EJBRAT 6(3) 2016
Volume 6
Number 3
July-September 2016
European
Journal
of Biological
Research
formerly
European Journal of Biological Research, Volume 6, Issue 3, July-September 2016 European Journal of Biological Research
ISSN 2449-8955
Editor-in-Chief Tomasz M. Karpiński
Poznań University of Medical Sciences, Poznań, Poland
Co-Editor Artur Adamczak
Institute of Natural Fibres and Medicinal Plants, Poznań, Poland
Editorial Secretary
Joanna Bródka, Poznań, Poland
Statistical Editor
Paweł Zaprawa, Lublin, Poland
Language Editor Jan Nowacki, London, UK
Scientific Editorial Board Tamara Bayanova, Apatity, Russia Alexander Ereskovsky, Marseille, France Agnieszka Gałuszka, Kielce, Poland Vittorio Gentile, Naples, Italy Stanisław Hałas, Lublin, Poland Fadi Hage Chehade, Beirut, Lebanon Afaf M. Hamada, Stockholm, Sweden Sven Herzog, Tharandt, Germany Liviu Holonec, Cluj-Napoca, Romania Miłosz A. Huber, Lublin, Poland Shri Mohan Jain, Helsinki, Finland Wouter Kalle, Wagga Wagga, Australia Tomasz Klepka, Lublin, Poland Nikolaos Labrou, Athens, Greece Igor Loskutov, Sankt Petersburg, Russia Ákos Máthé, Sopron, Hungary
Ahmed El-Mekabaty, Mansoura, Egypt Artem V. Mokrushin, Apatity, Russia Shahid M. Mukhtar, Birmingham, USA Robert Pal, Pécs, Hungary
Amal K. Paul, Kolkata, India Rajiv Ranjan, Narkatia Ganj, India Antonio Tiezzi, Viterbo, Italy
Timotej Verbovšek, Ljubljana, Slovenia Vladimir K. Zhirov, Apatity, Russia
List of Peer-Reviewers
http://www.journals.tmkarpinski.com/index.php/ejbr/pages /view/reviewers
Author Guidelines
http://www.journals.tmkarpinski.com/index.php/ejbr/about /submissions
More information
www.journals.tmkarpinski.com/index.php/ejbr
DISCLAIMER
The Publisher and Editors cannot be held responsible for errors and any consequences arising from the use of information contained in this journal; the views and opinions expressed do not necessarily reflect those of the Publisher and Editors, neither does the publication of advertisements constitute any endorsement by the Publisher and Editors of the products advertised.
Cover: http://openwalls.com/image?id=20115, Licence Creative Commons Attribution 3.0 Unported (CC BY 3.0)
Copyright: © The Author(s) 2016. European Journal of Biological Research © 2016 T.M.Karpiński. All articles and abstracts are open-access, distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted, non-commercial use, distribution and reproduction in any medium, provided the work is properly cited.
European Journal of Biological Research, Volume 6, Issue 3, July-September 2016
Contents
135-144
145-151
152-169
170-175
176-185
186-192
193-200
201-208
209-217
218-225
Antimicrobial and L-asparaginase activities of endophytic fungi isolated from
Datura innoxia and Hyoscyamus muticus medicinal plants
Ahmed H. M. El-Said, Yassmin M. Shebany, Mohamed A. Hussein, Eman G. A. El-Dawy
Seedling establishment in Scaligeria stewartiana (Nasir) Nasir (Apiaceae) from North-West Himalayas, India
Kulvinder Kour, Bachan Lal Bhellum, I. A. Hamal
Biosynthesis of anti-inflammatory immunosuppressive metabolite by Streptomyces
variabilis ASU319
Mohamed Hemida Abd-Alla, Abdel-Hamied M. Rasmey, El-Sayed A. El-Sayed, Ismail A. El-Kady, Ibrahim M. Yassin
Evaluation of the proliferative activity of aqueous extracts from three medicinal plants in murine spleen cells
Amit Gupta, Sushama R. Chaphalkar
Plant litter decomposition: drivers insight to the ecological process Gaurav Mishra, Rajesh Kumar
Flow cytometry: an overview in optical system and application in biological studies Amit Gupta, Deepak Vakhare, Sushama R. Chaphalkar
Significance of gut-blood barrier in health and disease Tomasz Huc, Kinga Pham, Janusz Skrzypecki, Marcin Ufnal
Impact of air pollutants on encountered plant foliage Himani Bisht, J. P. N. Rai, Krishna Giri
Growth space requirements models for Prosopis africana (Guill & Perr) Taub tree species in Makurdi, Nigeria
Henry Japheth Dau, B. I. Chenge
Oral administration of monosodium glutamate alters growth and blood parameters in female rabbits
of Biological Research
European Journal of Biological Research 2016; 6 (3): 135-144
Antimicrobial and L-asparaginase activities of endophytic
fungi isolated from Datura innoxia and Hyoscyamus
muticus medicinal plants
Ahmed H. M. El-Said
1,2, Yassmin M. Shebany
1,2, Mohamed A. Hussein
1*,
Eman G. A. El-Dawy
11
Botany Department, Faculty of Science, South Valley University, Qena, Egypt 2
Biology Department, Faculty of Science, Taif University, Saudi Arabia
*Corresponding author: Mohamed A. Hussein; E-mail: m.hussein@sci.svu.edu.eg
ABSTRACT
Thirty-six species and two varieties belonging to 16 genera of fungal endophytes were isolated from the leaves of Datura innoxia and Hyoscyamus muticus plants. The most prevailing fungi were: Asper-
gillus fumigatus, A. niger, A. terreus var. africanus, Cladosporium cucumerinum, C. oxysporum, Penicil-lium aurantiogriseum and P. chrysogenum.
Endo-phytic fungi from D. innoxia and H. muticus plants were tested for antibacterial and antifungal activities from which 68.98 and 78.26% respectively showed antibacterial against at least one of the tested microbe, but didn’t had effect on tested fungal isolates. Aspergillus niger SVUAn1 was in the top in producing L-asparaginase among tested isolates obtained from two plants. Maximum production of this enzyme obtained after 4 days of incubation with culture medium containing glucose as a carbon source. This study indicated that the endophytic fungi from D. innoxia and H. muticus plant another potential source of bioactive antimicrobial and anticancer agents.
Keywords: Datura innoxia; Hyoscyamus muticus; Endophytic fungi; Antimicrobial activity; L-aspara-ginase.
1. INTRODUCTION
Endophytic fungi colonize healthy living plant tissues without causing visible negative symptoms [1]. Endophytic fungi are known to be associated with medicinal plants and proved to be an important source of various secondary metabo-lites and bioactive compounds valuable for the pharmaceutical industry [2-4]. Datura innoxia and Hyoscyamus muticus belonging to Solanaceae
family, medicinally and economically these plants are important as it contains widely used tropane alkaloids, scopolamine, hyoscyamine and atropine [5]. Many investigations have been carried out on the endophytic mycobiota associated with various types of medicinal plants by several researches [6-8]. Many problems associated with using antibio-tics as antimicrobial agent including antibiotic resistance, host hypersensitivity, host immune-suppression and allergic reactions. Therefore, there is a need to develop alternative antimicrobial drugs Received: 21 March 2016; Revised submission: 08 June 2016; Accepted: 20 June 2016
Copyright: © The Author(s) 2016. European Journal of Biological Research © T.M.Karpiński 2016. This is an open access article licensed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits
European Journal of Biological Research 2016; 6 (3): 135-144 for the treatment of infectious diseases from fungi
[9]. Fungi are a good source of antimicrobial compounds used for medicine [10]. Numerous investigations have been carried out on the antimicrobial activity of endophytic fungi associated with various types of medicinal plants by several researches [11-13].
L-Asparaginase (EC 3.5.1.1) is a tetrameric protein [14] belonging to amidase group that ctalyses the hydrolytic deamination of asparagine to yield aspartic acid and ammonium ion [15]. L-asparaginase (EC 3.5.1.1), a medically important
enzyme possesses abroad spectrum of anticancer activity [16]. Using in treatment of different forms of cancer including acute lymphoblastic leukiemia [17]. L-asparaginase is produce by many micro-organisms including fungal species [18]. Several endophytic fungi appear to be a good source of this therapeutic enzyme including Alternaria tangelonis,
Cladosporium cladosporioides, Curvularia akaii
and Fusarium subglotinans [19] and Aspergillus
terreus [20]. Optimization of growth parameters
increases the yield of enzyme activity; several
workers have revealed that, incubation periods and carbon sources affecting the production of L- asparaginase by fungi [21-23].
