized inflorescences of TP1 and Tp2,
which possess structures from two very different de- velopmental programs: vegetative and reproductive.
Heterochronic Mutations in Maize 97 1
Because shoot growth is polar, structures produced at different times in development occupy different po- sitions along the shoot. Thus, changes in phase could just as well be regulated by the position of the shoot meristem relative to some external point, as by an endogenous counting or timing mechanism. T h e ob- servation that the effects of Tpl are non-cell-autono- mous is significant in this regard because it suggests that this locus controls the production or distribution of a diffusible substance (POETHIG 1987). Additional evidence that positional information may play an im- portant role in regulating phase change in maize is provided by the results of IRISH and NELSON (1988). These investigators have shown that the growth of the shoot can be prolonged by removing lower leaves and re-rooting the meristem before the tassel is initi- ated. T h e fact that plants derived from re-rooted shoots have the same number of leaves as seed-derived plants clearly suggests that the fate of the meristem is not entirely dependent on its history, but is deter- mined in part by factors originating outside it.
T h e phenotype of T p l , Tp2 and Tp3 is interesting from an evolutionary perspective because of its ata- vistic character. Indeed, the first mutation in this series, Teopod
I ,
was so named because its striking similarity to teosinte and to pod corn-plants which were thought to have played a major role in maize evolution (LINDSTROM 1925). But, although these mu- tations and Corngrass share several features with wild relatives of maize, the resemblance is far from perfect (GALINAT 1954a). While it is unlikely that teopod-like plants are the ancestors of the modern corn plant, the atavistic phenotype of these mutants raises the possi- bility that modern maize evolved by the progressive truncation of a juvenile developmental phase. T h e fundamental difference between maize and its highly tillered, small-eared, narrow-leafed relatives may sim- ply reside in the relative duration of the juvenile and adult phases of shoot development. Ears are initiated during the adult stage of development. Assuming that ear size is related to the duration of this stage, human selection for large ears might have led to the suppres- sion ofjuvenile traits in modern maize.The functions of T p l , T p 2 and T p 3 : As discussed above, the phenotypes of T p l , Tp2 and Tp3 suggest that these genes have major regulatory functions. Some clues about the nature of these functions are provided by the expression of these mutations in different genetic backgrounds. One of the striking features of T p l , Tp2 and Tp3 is their exquisite sensi- tivity to genetic modification; in each of the inbred backgrounds examined in this study, these mutations have different phenotypes. This feature suggests that T p l , Tp2 and Tp3 interact with numerous other loci, although it says nothing about the nature of these interactions. T h e isogenic stocks that have been gen-
erated in this study should make it possible to under- take a genetic analysis of this phenomenon. From a functional standpoint, the similar phenotypes of Tpl and Tp2 and their parallel response to genetic modi- fication suggest that these loci are closely related. Although it is possible that these mutations represent duplicate loci, the few but significant phenotypic dif- ferences between them and the nature of their re- sponse to variation in gene dose suggest this is not the case. While the expression of Tpl is suppressed by increasing doses of its wild-type allele, the expression of Tp2 is essentially indifferent to wild-type gene dose. These response patterns suggest that Tpl has an an- timorphic function, while Tp2 has a neomorphic one. Although this does not necessarily mean that these genes are functionally distinct, it seems unlikely that antimorphic and neomorphic mutations of the same function would have phenotypes as similar as those of Tpl and Tp2.
Tp3 appears to define a third, and more distantly related function. Although its phenotype is similar to that of Tpl and Tp2, Tp3 differs from these mutations in a number of important respects. Most significant is its dramatic effect on tiller development. Both Tpl and Tp2 stimulate the production of tillers, but this effect is secondary to their other effects on vegetative and reproductive morphology. Not only does Tp3 only rarely cause reproductive structures to become vegetatively transformed but, in contrast to Tpl and Tp2, tiller production is one of the most consistent aspects of its phenotype. Moreover, in at least some genetic backgrounds, Tp3 stimulates tiller production to a greater extent than Tpl and Tp2. Tp3 also has a modification pattern opposite to that of Tpl and Tp2. Its expression is suppressed by backgrounds that en- hance the expression of Tpl and Tp2 (A632 and Oh5 la) and is enhanced by backgrounds that suppress Tpl and Tp2 (W23 and Mo17).