This study is aimed to isolate endophytic fungi from Datura innoxia and Hyoscyamus muticus plants, identify them, and detect their antimicrobial potential and L-asparginase activity.
2. MATERIAL AND METHODS
2.1. Collection of plant samples
Twenty samples from each plant (Datura
innoxia and Hyoscyamus muticus) were chosen to
isolate endophytic fungi, which collected from desert habitat in Qena governorate, Egypt. Each sample was put in a sterile polyethylene bag [24]. Samples were transported in the same day to laboratory and were kept at 5˚C for mycological analysis.
2.2. Determination of Endophytic Fungi
Isolation of endophytic fungi from plant parts was done according to the method described by Rossman et al. [25]. Firstly, the plant leaves were
rinsed gently in running water to remove dust and debris. Then, leaves were cut into 1 cm in diameter with mid rib. The surface sterilization was done by sequel immersion in 75% ethanol for 1 min followed by sodium hypochlorite (5% available chlorine) for 2 min and treated with 75% ethanol for 1 min. Later the segments were rinsed three times with sterile distilled water and dried between sterile filter paper. Finally, four segments were inoculated on GPY plate amended with chloram-phenicol. The plates incubated at 28±2˚C for 2-3 weeks then the developing fungi were counted and identified morphologically, based on macro- and microscopic characters [26-30].
2.3. Crude extracts from fungi
Firstly, the endophytic fungi isolates were grown in GPY medium at 28±2˚C for 3-5 days. After that, 10 mm discs of the growth culture were introduced into 250 ml Erlenmeyer flasks containing 50 ml of GPY broth and incubated at 28±2˚C on a rotary shaker at 160 rpm with normal daily light and dark periods for 10 days. Then, the culture broth was filtrated through Whatman filter paper and the filtrate was extracted with chloroform (1:1 v/v) under constant shaking. The organic phase was concentrated under reduced pressure using a rotary evaporator at 45˚C and, finally, the concentrated extract was stored in a vacuum desiccator until constant weight [31].
2.4. Antimicrobial assay
The antimicrobial activity test was carried out by disk diffusion method [32] against the following bacteria (Enterobacter aerogenes, Enterococcus
faecalis, Escherichia coli, Klebsiella pneumonia, Pseudomonas aeruginosa, Salmonella typhi, Salmo-nella typhimurium, Shigella flexneri and Staphy-lococcus aureus) and fungi (Alternaria alternata, A. citri, Aspergillus niger, A. flavus, Cochliobolus spicifer, Stemphylium vesicarium and Ulocladium botrytis). The crude extracts of endophytic fungi
previou-European Journal of Biological Research 2016; 6 (3): 135-144 sly spread with bacterial suspension. Subsequently,
the Petri were incubated at 37±2˚C and the diameter of the inhibition zones was measured after 24 hr. For antifungal test, the fungal species were employed with GPY agar medium and the plates were incubated at 28±2˚C up to 5-7 days [34]. Chloroamphenicol and Nystatin used as positive control for the bacterial and fungal isolates, respectively.
2.5. L-asparaginase activity of fungal isolates
2.5.1. Screening of fungal isolates for L-aspara-ginase production
Based on isolation results Aspergillus niger was the most prevalent species. Six isolates from
A. niger isolated from Datura innoxia and Hyoscya-mus muticus were chosen randomly and screened
for their abilities to produce L-asparaginase as described by Gulati et al. [35]. Modified Czapek
Dox’s medium [36] contained (g/L) glucose 2.0, L-asparagine 10.0, KH2PO4 1.5, KCl 0.5, MgSO4.
7H2O 0.5, CuNO3. 3H2O 0.03, ZnSO4.7H2O 0.05,
FeSO4.7H2O 0.03, agar 18, initial pH 6.2
supple-mented with 0.09% phenol red as indicator was used to detect L-asparaginase activity by tested isoltes. The plates were inoculated with the 6 selected fungal isolates and incubated at 30°C for 48 h. The developing pink zones around the fungal colonies which indicated L-asparaginase production were measured.
2.5.2. Factors affecting L-asparaginase production
The effect of incubation periods and carbon sources on L-asparaginase production by
Asper-gillus niger SVUAn1 were studied; since isolate
was found to be highly active in L-asparaginase production.
2.5.2.1. Effect of incubation periods
The test isolate A. niger SVUAn1 was grown on the basal medium modified Czapek Dox’s liquid media with pH 6.2. The flasks were incubated at 30°C at different incubation periods (24-144 h). Uninoculated media served as controls. The cultures filterated through Whatman No. 1 filter paper. The
culture filtrate was used as crude enzyme to estimate enzyme activity.
2.5.2.2 Effect of carbon sources
The basal medium of modified Czapek Dox’s liquid media with pH 6.2 was supplemented with 0.2% of one of the following carbon sources: carboxymethylcellulose, fructose, maltose, starch, sucrose and yeast extract, in addition to glucose as control. After inoculation cultures were incubated at 30 ˚C for 96 h and the cultures were filtered, centrifuged and the filtrate was used for the detection of L-asparaginase activity according to method described by Imada et al. [37].
3. RESULTS
3.1. Endophytic mycobiota of Datura innoxia and
Hyoscyamus muticus plants
Thirty six and 2 varieties belonging to 16 genera were collected from 40 plant samples. The most prevalent genera on D. innoxia plant were
Aspergillus and Penicillium isolated from 65% and
50% of the samples comprising 25.92% and 24.69 of total fungi, respectively. From these genera, the most prevalent species were A. terreus var.
africanus, P. aurantiogriseum and P. chrysogenum,
they recovered from 25, 25 and 35% of the samples comprising 6.17, 8.64 and 12.34% of total fungi, respectively. Chaetomium and Cladosporium were the third frequent genera recovered from 30% of the samples contributing 12.34% and 16.04 of total fungi respectively (Table 1). Aspergillus and
Cladosporium were the most common genera on H. muticus plant were recovered from 90% and 80%
of the sample matching 45.61% and 27.20% of total fungi, respectively. From previous genera
A. fumigatus, A. niger, C. cucumerinum and C. oxysporum were the most prevalent species they
European Journal of Biological Research 2016; 6 (3): 135-144
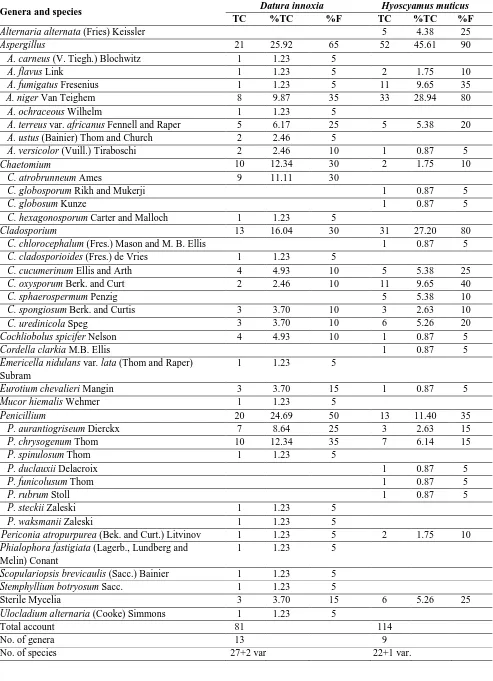
Table 1. Total counts (TC, calculated per 240 leaf segments), percentage of fungal counts (%TC, calculated per total fungi) and frequency of fungal species (%F, calculated per 20 samples) of various fungal genera and species recovered from leaves of Datura innoxia and Hyoscyamus muticus.
Genera and species Datura innoxia Hyoscyamus muticus TC %TC %F TC %TC %F Alternaria alternata (Fries) Keissler 5 4.38 25 Aspergillus 21 25.92 65 52 45.61 90 A. carneus (V. Tiegh.) Blochwitz 1 1.23 5
A. flavus Link 1 1.23 5 2 1.75 10 A. fumigatus Fresenius 1 1.23 5 11 9.65 35 A. niger Van Teighem 8 9.87 35 33 28.94 80
A. ochraceous Wilhelm 1 1.23 5
A. terreus var. africanus Fennell and Raper 5 6.17 25 5 5.38 20 A. ustus (Bainier) Thom and Church 2 2.46 5
A. versicolor (Vuill.) Tiraboschi 2 2.46 10 1 0.87 5 Chaetomium 10 12.34 30 2 1.75 10 C. atrobrunneum Ames 9 11.11 30
C. globosporum Rikh and Mukerji 1 0.87 5
C. globosum Kunze 1 0.87 5
C. hexagonosporum Carter and Malloch 1 1.23 5
Cladosporium 13 16.04 30 31 27.20 80 C. chlorocephalum (Fres.) Mason and M. B. Ellis 1 0.87 5 C. cladosporioides (Fres.) de Vries 1 1.23 5
C. cucumerinum Ellis and Arth 4 4.93 10 5 5.38 25 C. oxysporum Berk. and Curt 2 2.46 10 11 9.65 40 C. sphaerospermum Penzig 5 5.38 10 C. spongiosum Berk. and Curtis 3 3.70 10 3 2.63 10 C. uredinicola Speg 3 3.70 10 6 5.26 20 Cochliobolus spicifer Nelson 4 4.93 10 1 0.87 5 Cordella clarkia M.B. Ellis 1 0.87 5 Emericella nidulans var. lata (Thom and Raper)
Subram
1 1.23 5
Eurotium chevalieri Mangin 3 3.70 15 1 0.87 5 Mucor hiemalis Wehmer 1 1.23 5
Penicillium 20 24.69 50 13 11.40 35 P. aurantiogriseum Dierckx 7 8.64 25 3 2.63 15 P. chrysogenum Thom 10 12.34 35 7 6.14 15 P. spinulosum Thom 1 1.23 5
P. duclauxii Delacroix 1 0.87 5
P. funicolusum Thom 1 0.87 5
P. rubrum Stoll 1 0.87 5
P. steckii Zaleski 1 1.23 5 P. waksmanii Zaleski 1 1.23 5
Periconia atropurpurea (Bek. and Curt.) Litvinov 1 1.23 5 2 1.75 10 Phialophora fastigiata (Lagerb., Lundberg and
Melin) Conant
1 1.23 5 Scopulariopsis brevicaulis (Sacc.) Bainier 1 1.23 5 Stemphyllium botryosum Sacc. 1 1.23 5
Sterile Mycelia 3 3.70 15 6 5.26 25 Ulocladium alternaria (Cooke) Simmons 1 1.23 5
Total account 81 114
No. of genera 13 9
European Journal of Biological Research 2016; 6 (3): 135-144 Dos Santos et al. [41] reported that, among
the fungi isolated from Melia azedarach, the endophytic fungi belonging to Aspergillus and
Penicillium were predominant; on the other hand,
Selvi and Balagengatharathilagam [42] found that
Cladosporium sp. was commonly present in most
of the tested medicinal plants in Virudhunagar District. Also, Ramesha and Srinivas [43] isolated endophytic fungi from different parts of Plumeria
acuminata and Plumeria obtusifolia and identified
them morphologically from which Alternaria sp.,
Aspergillus sp., Chaetomium sp., Cladosporium sp., Cochliobolus sp., Curvularia sp., Mycelia Sterilia, Fusarium sp. and Penicillium sp.
3.2. Antimicrobial effects of endophytic fungal extracts isolated from Datura innoxia and
Hyoscyamus muticus
Generally, fungal endophytes isolated from
D. innoxia and H. muticus did not have any
antifungal effect against tested fungi. In total, 68.96% and 78.26% of the endophytic fungal isolates from D. innoxia and H. muticus exhibited antibacterial activities, respectively (Table 2 and 3). This result were in agreement with obtained by Mahdi et al. [44] they found fungal extract showed inhibition for bacterial growth but were not active against tested fungi. Ramesha and Srinivas [35] screened the potential of endophytic fungi from Plumeria obtusifolia and P. acuminate for antimicrobial activity and they found that, 66.6% and 58.8% recovered from P. obtusifolia and
P. acuminate plants, respectively, exhibited activity
against tested organism. 70 % of fungal endophytes from Celastrus paniculatus plant displayed antimicrobial activities against tested bacteria and fungi [45].
Endophytic fungi showed different antibac-terial activities against tested bacteria, the most potent fungi from D. innoxia plant were:
Asper-gillus fumigatus, Cochliobolus spicifer, Phialophora fastigiata and Stemphylium botryosum exhibited
antimicrobial activity ranged from 77.7-88.8 % with inhibition zone from 8-23 mm. Aspergillus flavus,
A. versicolor, Cordella clarkia, Eurotium chevalieri, Penicillium aurantiogriseum and P. funicolusum
were the most potent fungi from H. muticus plant with antimicrobial activity from 77.7-88.8 and
inhibition zone from 8-16 mm. A. fumigatus and A. versicolor isolated from D. innoxia showed
strongest inhibition against Enterobacter aerogenes growth (Table 2 and 3). Among 144 endophytic isolates, A. fumigatus isolates were the most effective fungi against Staphylococcus aureus and
Klebsiella pneumoniae [46]. The antimicrobial
activity of Aspergillus fumigatus against bacteria and fungi was previously reported [47]. Fumifungin and synerazol, new antibiotics, were recovered from the culture broth of A. fumigatus [48, 49]. Another antibiotic, fumagillin, is produced by certain strains of A. fumigatus and because it was reported as an angiogenesis inhibitor [50]. Two aroyl uridine derivatives kipukasins H and I from A. versicolor strain ATCC 9577 exhibited antibacterial activity against Staphylococcus epidermidis [51]. Many other compounds including anthraquinone deri-vatives, averantin, averufin, methyl-averantin nidurufin, sterigmatocystin and versiconol extracted from cultures broth of A. versicolor exhibited antibacterial activities [52-54]. Thus, it was clear that, chloroform extracts of endophytic fungi obtained from the same plant showed different antibacterial activity (Table 2 and 3). These diffe-rences in susceptibility could be attributed to the type of isolates and nature and level of the antimicrobial agents present in their extracts as well as their mode of action on different test microorganisms [55].
3.3. L-asparaginase activities of endophytic
Aspergillus niger isolates
European Journal of Biological Research 2016; 6 (3): 135-144 L-asparaginase production on glucose-asparagine
broth medium seven isolates showed higher enzyme activity. Twelve fungal strains isolated from wheat flour including A. niger exhibited high L-aspara-ginase activity [61].
High enzyme producer Aspergillus niger (SVUAn1) was chosen to study the effects of incubation time and carbon source on enzyme activity. Maximum production of L-asparaginase by A. niger (SVUAn1) isolate was achieved at 96 h (4 days) with incorporation of glucose as carbon source in the culture medium (Figs.1, 2). These finding are almost in agreement with those results reported by several workers with different fungal species [22, 62-64].
Figure 1. Production of L-asparaginase by A. niger (SVUAn1) at different time intervals.
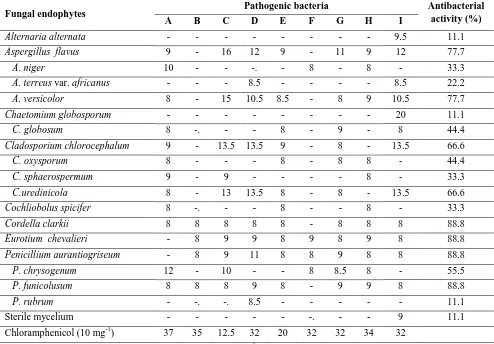
Table 2. Antibacterial effects (in mm diameter) of selected fungal endophytes from Datura innoxia against different nine pathogenic bacteria.
Fungal endophytes Pathogenic bacteria Antibacterial activity (%) A B C D E F G H I
Aspergillus flavus - - - 9 - - 10 22.2 A. fumigatus 23 19.5 10 - - 8.5 15 17.5 23 77.7 A. niger 10 8 7.5 16 - - 11 8 - 66.6 A. versicolor 40 - - - - 9 - - 11 33.3 A. ochraceous 9 - - - 8.5 - 22.2 A. ustus - - - 12 11.1 Chaetomium atrobrunneum - - - 11.5 11.1 C. hexagonosporum 10 - - - -. - - - - 11.1 Cladosporium spongiosum 10.5 9 10 - - 9 10.5 10 10 77.7 C. uredinicola - - - 11 11.1 Cochliobolus spicifer 10 8 11 13.5 8 9.5 11 8 - 88.8 Emericella nidulans var. lata 9 - - - 11.1 Mucor hiemalis - - - 8 - 11.1 Penicillium aurantiogriseum - - - 8 - 11.1 P. chrysogenum 11 - - - -. - - - - 11.1 P. steckii - 10 10 - - 9 8.5 - 9.5 55.5 P. waksmanii - - - - 9 - - - - 11.1 Phialophora fastigiata 9.5 8 - 10 8 9 11 8 - 77.7 Stemphylium botryosum 14.5 11 10 - - 8 9 9 10.5 77.7 Ulocladium alternariae - - - 11.5 - - - - 12.5 22.2 Chloramphenicol (10 mg-1) 37 35 12.5 32 20 32 32 34 32
European Journal of Biological Research 2016; 6 (3): 135-144
Table 3. Antibacterial effects (in mm diameter) of selected fungal endophytes from Hyoscyamus muticus against different nine pathogenic bacteria.