972
R.
S. Poethigbud-prophyll-leaf). Here, we chose to use yet another form of the phytomer. For the purposes of this study, the phytomer was considered to consist of a leaf, bud and prophyll and the internode at the base of this complex (bud-prophyll-leaf-internode). This associa- tion is based on the observation that a leaf has the same cell lineage as its subtending internode (SHAR-
MAN 1942; JOHRI and COE 1982; MCDANIEL and POETHIG 1988), and the observation that the axillary bud and prophyll always develop in association with a subtending leaf, not in association with a more apical one. This is particularly obvious when mutant plants exhibit whorled phyllotaxis. In these situations the number of axillary buds present at a node reflects the number of leaves at that node, not at the node above it
(R.
S. POETHIG, unpublished observations). Similar observations have been made in the cases of phyllo- tatic transformations produced by microsurgical ma- nipulations (E. IRISH, personal communication).T h e fact that more than one leaf-bud-prophyll com- plex can be associated with a single internode reveals the fundamental weakness of the phytomer concept of shoot development. If the phytomer is the basic unit of shoot construction, then each and every leaf- bud-prophyll complex should be associated with a unique internode. Since this is not the case, the de- velopmental algorithm for this complex must be sep- arate from that of the stem. T o what extent these appendages are themselves a developmentally inte- grated unit is still unclear. At various points along the shoot one or another of these appendages is absent. Normally, axillary buds and prophylls are absent from the axils of leaves above the ear node, while leaves fail to develop in both the tassel and ear. T h e phenotype of the Tp mutations demonstrates, however, that the potential to form these structures has not been lost, just suppressed. This observation supports the hypoth-
esis that shoot development in maize is based on two
fundamental programs, one for the development of the stem, and another for the development of the leaf, bud and prophyll.
This research was initiated in the laboratory of E. H. COE, JR., and continues to benefit from his insight, constant support, and
encyclopedic knowledge of corn genetics; for all these things, I am
deeply indebted. It could not have been accomplished without the
dedicated assistance Of GENE SZYMKOWIAK, TRACY BYFORD, CHUCK
BRONK, KIRSTEN SAVINESE and MARY Lou OEHLERT. ABDOLLAH
BASSIRI, J A N NADEAU, CARL MCDANIEL and WALTON GALINAT
provided helpful comments on the manuscript. This research was supported by a grant from Pioneer Hi-Bred, Inc., U.S. Department of Agriculture Competitive Research grant 83-CRCR-1-1274, and National Science Foundation grant DCB-85 175 15.
L I T E R A T U R E C I T E D
ALBERCH, P., S. J. COULD, G. OSTER and D. WAKE, 1979 Size and
ALLSOPP, A., 1963 Morphogenesis in Marsilea. J. Linn. SOC. (Bot.)
shape in ontogeny and phylogeny. Paleobiology 5: 296-3 17.
58: 417-427.
ALLSOPP, A., 1965 Heteroblastic development in cormophytes.
pp. 1 172-1 221. In: Handbuch der Pjlanzenphysiologie, Vol 15,
Edited by W. RUHLAND. Springer, Berlin.
AMBROS, V., and H. R. HORVITZ, 1984 Heterochronic mutations
of the nematode, Caenorhabditis elegans. Science 2 2 6 409-4 16.
AMBROS, V., and H. R. HORVITZ, 1987 The lin-14 locus of Cae-
norhabditis elegans controls the time of expression of specific
postembryonic developmental events. Genes Dev. 1: 398-414.
ARBER, A., 1923 Leaves of the Gramineae. Bot. Gaz. 76: 374-
388.