Fungal endophytes Pathogenic bacteria Antibacterial activity (%) A B C D E F G H I
Alternaria alternata - - - 9.5 11.1 Aspergillus flavus 9 - 16 12 9 - 11 9 12 77.7 A. niger 10 - - -. - 8 - 8 - 33.3 A. terreus var. africanus - - - 8.5 - - - - 8.5 22.2 A. versicolor 8 - 15 10.5 8.5 - 8 9 10.5 77.7 Chaetomium globosporum - - - 20 11.1 C. globosum 8 -. - - 8 - 9 - 8 44.4 Cladosporium chlorocephalum 9 - 13.5 13.5 9 - 8 - 13.5 66.6 C. oxysporum 8 - - - 8 - 8 8 - 44.4 C. sphaerospermum 9 - 9 - - - - 8 - 33.3 C.uredinicola 8 - 13 13.5 8 - 8 - 13.5 66.6 Cochliobolus spicifer 8 -. - - 8 - - 8 - 33.3 Cordella clarkii 8 8 8 8 8 - 8 8 8 88.8 Eurotium chevalieri - 8 9 9 8 9 8 9 8 88.8 Penicillium aurantiogriseum - 8 9 11 8 8 9 8 8 88.8 P. chrysogenum 12 - 10 - - 8 8.5 8 - 55.5 P. funicolusum 8 8 8 9 8 - 9 9 8 88.8 P. rubrum - -. -. 8.5 - - - 11.1 Sterile mycelium - - - -. - - 9 11.1 Chloramphenicol (10 mg-1) 37 35 12.5 32 20 32 32 34 32
A: Enterobacter aerogenes (ATCC13048), B: Enterococcus faecalis (ATCC29212), C: Escherichia coli (ATCC25922), D: Klebsiella pneumonia (ATCC13888), E: Pseudomonas aeruginosa (ATCC278223), F: Salmonella Typhi (ATCC19430), G: Salmonella typhimurium (ATCC14028), H: Shigella flexneri (ATCC12022) and I: Staphylococcus aureus (ATCC43300).
Table 4. Degree of L-asparaginase activities (calcu- lated as average diameter of pink color zone around the colony mm) of the tested Aspergillus niger isolates.
A. niger
isolates Source of isolation
Activity remarks SVUAn1 Datura innoxia 67.5 H SVUAn2 Datura innoxia 25 M SVUAn3 Datura innoxia 30 M SVUAn4 Hyoscyamus muticus 32.5 M SVUAn5 Hyoscyamus muticus 25 M SVUAn6 Hyoscyamus muticus 20 M Activity remarks: high activity, H = 35-70 mm; moderate activity, M = 20-34 mm and weak activity, W = less than 20 mm.
Figure 2. The effect of carbon sources on the production of L-asparaginase by A. niger (SVUAn1).
decom-European Journal of Biological Research 2016; 6 (3): 135-144 position by proteolytic enzymes while, the
prolon-ged incubation time led to a decrease in L-asparagi-nase secretion and this may be due to the exhaustion of some medium constituents or the production of inhibitory compounds [66].
4. CONCLUSIONS
From above results, it can conclude that medicinal plants are a reservoir for many endo-phytic fungi which consider a good source for antimicrobial and anticancer compounds. The maximum production of L-asparaginase by
Asper-gillus niger SVUAn1 was achieved at shorter
incubation time with glucose as carbon source.
AUTHORS’ CONTRIBUTION
YMS: Designed and collect the samples, EGAE: laboratory work and recorded the experimental data. MAH: participate in collections of samples, fungal identification and wrote the research and editing. AHME; Supervision, revision. The final manuscript has been read and approved by all authors.
TRANSPARENCY DECLARATION The authors declare no conflicts of interest.
REFERENCES
1. Hyde KD, Soytong K. The fungal endophyte dilemma. Fungal Divers. 2008; 33: 163-173.
2. Zhang HW, Song YC, Tan RX. Biology and chemistry of endophytes. Natural Prod Rep. 2006; 23: 753-771.
3. Krishnamurthy YL, Naik SB, Jayaram S. Fungal communities in herbaceous medicinal plants from the Malnad Region, Southern India. Microbes Environ. 2008; 23(1): 24-28.
4. Khan R, Shahzad S, Choudhary I, Khan SA, Ahmad A. Communities of endophytic fungi in medicinal plant Withania somnifera. Pak J Bot. 2010; 42(2): 1281-1287.
5. Niber BT, Helenius J, Varis AL. Toxicity of plant extracts to three storage beetles (Coleoptera). J Appl Entomol. 1992; 113(2): 202-208.
6. Bharathidasan R, Panneerselvam A. Biodiversity of the endophytic fungi isolated from Avicennia marina in Ramanathapuram District, Karankadu. World J Sci Technol. 2011; 1(9): 1-5.
7. Abdou R. Bioactive metabolites from the endophyte Botryospheria obtuse of the medicinal plant Bidens pilosa. Int J Pharmacy Pharm Sci. 2013; 5(3): 579-584.
8. Devi NN, Prabakaran JJ. Bioactive metabolites from an endophytic fungus Penicillium sp. isolated from Centella asiatica. Curr Res Environ Appl Mycol. 2014; 4(1): 34-43.
9. Supriya GNR, Audipudi AV. Screening for antimicrobial activities of endophytic fungi isolated from ripened fruit of Capsicum frutescence L. World J Pharm Sci. 2015; 3(2): 258-262.
10. Newman DJ, Cragg MG. Natural products as source of new drugs over the last 25 years. J Nat Prod. 2007; 70: 461-477.
11. Kumala S, Izzati H. Isolation IPG3-1 and IPG3-3, endophytic fungi from Delima (Punica granatum Linn.) twigs and in vitro assessment of their antimicrobial activity. Int Res J Pharmacy. 2013; 4(6): 49-53.
12. Kumar S, Aharwal RP, Kumar S, Sandhu SS. Isolation and detection of anti-bacterial activity of endophytic fungi from Bombex cebia and Argemone Mexicana. J Chem Pharm Res. 2014; 6(11): 95-100. 13. Raju DC, Victoria TD. Isolation, characterization
and antibacterial activeity of endophytic fungi from Calophyllum inophyllum L. Der Pharma Chemica. 2015; 7(7): 250-254.
14. David SG. The molecular perspective: L-aspara-ginase. Oncologist. 2005; 10: 238-239.
15. Ghasemi Y, Ebrahimminezhad A, Amini SR, Zarrini G. Ghoshoon MB, Raee MJ, et al. An optimized medium for screening of L-asparaginase production by Escherichia coli. Am J Biochem Biotechnol. 2008; 4(4): 422-424.
16. McCredie KB, Wang Ho DH, Freireich EJ. L-aspara-ginase for the treatment of cancer. A Cancer J Clin. 2008; 23(4): 220-227.
17. Jain R, Zaidi KU, Verma Y, Saxena P. L-aspara-ginase: A promising enzyme for treatment of acute lymphoblastic leukiemia. J Sci Res. 2012; 5(1): 29-35.
18. Yadav CN, Sarkar S. Production of L-asparagi- nase by Fusarium oxysporum using submerged fermentation. Int J Pharm Sci Invention. 2014; 3(6): 32-40.
19. Masumi S, Mirzaei S, Kalvandi R, Zafari D. Asparaginase and amylase activity of thyme endophytic fungi. J Crop Prot. 2014; 3: 655-662. 20. Kalyanasundaram I, Nagamuthu J, Srinivasan B,
European Journal of Biological Research 2016; 6 (3): 135-144
purification and characterisation of extracellular L-asparaginase from salt marsh fungal endophytes. World Journal Pharm Pharmac Sci. 2015; 4(3): 663-677.
21. Thirunavukkarasu N, Suryanarayanan TS, Murali TS, Ravishankar JP, Gummadi SN. L-asparaginase from marine derived fungal endophytes of seaweeds. Mycosphere. 2011; 2(2): 147-155.
22. Rani SA, Sundaram L, Vasantha PB. Isolation and screening of L-asparaginase producing fungi from soil samples. Int J Pharmacy Pharm Sci. 2011; 4: 279-282.
23. Lincoln L, More SS. Isolation and production of clinical and food grade L-asparaginase enzyme from fungi. J Pharmacognosy Phytochem. 2014; 3(3): 177-183.
24. Bills GF. Isolation and analysis of endophytic fungal communities from woody plants. In: Redlin SC, Carris LM, eds. Endophytic fungi in grasses and woody plants: systematics, ecology and evolution. APS Press, St. Paul, 1996.
25. Rossman AY, Tulloss RE, O’Dell TE, Thorn RG. Protocols for an all taxa biodiversity inventory of fungi in a Costa Rican conservation area. Parkway Publications, Inc., Boone, North Carolina, 1998. 26. Ames LA. A monograph of the chaetomiaceae.
Wheldon and Wasley L.T.D. New York, 1969. 27. Ellis MB. Dematiaceous hyphomycetes.
Common-wealth Mycological Institute, Kew, England, 1971. 28. Raper KB, Fennell DJ. The genus Aspergillus.
Williams & Wilkins, Baltimore, USA, 1965.
29. Raper KB, Thom C. A manual of the Penicillium. Williams & Wilkins, Baltimore, USA, 1949.
30. Domsch KH, Gms W, Anderson TH. Compendium of soil fungi. Acad Press, London, 1980.
31. Silva MRO, Almeida AC, Arruda FVF, Gusmao N. Endophytic fungi from Brazillian mangrove plant Laguncularia racemosa (L.) Gaertn. (Combre-taceae): their antimicrobial potential. In: Science against microbial pathogens: communicating current research and technological advances. Mendez-Vilas A, ed. 2011: 1260-1266.
32. Bauer AW, Kirby WMM, Sherris JC, Turck M. Antibiotics susceptibility test by a standardized single disc method. Am J Clin Pathol. 1966; 45: 493-496.
33. Tripathi NK, Shrivastva A, Biswal KC, Rao PVL. Optimization of culture medium for production of recombinant dengue protein in Escherichia coli. Indust Biotechnol. 2009; 5(3): 179-183.
34. Maria GL, Sridhar KR, Raviraja NS. Antimicrobial and enzyme activity of mangrove endophytic fungi of southwest coast of India. J Agric Technol. 2005: 67-80.
35. Gulati R. Saxena RK, Gupta R. A rapid plate assay for screening L-asparaginase producing micro-organisms. Lett Appl Microbiol. 1997; 24: 23-26. 36. Saxena RK, Sinha U. L-asparaginase and
gluta-minase activities in culture filtrates of Aspergillus nidulans. Curr Sci. 1981; 50: 218-219.
37. Imada A, Igarasi S, Nakahama K, Isono M. Asparaginase and glutaminase activities of micro-organisms. J General Microbiol. 1973; 76: 85-99. 38. El-Morsy EM. Fungi isolated from the
endo-rhizosphere of halophytic plants from the Red Sea Coast of Egypt. Fungal Divers. 2000; 5: 43-54. 39. Abdel-Motaal FF, El-Zayat Y, Kosaka Y, El-Sayed
M A, Nassar MSM, Ito SI. Antifungal activity of endophytic fungi isolated from Egyptian henbane (Hyoscyamus muticus L.). Pak J Bot. 2010; 42(4): 2883-2894.
40. Singh SK. Endophytic fungi from Jatropha curcus: A preliminary study. J Pharm Sci Innov. 2013; 2(2): 26-29.
41. Dos-Santos RMG, Rodrigues-Fo E, Rocha WC, Teixeira MFS. Endophytic fungi from Melia azedarach. World J Microbiol Biotechnol. 2003; 19: 767-770.
42. Selvi KB, Balagengatharathilagam P. Endophytic fungi from medicinal plants of Virudhunagar district for antimicrobial activity. Int J Sci Nature. 2014; 5(1): 147-155.
43. Ramesha A, Srinivas C. Antimicrobial activity and phytochemical analysis of crude extracts of endophytic fungi isolated from Plumeria acuminata L. and Plumeria obtusifolia L. Eur J Exp Biol. 2014; 4(2): 35-43.
44. Mahdi T, Mohamed I, Yagi S. Endophytic fungal communities associated with ethno-medicinal plants from Sudan and their antimicrobial and antioxidant prospective. J Forest Prod Industr. 2014; 3(6): 248-256.
45. Powar PV, Patil KS. Screening of endophytic fungi isolated from Celastrus paniculatus for antimicrobial potential. World J Pharmacy Pharm Sci. 2015; 4(7): 717-722.
European Journal of Biological Research 2016; 6 (3): 135-144
47. Shaaban M, Nasr H, Hassan AZ, Asker MS. Bioactive secondary metabolites from endophytic Aspergillus fumigatus: structural elucidation and bioactivity studies. Rev Latinoam Quím. 2013; 41(1): 50-60.
48. Mukhopadhyay T, Roy K, Coutinho L, Rupp RH, Ganguli BN. Fumifungin, a new antifungal antibiotic from Aspergillus fumigatus Fresenius 1863. J Antibiot. 1987; 40: 1050-1052.
49. Ando O, Satake H, Nakajima M, Sato A, Nakamura T, Kinoshita T. Synerazol, a new antifungal antibiotic. J Antibiot. 1991; 44: 382-389.
50. Han SK, Choi ANS, Hong RK, Moon SK, Chun HS, Lee SJ. Design and synthesis of highly potent fumagillin analogues from homology modeling for a human metAP-2. Bioorg Med Chem Lett. 2000; 10: 39-43.
51. Xu L, Meng W, Cao C, Wang J, Shan W, Wang Q. Antibacterial and antifungal compounds from marine fungi. Marine Drugs. 2015; 13: 3479-3513.
52. Lee YM, Li H, Hong J, Cho HY, Bae KS, Kim MA, et al. Bioactive Metabolites from the Sponge-Derived Fungus Aspergillus versicolor. Arch Pharm Res. 2010; 33(2): 231-235.
53. Zhang Y, Li XM, Wang BG. Anthraquinone derivatives produced by marine-derived fungus Aspergillus versicolor EN-7. Biosci Biotechnol Biochem. 2012; 76: 1774-1776.
54. Song FH, Ren B, Chen CX, Yu K, Liu XR, Zhang YH, et al. Three new sterigmatocystin analogues from marine-derived fungus Aspergillus versicolor MF359. Appl Microbiol Biotechnol. 2014; 98: 3753-3758.
55. Barbour EK, Sharif MA, Sagherian VK, Habre AN, Talhouk RS, Talhouk SN. 2004. Screening of selected indigenous plants of Lebanon for antimicrobial activity. J Ethnopharmacol. 2004; 93: 1-7.
56. Sidalingeshwara KG, Lingapa K. Key fermentation factors for the synthesis of L-asparaginase - an anti tumour agent through ssf methodology. Int J Pharm Sci. 2010; 1(1): 103-112.
57. Siddalingeshwara KG, Lingappa K. Production and characterization of L-asparaginase - a tumour inhibitor. Int J PharmTech Res. 2011; 3(1): 314-319. 58. Audipudi AV, Supriya GNR, Pallavi R, Mani PG.
Characterization of L-asparaginase producing endophytic fungi isolated from ripened fruit of Capsicum frutescence. Int J Pharm Develop Technol. 2014; 4(1): 52-57.
59. El-Hefnawy MAA, Attia M, El-Hofy ME, Ali MAS. Optimization Production of L asparaginase by locally isolated filamentous fungi from Egypt. Curr Sci Int. 2015: 4(3): 330-341.
60. Patro KKR, Satpathy S, Gupta N. Evaluation of some fungi for L-asparaginase production. Indian J Fund Appl Life Sci. 2011; 1(4): 219-221.
61. Alhussaini MS. Mycobiota of wheat flour and detection of α- amylase and L-asparaginase enzymes. Life Sci J. 2013; 10(1): 360-371.
62. Tippani R, Sivadevuni G. Nutritional factors effecting the production of L asparaginase by the Fusarium sp. Afr J Biotechnol. 2012; 11(15): 3692-3696.
63. Vijay B, Raju KJ. Production of L-asparaginase by Aspergillus terreus MTCC 1782 under solid state fermentation using pearl millet and finger millet as mixed substrate. J Chem Biol Phys Sci. 2014; 5(1): 366-377.
64. Aparna C, Raju KJ. Optimization of process parameters for L-asparaginase production by Aspergillus terreus MTCC1782 under solid state fermentation using mixed substrate. Int J Res Eng Technol. 2015; 4(5): 354-360.