ARBER, A., 1934 The Gramineae. J. Cramer, Weinheim.
BECKETT, J. B., 1978 B-A translocations in maize. I. Use in locat-
ing genes by chromosome arms. J. Hered. 6 9 27-36.
BIRCHLER, J. A., 1981 The genetic basis of dosage compensation
of alcohol dehydrogenase-1 in maize. Genetics 97: 625-637.
BIRCHLER, J. A., 1983a Allozymes in gene dosage studies. pp. 85-
108. In: Isozymes in Plant Genetics and Breeding, Edited by S. D.
TANKSLEY and T. J. ORTON. Elsevier, Amsterdam.
BIRCHLER, J. A,, 1983b Chromosomal manipulation in maize. pp.
379-403. In: Cytogenetics of Crop Plants, Edited by M. S. SWA-
MINATHAN, P. K. GUPTA and U. SINHA. Macmillan India Ltd, Delhi.
BONNETT, 0. T., 1953 Developmental morphology of the vege-
tative and floral shoots of maize. Ill. Agr. Exp. Sta. Bull. No.
568.
COE, E. H., JR., and M. G. NEUFFER, 1976 The genetics of corn.
pp. 11 1-223. In: Corn and Corn Improvement, Edited by G. F.
SPRAGUE. American Society of Agronomy, Madison, Wisc.
COE, E. H., JR., and R. S. POETHIG, 1982 Genetic factors affecting
plant development. pp. 295-300. In: Maize f o r Biological Re-
search, Edited by W. F. SHERIDAN. Plant Molecular Biology Assn., Charlottesville, Va.
COLLINS, G. N., 1924 The prophyllum of grasses. Bot. Gaz. 78:
353-354.
FRISCHER, L. E., F. S. HAGEN and R. L. GARBER, 1986 An
inversion that disrupts the Antennapedia gene causes abnormal
structure and localization of RNAs. Cell 47: 101 7-1023.
GALINAT, W. C., 1954a Corn grass. I. Corn grass as a prototype or
a false progenitor of maize. Am. Nat. 88: 101-104.
GALINAT, W. C., 1954b Corn grass. 11. Effect of the corn grass
gene on the development of the maize inflorescence. Am. J.
GALINAT, W. C., 1959 The phytomer in relation to the floral
homologies in the American Maydea. Bot. Mus. Leaflets, Har- vard U. 19: 1-32.
GALINAT, W. C., 1963 Form and function of plant structures in
the American Maydea and their significance for breeding.
Econ. Bot. 17: 51-59.
GALINAT, W. C., 1966 The corn grass and teopod loci involve
phase change. Maize Genet. Coop. News Lett. 4 0 102-103.
GEHRING, W. J., and Y. HIROMI, 1986 Homeotic genes and the
homeobox. Annu. Rev. Genet. 2 0 147-173.
COULD, S. J., 1982 Change in developmental timing as a mecha-
nism of macroevolution. pp. 333-346. In: Evolution and Deoel-
opment, Edited by J. T . BONNER. Springer-Verlag, New York.
GREYSON, R. I., D. B. WALDEN and W. J. SMITH, 1982 Leaf and
stem heteroblasty in Zea. Bot. Gaz. 143: 73-78.
IRISH, E. E., and T . M. NELSON, 1988 Development of maize
plants from cultured shoot apices. Planta. In press.
JOHRI, M. M., and E. H. COE, JR., 1982 Genetic approaches to
meristem organization. pp. 30 1-3 10. In: Maize f o r Biological
Research, Edited by W. F. SHERIDAN. Plant Molecular Biology Assn., Charlottesville, Va.
KIESSELBACH, T. A., 1949 The Structure and Reproduction of Corn.
U. Nebraska Press, Lincoln, Nebraska.
LIN, B-Y., 1982 Association of endosperm reduction with parental
imprinting in maize. Genetics 1 0 0 475-486.
LINDSTROM, E. W., 1925 Heritable characters of maize. XVI. A