65. Zia MA, Bashir R, Ahmed I, Iftikhar T. Production of L-asparaginase from Aspergillus niger using agro wastes by-products in submerged fermentation process. J Teknologi. 2013; 62(2): 47-51.
of Biological Research
European Journal of Biological Research 2016; 6 (3): 145-151
Seedling establishment in
Scaligeria stewartiana
(Nasir)
Nasir (Apiaceae) from North-West Himalayas, India
Kulvinder Kour
1, B. L. Bhellum
1*, I. A. Hamal
21
Department of Botany, Govt. College for Woman Parade, Jammu - 180001, Jammu & Kashmir State, India
2
BGSB University, Rajouri, Jammu & Kashmir State, India
*Corresponding author: B. L. Bhellum; E-mail: blbhellum@gmail.com
ABSTRACT
The present paper deals with the morphological diversity of seedling establishment in different populations of Scaligeria stewartiana (Nasir) Nasir, inhabiting in North-West Himalayas. The seedling at different stages in the population of the species revealed marked variability. Seed treatments for germination resulted in understanding of seed dormancy and time required for the seedling establishment. The species develops variability in the genetic level that is transmitted and reflected in the form of morphology in order to adapt itself in the extremes of biotic and abiotic variables. Photographs of the seedling and data compiled in tables catch the specific stages of their seedling development after germination as well variability of the chromosomes. The initiation of tuber formation under the ground level in the soil and allocation of resources perform indispensable role in the life span as well as in paving the way for survival, conservation and distribution of the species.
Keywords: Seed germination; Seedlings; Seed set; Tuber; Scaligeria stewartiana; India.
1. INTRODUCTION
Scaligeria stewartiana (Nasir) Nasir (Tribe
pyramidoptereae, Apiaceae) has undergone nume-rous nomenclatural changes both at genus and species level. Currently, the number of species has been reduced to three. The adaptation of living organisms to the ecological amplitude of any habitat depends upon their genetic composition, which in turn is controlled by various components of their genetic system and finally translated and expressed in the form of morphology. Members of the family Apiaceae have exploited several strategies in order to achieve tremendous diversity in their habitat and geographical distribution in the Himalayas that support rich umbellifer diversity [1]. Scaligeria
stewartiana (Nasir) Nasir was recorded from
Shiwaliks in Jammu region as a new record for India [2]. The species inhabits isolated populations and turned out to be a diploid, having 2n=20 besides carrying 1-4 extra chromosomes [3]. It is locally known as Jangli Ajwain, the fruit of the species is used as carminative and spice. Plant has thrived high for its conservation by naturally allocating most of its resources towards below ground part, tuber for its survival. Another threat towards its diminishing population is its long period of seedling establishment which takes about four years to estab-lish and a seed dormancy period of full one year. Received: 25 April 2016; Revised submission: 10 June 2016; Accepted: 21 June 2016
Copyright: © The Author(s) 2016. European Journal of Biological Research © T.M.Karpiński 2016. This is an open access article licensed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits
European Journal of Biological Research 2016; 6 (3): 145-151 The period of production of seeds year after
year is also restricted exactly to four years in which the environmental disturbances, effect of local acti-vities has imposed a lot on its survival. The seeds as well as the leaves are used for various types of gastrointestinal disorders by the locals. The seeds are also used for treatment of digestive disorders of live-stock.
2. MATERIALS AND METHODS
Scaligeria stewartiana (Nasir) Nasir of the
family Apiaceae is growing in wild situations of different parts of Jammu Province expanding to the different locations at a distance of over 100 Kms, ranging in altitude from 600 to 1200 mts. The sources of the tubers are confined to different localities namely Mansar, Tikri and Reasi. The tubers were transplanted in the botanical garden. Seeds were collected from the different populations and were treated for their germination. Seed germination has been studied under laboratory as well as in the experimental plots of the University Botanic Garden. The seeds collected during May 1995, were treated with 0.1% mercuric chloride for two minutes. Thereafter, the seeds were given a wash in 70 percent alcohol. All traces of mercuric
chloride and alcohol were removed by washing the seeds thoroughly with distilled water. For
germination in the laboratory, the seeds were divided into replicates of 50 seeds each and then subjected to following physical and chemical treatments.
a) Chilling at ± 50C for 15, 20, 30 and 50 days using refrigerator
b) Treatment with conc. sulphuric acid for two minutes.
c) Hard water treatment for 2 minute. d) Seed coat puncturing
e) Seed coat scarification.
In all the above treatments including control, no seed germination was recorded under laboratory conditions. However, from the seeds collected during May, 1995, 53 seeds germinated in the experimental plots of the Botanic Garden during January 1997, after sowing during October 1995. There was no germination of the seeds from October 1995 to December 1996. The germinated seeds formed well-established seedlings and some of these
seedlings developed into mature plants and flowered in the fourth year. The critical period starts even before germination with predation of seeds, followed by the germination phase that usually allows only less than half of the viable seed to viable seed to emerge, predation on germination and seedlings.[4]
Seed germinator was also used in order to germinate seeds. However seeds did not respond to the different treatments of variable temperature provided to facilitate germination. Observation of Botanical Garden population where tubers were transplanted three years earlier, seedlings were observed growing. The measurements of seedlings were made and data was collected. All photos were done by using Olympus PM6 35 Camera.
3. RESULTS
3.1. Observations
The seeds from natural population growing at Mansar were collected and treated for germination and showed dormancy of one year (Fig. 1). The germination of seed after dormancy resulted in the formation of radical, which is first to emerge and grow geotropically. After the emergence of the radical two cotyledons are formed which on reaching above ground start turning green. Both the cotyledons were unequal in size in majority of the seedlings and remain green for 20 to 30 days. Seedlings attained a length of 5.0-5.4 cm and the primary root also grow in size which varies from 3.0-3.4 cm. The elongation of primary root resulted in initiation of a tuber in the form of a small swelling at the sub apical position. However, there is no shoot formation. At the end of the growing season the cotyledons wither and the seedlings perennate through the tuber. The epidermis of the tuber starts turning light brown. As the tuber sprouts during next growing period, a basal leaf emerges from tuber above ground (Fig. 1 and Fig. 2 A-B).
European Journal of Biological Research 2016; 6 (3): 145-151 below the ground level. The tubers attain almost the
normal size and perennate for the next year (Fig. 2 C). During third year growing season, the tuber sprouted again which resulted in the formation of 2-3 basal leaves as shown in (Fig. 2 D). After further growth the basal leaves emerged above the soil and turned green. The mature leaves formed during third year are larger in size more dissected and remained above ground for 35-45 days. All the resources gathered during growing season were allocated towards below ground tuber [5].
Figure 1. Population of Scaligeria stewartiana (Nasir) Nasir in Mansar.
Figure 2. Photographs of seedling of Scaligeria stewartiana (Nasir) Nasir showing different stages A-C: Seedlings at first year after seed germination; D - Second year seedling showing one basal leaf arising from the tuber; E - Third year seedling showing more than one leaf; F - Fourth year tuber showing numerous basal leaves above ground.
Figure 3. Scaligeria stewartiana (Nasir) Nasir: a matured tuber exposed to show under the ground level.
The tuber that was rounded in the first and second year of growing season attains irregular shape. The leaves after the completion of the growing season wither and tubers enter into dormancy. The tuber which is irregular in shape attaining brownish to black colour in its outer covering again perennates for fourth year and sprouted in the forthcoming season of growth. This fourth year tuber produced 5-9 basal leaves (Fig. 2 E-F and Fig. 3) and their size varied from 10-12 cm. The dissections of the leaves increased and the size of the ultimate leaf segments were observed to be variable. During fourth year of growing season, the tuber turned darker in color and attained diameter up to 3.5 cm. The size of the fourth year tuber was comparable to the size of the tuber transplanted from natural populations. During the same year (4th years) these tubers initiated shoot development, which subsequently developed flowers in the orders starting from primary to higher orders.
3.2. Seed morphology
European Journal of Biological Research 2016; 6 (3): 145-151 mericarps. Each mericarp alongwith the seed forms
a single dispersal unit. The fruit is subdidymous, broad at base and narrow towards apex where it is capped by a stlypodium from which two recurved styles emerge. Each mericarp carries five primary ridges, one dorsal, two commissural and two intermediate. The ridges are corky, obtuse and run parallel to the longitudinal axis of the mericarp. In between the primary ridges, are present vallecular spaces.
Seed weight: The seeds are small in size; each mericarp measures 1.5-2.3 (x̄ 2.1) mm in length and 0.8-1.1 (x̄ 0.09) in breadth, ( commissural face). The seed weight of 100 mericarps varies between 70-95 (x̄ 90) mg.
3.3. Seed set
Scaligeria stewartiana (Nasir) Nasir exhibits
variation in seed set at intra- as well as at inter population level. The umbels of different order portray variation in their contribution to the total fruit yield of an individual plant. In Mansar population, the tertiary umbels contribute maximum
to the total fruit yield of the individual plant. On the contrary secondary umbels are the major
contributors to the fruit yield per plant in Tikri and Reasi populations. However, irrespective of the contribution of different umbel orders to the total fruit yield, the fruit set decreases from primary to the higher order umbels in all the three population.
The average fruit set in primary umbel of all the three populations was above 90 percent. It was x̄ 90.62 ± 0.55 (77.7-100) percent in Mansar popu-lation, x̄ 93.95 ± 0.48 (75.0-100) percent in Tikri population and x̄ 93.38 ± 0.26 (80.0-100) percent population. Percentage fruit set in secondary order umbels varied between 88-90 percent, being x̄ 88.9 ± 0.35 (72.0-100) in Mansar, x̄ 88.48 ± 0.32 (81.8-95.0) in Tikri, x̄ 90.94 ± 0.23(79.0-100) percent in Reasi population.
The fruit set in third order umbels ranged between 70-83.38 percent. The Mansar population had 70.31 ± 1.49 (49.6-100) percent whereas in Tikri and Reasi populations, fruit set was x̄ 83.38 ± 0.71 (70-100) and x̄ 81.24 ± 0.36 (36.2-90) percent respectively. There was wide variation in the fruit set of fourth order umbels. It was x̄ 65.72 ± 1.36 (0-90.6) percent in Mansar, x̄ 78.1 ± 0.49 (0-88.8)
percent in Tikri and x̄ 60.2 ± 0.51 (0-65.0) in Reasi population. The fruit set was also recorded separa-tely for normal disomics and those bearing 1-4 extra chromosomes, beside the norma complement (Table 1).
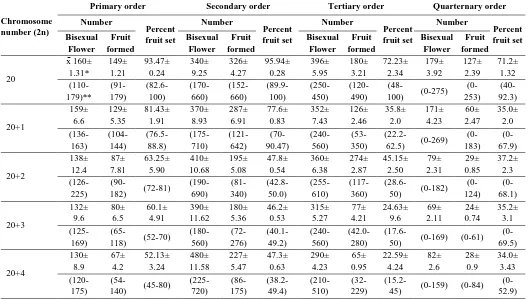
The data given in Table 18 reveals that in comparison to normal disomics, the percentage fruit set reduces, with increase in the number of extra chromosomes, in all the umbel orders. In primary umbel it reduced from 93.47 in disomics to 52.13 percent in plants with 2n=20 ± 4 extra chromo-somes. Similar trend was observed in the fruit set of secondary, tertiary and quarternary umbel orders.
3.4. Seedling establishment
As the seed germinate, the radical is the first to emerge and grow geotropically. Subsequently, the two cotyledons emerge and on reaching above ground (Fig. 2 A-B) start turning green. The cotyle-dons are unequal sized in majority of the seedlings and remain green for 20-30 days. During this period the seedlings attain a length of 5.0-5.4 cm. The primary root also grows in size, which varies from 3.0-3.4 cm. As the elongation of primary root is taking place; the development of a tuber is initiated in the form of a small swelling at its sub-apical position (Fig. 2 C). However, there is no shoot formation. Towards the end of the growing season, the cotyledons wither and the seedlings perennate through the tuber. The epidermis of the tuber turns light brown. As the tuber sprouts during next growing period, a basal leaf emerges from the tuber and reaches above ground (Fig. 2 D).
Although all seedlings at the stage carried a single basal leaf but the dissection of the leaf was variable. The basal leaf of these second seedlings remained green for 30-40 days. During this period, photosynthesis in leaves provided nutrition to developing tubers, which attained a size of 8 to 9.5 mm. The leaf withered towards end of the growing period and the tuber perennated in the soil, upto 15 cm deep from the ground level.
European Journal of Biological Research 2016; 6 (3): 145-151
Table 1. Fruit set at umbel in Scaligeria stewartiana (Nasir) Nasir.
Chromosome number (2n)
Primary order Secondary order Tertiary order Quarternary order Number Percent fruit set Number Percent fruit set Number Percent fruit set Number Percent fruit set Bisexual Flower Fruit formed Bisexual Flower Fruit formed Bisexual Flower Fruit formed Bisexual Flower Fruit formed 20
x̄ 160± 1.31* 149± 1.21 93.47± 0.24 340± 9.25 326± 4.27 95.94± 0.28 396± 5.95 180± 3.21 72.23± 2.34 179± 3.92 127± 2.39 71.2± 1.32 (110-179)** (91-179) (82.6-100) (170-660) (152-660) (89.9-100) (250-450) (120-490)
(48-100) (0-275)
(0-253) (40-92.3) 20+1 159± 6.6 129± 5.35 81.43± 1.91 370± 8.93 287± 6.91 77.6± 0.83 352± 7.43 126± 2.46 35.8± 2.0 171± 4.23 60± 2.47 35.0± 2.0 (136-163) (104-144) (76.5-88.8) (175-710) (121-642) (70-90.47) (240-560) (53-350)
(22.2-62.5) (0-269) (0-183) (0-67.9) 20+2 138± 12.4 87± 7.81 63.25± 5.90 410± 10.68 195± 5.08 47.8± 0.54 360± 6.38 274± 2.87 45.15± 2.50 79± 2.31 29± 0.85 37.2± 2.3 (126-225)
(90-182) (72-81)
(190-690) (81-340) (42.8-50.0) (255-610) (117-360)
(28.6-50) (0-182)
(0-124) (0-68.1) 20+3 132± 9.6 80± 6.5 60.1± 4.91 390± 11.62 180± 5.36 46.2± 0.53 315± 5.27 77± 4.21 24.63± 9.6 69± 2.11 24± 0.74 35.2± 3.1 (125-169)
(65-118) (52-70)
(180-560) (72-276) (40.1-49.2) (240-560) (42.0-280)
(17.6-50) (0-169) (0-61)
(0-69.5) 20+4 130± 8.9 67± 4.2 52.13± 3.24 480± 11.58 227± 5.47 47.3± 0.63 290± 4.23 65± 0.95 22.59± 4.24 82± 2.6 28± 0.9 34.0± 3.43 (120-175)
(54-140) (45-80)
(225-720) (86-175) (38.2-49.4) (210-510) (32-229)
(15.2-45) (0-159) (0-84)
(0-52.9)
Table 3. Dry biomass of Scaligeria stewartiana (Nasir) Nasir above and below ground parts of the seedings at different stages of development.
Seedings
stage (N)
Total dry biomass (mg)
a
Dry biomass of below ground parts (mg)
b
Dry biomass of above ground parts (mg)
c
Ratio b:c
Ist year 20 x̄ 0.2330±0.0005* (0.2021-0.2497)**
0.1156 ± 0.0003 (0.1039-0.1251)
0.1174±0.0005
(0.1094-0.1279) 1.01 IInd year 20 0.2286±0.0006
(0.1935-0.2481)
0.1137±0.0005 (0.1120-0.1296)
0.1149±0.0007
(0.1012-0.1256) 1.01 IIIrd year 20 0.1353±0.0003
(0.1151-0.1532)
0.1190±0.0005 (0.1036-0.1295)
0.1163±0.0006
(0.1020-0.1347) 0.97 IV year 10 0.2570±0.0005
(0.2193-0.2695)
0.1230±0.0006 (0.936-0.1435)
0.1110±0.0005
(0.996-0.1239) 0.90 * Mean, S.E ** Range
These remained green and above ground for 35-40 days. During this period, the tuber also grew in size upto 1.5 cm in diameter. During this period, tuber also developed adventitious roots. The tuber, which was almost globular during first and second year, assumed irregular shape in the third year. Towards the end of the growing season, the leaves withered and tubers again entered into dormancy.
After perennation of the third year, the tubers sprouted again during the growing season of fourth
European Journal of Biological Research 2016; 6 (3): 145-151 same year (4th year) these tuber initiated shoot
development, which subsequently produced flowers in the same order as was observed in case of tubers transplanted from natural populations.
3.5. Seed viability
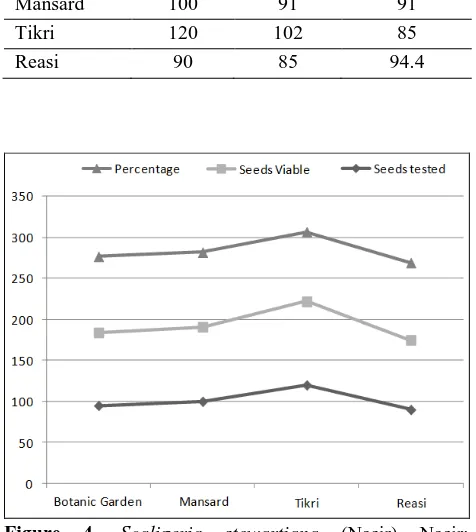
The viability of the seeds was determined by tetrazolium test (Tz-test). The seeds of various populations exhibited variation in their percentage viability. In all the populations, seed viability was above 85 percent (Table 4).
The strategy of resource allocation in the species has been assessed after analyzing dry biomass at various stages of seedling establishment and seed set. The different stages of seedling exhibit distinct morphology. In the subsequent years, seedlings produce shoots and umbels of different orders. The dry biomass of the seedlings at different stages of development is represented in the Table 3.
Table 4. Seed viability in Scaligeria stewartiana (Nasir) Nasir.
Population Seeds tested
Seeds
viable Percentage Botanic
Garden 95 89 93 Mansard 100 91 91 Tikri 120 102 85 Reasi 90 85 94.4
Figure 4. Scaligeria stewartiana (Nasir) Nasir: representing comparison of seed viability at different locations in Jammu and Kashmir.
4. DISCUSSION
The life history pattern of a species is the result of evolutionary decision in order to achieve successful establishment, adaptation and future evolutionary diversification [6]. The perpetuation of a species depends at the rate at which individuals of population contribute to future generations, which in turn is influenced by the number of reproductive attempts during its life time, age of first reproduc-tion and reproductive rate. The herbaceous perennial
Scaligeria stewartiana (Nasir) Nasir produces
leaves and flowers every year from the underground tuberous root which however it is not an organ of vegetative multiplication [7]. The observations on seed biology of the species have revealed that the seed undergoes germination after attaining dor-mancy for the period of one year. After germination of seeds the seedlings get established in four years, after that every individual plant flowers for 4 to 5 years before perishing. The reproductive output also increases with age, as the tuber increase in size. As compared to only two umbel orders during first year of reproductive age, the plant in the subsequent years produce umbels upto fourth order [8]. During first two years of seedling establishment, the species invests more resources (dry biomass) in the forma-tion of aerial parts and in third and fourth year, the investment is more in the production of tuber. Long gestation period in seedling establishment enables the species to reduce competition and maintain genetic composition of the populations. The weather conditions are main cause of mortality. However, permanent snow cover can protect seedling from frost and heaving injuries [9-11].
5. CONCLUSION
European Journal of Biological Research 2016; 6 (3): 145-151 established individual and fresh recruits. However,
the species needs special attention due to a number of threats.
ACKOWLEDGEMENTS
Authors are grateful to Prof. A. K. Koul, Dean Academics, BGSB University, Rajouri for helpful suggestions and thankful to Principal Govt. College for Women, Parade Jammu for laboratory facilities.
AUTHORS’ CONTRIBUTION
KK: Data collection, Interpretation, Writing, Dis-cussion; BLB: Manuscript designing, DisDis-cussion; IAH: Guidance, Interpretation. The final manuscript has been read and approved by all authors.
TRANSPARENCY DECLARATION The authors declare no conflicts of interest.
REFERENCES
1. Sarukhan J. Demography problems in tropical system. Bot Monogr. 1980; 15: 161-188.
2. Hamal, I. A. Cytotaxonomy of the umbellifers of Kashmir Himalayas. Ph. D. Thesis. Jammu University, 1981.
3. Kour K, Hamal IA, Gupta SK. Chromosomal variability in Scaligeria stewartiana (Nasir) E. Nasir. J Cytol Genet. 1992; 27: 43-45.
4. Willson MF. Plant reproductive ecology. John Wiley and Sons, Inc., New York, 1983.
5. Koul V. Resource allocation in relation to floral structure and breeding system in some member of commelinaceace. Ph.D. Thesis, University of Jammu, 1998.
6. Putz N, Sakkau I. Seedling establishment bud movement and subterranean diversity of subterra-nean system in Apiaceae. Flora. 2002; 197: 385-393.
7. Constance L, Chuang T, Bell CR. Chromosome numbers in Umbelliferae. Am J Bot. 1971; 58(6): 577-587.
8. Mukerjee PK, Constance L. Umbliferae of India. Oxford and IBH. Publishing, New Delhi, 1993. 9. Bergsten U, Nilsson JE. Early seedling growth of
Pinus sylvestris (L.) after sowing with a mixure of stand and orchard seed in dence spacings. Can J Forest Res. 2001; 31(7): 1184-1194.
10. Bergsten U, Nilsson JE. Effects of seed weight and seed type on early seedling growth of Pinus sylvestris under harsh and optimal conditions. Scand J Forest Res. 2002; 17: 118-130.
of Biological Research
European Journal of Biological Research 2016; 6 (3): 152-169
Biosynthesis of anti-inflammatory immunosuppressive
metabolite by Streptomyces variabilis ASU319
Mohamed H. Abd-Alla
1*, Abdel-Hamied M. Rasmey
2, El-Sayed A. El-Sayed
3,
Ismail A. El-Kady
1, Ibrahim M. Yassin
11
Botany and Microbiology Department, Faculty of Science, Assiut University, Egypt 2
Botany and Microbiology Department, Faculty of Science, Suez University, Egypt 3
Botany Department, Faculty of Science, Zagazig University, Egypt
*Corresponding author: Mohamed H. Abd-Alla; E-mail: mhabdalla2002@yahoo.com; mhabdalla@aun.edu.eg
ABSTRACT
Most immunosuppressive agents were initially developed as antibiotics produced by the genus
Streptomyces. This investigation was devoted to
explore the bioactive metabolite of the Streptomyces
variabilis ASU319 extract and testing the purified
active compound of this extract as an immuno-suppressive agent in rats blood. Elucidation of the chemical structure and optimization of the active compound were studied as well. Antimicrobial activity was conducted using agar-well diffusion and disc diffusion assays. The antimicrobial metabolite was extracted from the fermentation broth by ethyl acetate and purified by TLC and silica gel column chromatography. The pure active compound was then subjected to spectroscopic analyses: 1H NMR, Elemental analysis, IR and Mass spectra. The active antimicrobial compound was tested as an immunosuppressive agent by injection in the rat blood and the complete blood count (CBC) was determined. The crude extracts of the selected active antagonistic five isolates were tested to prevent the inflammation and proliferation of lymphocytes of the rats blood. The active antimicrobial compound of Streptomyces ASU319
was purified and proven as an immunosuppressive agent. The tested compound decreased each of the neutrophils, lymphocytes and monocytes than the positive control. The compound was of molecular weight 458 g/mol and had given the proposed chemical formula C24H46O8. The most potent
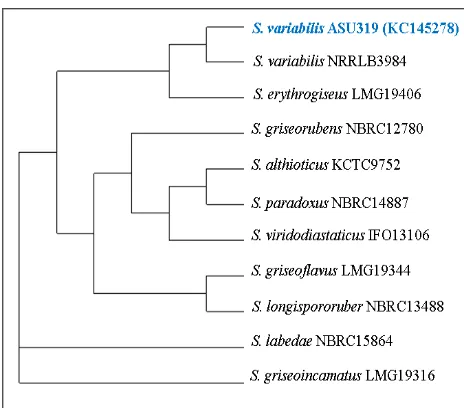
bacterial isolate was identified by 16SrRNA sequencing as Streptomyces variabilis ASU319 with accession number [GenBank: KC145278]. These results revealed that Streptomyces variabilis ASU319 is potential microbial for production of active antimicrobial compound that has the ability to decrease the proliferation of the lymphocytes cells in the blood and may be a good immunosuppressive agent.
Keywords: Streptomyces variabilis; Antimicrobial; Anti-inflammatory; Immunosuppressive; 16S rRNA; Optimization.
1. INTRODUCTION
Nature is an attractive source of new thera-peutic candidate compounds since a tremendous chemical diversity is found in millions of species of plants, animals, marine organisms and microorga-Received: 04 May 2016; Revised submission: 01 July 2016; Accepted: 11 July 2016
Copyright: © The Author(s) 2016. European Journal of Biological Research © T.M.Karpiński 2016. This is an open access article licensed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits