Salters-Nuffield Advanced Biology Resources
Activity 6.1 Student SheetDNA PHOTOCOPYING: THE POLYMERASE CHAIN
REACTION
Purpose
To understand how the polymerase chain reaction amplifies DNA.
Replicating DNA millions of times
The polymerase chain reaction (PCR) is used to produce millions of copies of the short tandem repeat (STR) fragments used in producing a DNA profile. PCR is also used to amplify DNA for use in many areas of genetic analysis and research, such as genetic testing, genetic modification, DNA sequencing, gene expression and diagnosis of infectious disease. Work through the interactive tutorial on PCR that accompanies this activity and use section 6.1 in Student Book 2 to help you complete the following exercise.
Q1 Draw a flowchart with simple diagrams to show the first two cycles of PCR, starting with one double-stranded DNA molecule. Colours will help you identify the different strands of DNA.
Q2 The enzyme polymerase is only added during the first PCR cycle, but continues to catalyse DNA replication through many cycles. Considering the treatment of the DNA during PCR, what property does polymerase show that is unusual for an enzyme?
Q3 What feature of a DNA molecule ensures accurate replication of the strands during each PCR cycle?
Q4 Explain how PCR has revolutionised criminal investigations.
Q5 The graph in Figure 1 shows the potential number of copies of DNA produced during PCR. Use your understanding of the process to explain how so many copies can be produced in relatively few cycles.
Figure 1 PCR: expected amplification graph.
Salters-Nuffield Advanced Biology Resources
Activity 6.1 Student SheetPractical PCR
SAFETYA risk assessment should be undertaken when planning any investigation, and both the plan and the risk assessment should be checked by your teacher/lecturer.
Scientists in forensics laboratories carry out PCR using a machine called an automated thermal cycler. This is a programmable heating unit in which the DNA to be amplified is incubated in a buffer solution with thermo-stable DNA polymerase, primers and deoxyribonucleotides. The unit maintains the cyclical sequence of temperatures for the PCR process.
Salters-Nuffield Advanced Biology Resources
Activity 6.1 Teacher SheetDNA PHOTOCOPYING: THE POLYMERASE CHAIN
REACTION
Purpose
To understand how the polymerase chain reaction amplifies DNA.
This activity is in two parts. The first part uses an interactive tutorial to show what is happening during the polymerase chain reaction. The second part refers to experimental work using PCR. The use of PCR could be combined with Activity 6.2.
Modelling clay models of the process could be made to help confirm understanding.
Replicating DNA millions of times
Students should read section 6.1 of Student Book 2 and work through the interactive tutorial on PCR to help them complete this exercise.
Answers
Salters-Nuffield Advanced Biology Resources
Activity 6.1 Teacher SheetQ2 The enzyme must be thermo-stable. Most enzymes would be denatured at the temperatures required for DNA denaturation.
Q3 Complementary base pairing between the two strands.
Q4 PCR allows minute samples of DNA, for example, from a speck of blood, to be amplified. DNA profiles can then be prepared from the amplified sample.
Q5 The number of DNA molecules is doubled during each cycle, producing a rapid (exponential) increase in the number of copies. Note that the y axis is a log10 scale.
Q6 Both methods use enzymes to produce STR fragments of DNA. PCR uses DNA polymerase rather than restriction enzymes/endonucleases. The restriction enzymes cut DNA directly at specific sequences to produce fragments whereas DNA polymerase allows replication of the DNA.
Practical
SAFETYReview the students’ risk assessments and discuss any safety considerations.
It is possible to carry out practical PCR using equipment available from a number of suppliers including NCBE (National Centre for Biotechnology Education), Bio-Rad and Discovering DNA. Although all these organisations supply automated PCR thermal cyclers, they are not absolutely necessary. Instead, PCR can be undertaken using three separate thermostatically controlled water baths, although care must be taken with the hottest as a risk of scalding exists. Note that during Science Year (September 2001–July 2002) many schools, colleges and initial teacher training institutions received free PCR equipment. This may have been ‘mislaid’ or tidied away, so check the prep. room before investing in any new kit.
Student and teacher/technician protocols for the PCR practicals can be downloaded as pdf files from the suppliers’ websites. These protocols often contain many coloured images, so they may take a long time to download and print out. Save them onto the hard disk first, rather than viewing through a browser.
Equipment can be purchased either in class sets or individually, with consumables also available in class-sized batches. All three organisations listed offer training courses, frequently in association with other institutions like botanic gardens or science centres, giving teachers and technicians the
Salters-Nuffield Advanced Biology Resources
Activity 6.2 Student SheetRESTRICTION ENZYMES AND GEL
ELECTROPHORESIS
Purpose
To describe how restriction enzymes can be used to create fragments of DNA.
To describe how gel electrophoresis can be used to separate DNA fragments of different lengths.
Additional resources
As you work through this sheet you will need to use some additional interactive resources. A
Microsoft Word document containing the DNA sequence of the lambda virus is needed for part 2 and an interactive tutorial is used with part 4. Do not be tempted to do the interactive tutorial first.
Procedure
1 Restriction enzymes
Genetic manipulation often involves cutting a section out of a length of DNA. This section may be a desired gene to be inserted into the DNA of another organism, via a vector, such as a plasmid or a virus. Cutting up DNA into fragments is also necessary for genetic screening and DNA profiling. Restriction enzymes are used to cut DNA at precise points. Like all enzymes, restriction enzymes are specific. They cut at a recognition site, which consists of a particular sequence of bases. Restriction enzymes are also called endonucleases because they cut the DNA at sites within a strand of DNA. In contrast, exonucleases cut DNA at the end of a strand.
The enzyme AluI recognises AGCT and cuts between G and C to produce blunt ends.
1 Draw an arrow down through where AluI would cut in the following DNA base sequence:
The enzyme KpnI recognises GGTACC and cuts between C and C to produce sticky ends with a four base overhang:
2 On the example DNA strands on page 2, show the cuts that each enzyme below would make in the DNA. For each of these enzymes, the overhang is four bases long.
Enzyme recognition site cutting site
EcoRI GAATTC between G and A
HindIII AAGCTT between A and A
Salters-Nuffield Advanced Biology Resources
Activity 6.2 Student Sheet2 Restriction enzyme simulation using lambda DNA base sequence
This activity simulates the cutting of DNA into fragments by restriction enzymes.
1 Open the lambda Microsoft Word document that accompanies this activity. This is the sequence of bases from a lambda virus. If you carry out practical work on DNA, such as running
electrophoresis gels, you may actually use lambda DNA.
2 Click on the Replace option from the Home menu.
3 Type the recognition sequence (in lower case letters) into Find? For example, for EcoRI in Find?
enter gaattc.
4 In the Replace box type the recognition sequence in capitals, with a space between the first two letters. For example, for EcoRI enter Gsinglespacehere AATTC, i.e. G AATTC.
This will introduce a carriage return where the ‘cut’ has been made, so that you can clearly see the fragments produced by cutting through every recognition sequence.
5 Click on Replace All.
Q1 How many recognition sequences for EcoRI were there in lambda DNA?
Q2 How many fragments will these cuts produce?
6 Close the Replace window.
7 Start at the top of the text and select the text by clicking the left mouse button and dragging down until you reach the first carriage return. This will have highlighted the first ‘fragment’. (Be careful that you do not highlight the space after the capital G.)
8 From the Review menu select WordCount. Record the number of characters in the highlighted text. Click on Close.
9 Highlight each fragment in turn and count the number of bases present in each. The final number should add up to 48 502. These figures represent the sizes of the fragments produced by cutting up the DNA with the restriction enzyme.
10 Perform this restriction enzyme simulation using the enzyme HindIII. For the ambitious, perform a double-digest. You ‘cut’ using one enzyme, EcoRI, and then continue with a second ‘replace’, giving the recognition code of the second enzyme, HindIII.
Q3 Record the number of cuts and fragments produced in each case.
Salters-Nuffield Advanced Biology Resources
Activity 6.2 Student Sheet3 Electrophoresis
Gel electrophoresis is a process used to separate molecules or fragments of molecules of different sizes. When DNA is cut with certain restriction enzymes, the lengths of the fragments produced will be different for each individual; see Student Book 2, page 72. The patterns these fragments make on the gel are the basis of genetic profiling. Separation using electrophoresis can also be used to isolate a particular gene from other fragments of DNA. This is how the genetic testing and genetic screening described in Topic 2 Student Book 1 are carried out.
1 Using the data from your restriction enzyme simulation above with EcoRI on lambda DNA, position a horizontal bar for each size fragment onto a vertical axis as shown in Figure 1.
Figure 1 Use your own scale on the vertical axis for lambda DNA cut with EcoRI.
4 Simulation of gel electrophoresis
Complete experiment 1 in the interactive tutorial that accompanies this activity and answer the questions below
Q1 The restriction enzymes in the coloured tubes are suspended in a buffer solution. Why is this important?
Q2 Why are the reaction tubes containing enzyme and DNA incubated at 37 °C?
Q3 Do the DNA fragments move towards the anode (positive electrode) or the cathode (negative electrode)?
Q4 What is the charge on a DNA fragment?
Q5 At which end of the gel are the smallest DNA fragments? Explain your answer.
Q6 How does the unrestricted DNA in track one differ from the restricted DNA in track two?
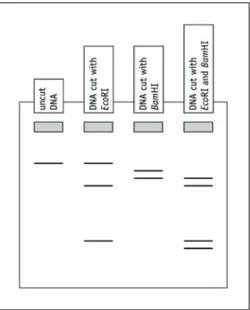
Q7 On Figure 2:
a circle any fragments of the EcoRI single digest that do not have a BamHI recognition site
b circle any fragments in the double digest that have been produced as a result of the DNA being cut by one enzyme and then the other enzyme.
Figure 2 A gel produced from DNA cut with EcoRI and BamHI. Grey rectangles represent wells.
Using size markers
Salters-Nuffield Advanced Biology Resources
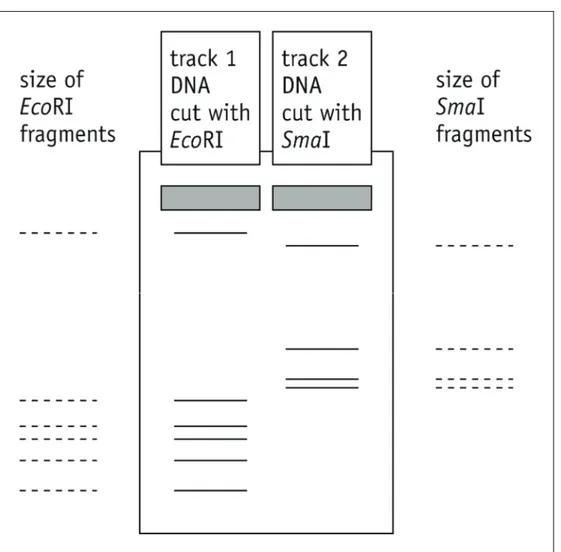
Activity 6.2 Student SheetQ8 Use the data collected in the lambda DNA restriction enzyme simulation to fill in the fragment sizes in track 1 of Figure 3 below. Then predict the sizes of the fragments in track 2, produced when lambda DNA is cut with the restriction enzyme SmaI. You can check if you were right by using the restriction enzyme simulation with this enzyme, which has a recognition sequence of CCCGGG and cuts between the C and G.
Salters-Nuffield Advanced Biology Resources
Activity 6.2 Teacher SheetRESTRICTION ENZYMES AND GEL
ELECTROPHORESIS
Purpose
To describe how restriction enzymes can be used to create fragments of DNA.
To describe how gel electrophoresis can be used to separate DNA fragments of different lengths.
Notes on the procedure
Students should work through the Student Sheet, completing the various tasks as they appear. It is best if they do the sections in the order they are presented so that they have covered the idea of restriction enzymes before completing the interactive tutorial. Part 2 requires students to use a Microsoft Word document containing the DNA sequence of the lambda virus; it accompanies this activity. Part 4 uses the interactive tutorial of gel electrophoresis that accompanies the activity. This will allow students to become familiar with the technique. It could be completed in advance of practical work so that students have a better understanding of what is going on in the practical session.
1 Restriction enzymes
Salters-Nuffield Advanced Biology Resources
Activity 6.2 Teacher Sheet2 Restriction enzyme simulation using lambda DNA base sequence
Q1 Five.Q2 The EcoRI enzyme will make five ‘cuts’ in the lambda DNA document, producing six fragments.
The EcoRI fragment lengths are 21 226, 4878, 5643, 7421, 5804 and 3530 bases.
HindIII makes seven cuts, producing eight fragments with lengths of 23 130, 2027, 2322, 9416, 564, 125, 6557 and 4361 bases.
A double-digest using EcoRI followed by HindIII produces fragments as outlined in the table below.
EcoRI fragment lengths Number of cuts with HindIII Fragment lengths with EcoRI and HindIII
21 226 0 21 226
4878 2 1904 2027
947
5643 1 1375 4268
7421 3 5148 564
125 1584
5804 1 4973 831
3530 0 3530
3 Electrophoresis
Salters-Nuffield Advanced Biology Resources
Activity 6.2 Teacher Sheet4 Simulation of gel electrophoresis
Q1 To keep the enzymes at their optimum pH.Q2 To keep the enzymes at their optimum temperature.
Q3 Anode.
Q4 Negative.
Q5 End furthest from the wells – the smaller fragments can travel more quickly through the gel.
Q6 The unrestricted DNA shows a single fragment that has not moved very far from the wells. The restricted sample contains some uncut DNA, plus two smaller fragments produced by a single cut to the DNA.
Q7 a Neither of the EcoRI fragments has a BamHI recognition site.
b The larger of the two BamHI fragments has been cut by EcoRI. There is only one additional fragment on the double digest so the two fragments are the same size or one is the same size as one of the other fragments.
Salters-Nuffield Advanced Biology Resources
Activity 6.3 Student Sheet Core PracticalPRACTICAL DNA GEL ELECTROPHORESIS
Purpose
To use restriction enzymes to create DNA fragments and gel electrophoresis to separate DNA fragments of different sizes.
SAFETY
Write a risk assessment detailing any safety precautions. Discuss this with your teacher before starting.
Be sure to use only the HT (high tension) power supply provided, limited to <5 mA. This delivers voltages up to 500 V, so take extra care that you do not touch any parts of the apparatus connected to such a high voltage.
Ensure the HT is turned off as soon as the experiment is complete.
Procedure
Salters-Nuffield Advanced Biology Resources
Activity 6.3 Teacher Sheet Core PracticalPRACTICAL DNA GEL ELECTROPHORESIS
Purpose
To use restriction enzymes to create DNA fragments and gel electrophoresis to separate DNA fragments of different sizes.
SAFETY
If gel electrophoresis is to be used, a full risk assessment should be obtained for the procedure and carefully followed by both staff and students.
Review the students’ risk assessments and discuss any safety precautions.
Ensure only the HT (high tension) power supply provided is used and is limited to <5 mA. This delivers voltages up to 500 V, so take extra care that no-one touches any parts of the apparatus connected to such a high voltage.
Ensure the HT is turned off as soon as the experiment is complete.
Restriction enzymes and gel electrophoresis
Note that gel electrophoresis also occurs in the second part of Activity 6.1, DNA photocopying: the polymerase chain reaction. However, digestion with restriction enzymes is only covered in this activity. All three aspects could be combined in this activity.
The use of restriction enzymes to cut DNA and electrophoresis to separate the resulting fragments is possible using equipment available from a number of suppliers including NCBE (National Centre for Biotechnology Education), Discovering DNA and Bio-Rad. Student and teacher protocols for these practicals can be downloaded as PDF files from their websites. See the weblinks accompanying this activity. Some of these practicals are presented within a context, for example, NCBE’s Nature’s Dice
investigates the inheritance of a single gene. Note that as these protocols often contain many coloured images they can be large and take a long time to download and print so it is worth laminating them for use with successive groups. Some printed copies of the protocols usually accompany any kits purchased. All of the companies supply the hardware (gel tanks, combs, micro-pipettes, etc.) and consumables (DNA, restriction enzymes, agarose, etc.). Some also supply power packs or batteries. Equipment can either be purchased in class sets or individually, with consumables also available in class-sized
batches. All three organisations listed below offer training courses, frequently in association with other institutions like botanic gardens or science centres, giving teachers and technicians the opportunity to carry out the practicals themselves.
Contact details and examples of the protocols offered by NCBE, Bio-Rad and Discovering DNA:
Organisation Website and phone number
E-mail Example of suitable protocols
NCBE
National Centre for Biotechnology Education Science and Technology Centre
The University of Reading 2 Earley Gate
Whiteknights Reading RG6 6AU
www.ncbe.reading.ac.uk 01189 873743
NCBE@reading.ac.uk Lambda DNA protocol Nature’s Dice – familial genetic screening simulation
Plant evolution PCR
Discovering DNA Discovering DNA, Ltd PO Box 280
Hertford SG13 9DG
www.discoveringdna.com Follow links to kits and/or equipment
Phone: 01992 410140 Fax: 01992 410106
All enquiries:
info@discoveringdna.co m
Salters-Nuffield Advanced Biology Resources
Activity 6.3 Teacher Sheet Core PracticalOrganisation Website and phone
number E-mail Example of suitable protocols Bio-Rad
Bio-Rad Laboratories, Ltd Bio-Rad House Maxted Road Hemel Hempstead Hertfordshire HP2 7DX www.bio-rad.com Follow the links to Life Science education, then About Biotechnology Explorer.
See outlines of each practical with the relevant kit’s contents and additional items needed using the link to Classroom kits or if you know what you want to buy then go to Equipment and supplies
Freephone: 0800 181134
Salters-Nuffield Advanced Biology Resources
Activity 6.4 Student SheetDNA PROFILING SUMMARY
Purpose
To summarise the role of PCR, use of restriction enzymes, gel electrophoresis and DNA probes in DNA profiling.
Preparing a DNA profile
Match the descriptions of each sequence with the correct stage on the diagram flowchart. You could cut out the flow chart and stick the correct description next to each picture to provide a summary of DNA profiling.
A. Denaturation: the double-stranded DNA fragments are immersed in alkali to convert them to single strands (so that the ‘probes’ can bind to them later).
B. Hybridisation: incubation of the nylon membrane with a single-stranded DNA probe. The probe will only bind to single-stranded DNA that contains the complementary nucleotide sequence of the STRs.
C. Gel electrophoresis: fragments of DNA are separated according to size. The sample of DNA fragments is placed in a well cut into an agarose gel and an electric current is applied. The smaller fragments of DNA pass more quickly through the gel towards the positive electrode (all the DNA
fragments are negatively charged).
D. Extraction of DNA: shake a tissue sample with water-saturated phenol and chloroform at pH 7.8. Protein is precipitated and DNA enters the aqueous layer.
E. Visualisation: the probes bound to DNA bands on the nylon membrane are revealed using a chemical label.
F. DNA fragments created: bacterial restriction endonucleases (restriction enzymes) cut the long DNA molecules into fragments. Alternatively, the fragments are produced using the polymerase chain reaction (PCR). In DNA profiling these fragments contain unique non-coding regions of STRs.
Salters-Nuffield Advanced Biology Resources
Activity 6.4 Teacher SheetDNA PROFILING SUMMARY
Purpose
To summarise the role of PCR, use of restriction enzymes, gel electrophoresis and DNA probes in DNA profiling.
Preparing a DNA profile
This is a straightforward matching exercise that could be used at the outset to outline the process or at the end to consolidate the ideas.
Students could either link the correct description and picture with a line, or cut and stick as suggested in the worksheet.
Answers
1 D
2 F
3 C
4 A
5 G
6 B
Salters-Nuffield Advanced Biology Resources
Activity 6.5 Student SheetCRIME INVESTIGATION
Purpose
To explore the use of forensic techniques in an applied context.
Body discovered
A body has been discovered at a house in Redhill, Surrey. The body was found outside, near the house and the immediate cause of death was thought to be a stab wound to the abdomen. From the state of decomposition of the body it would appear the victim had been dead for at least a week when discovered.
Experts investigate
The crime is investigated by various forensic ‘experts’. You need to assign members of the group into each expert team and then, using the appropriate handouts and online resources provided, the members of each team must prepare a report to be presented at the investigation team meeting.
Microsoft PowerPoint presentation templates are provided to help in the preparation of some of the reports. You can access them from the learning aids in the interactive tutorial that accompanies this activity.
Crime investigation team meeting
At the investigation team meeting, appoint one member of the group to act as the Crime Investigation Officer. He or she must call on the experts to each present a report and then lead a discussion on the possible explanations for the death, based on the evidence presented. At the end of the meeting the team must try to decide what happened and who committed the murder.
The experts and handouts available to each team are outlined in Table 1.
Expert team Handouts
Forensic pathologist (one student) ● Forensic pathologist’s brief ● Autopsy report
● Toxicology report Scene of Crime Officer (SOCO) (one student) ● SOCO brief
● SOCO Microsoft PowerPoint presentation for report
DNA analysis (small group) ● DNA profile brief ● DNA profiling animation
● DNA profiling Microsoft PowerPoint presentation
Entomologist (small group) ● Entomology briefing sheet
● Entomology Microsoft PowerPoint presentation Fingerprinting expert (small group) ● Fingerprint analysis briefing sheet
Crime Investigation Officer (one student) ● Crime Investigation Officer briefing sheet
Table 1 Experts and handouts for each team.
In a larger class you might have two rival investigation teams. Do they both come to the same conclusion?
Salters-Nuffield Advanced Biology Resources
Activity 6.5 Student SheetForensic pathologist briefing document
A body was discovered at a house in Redhill, Surrey, on May 11, 2009. The body was found outside, near the house and the immediate cause of death was thought to be a stab wound to the abdomen. From the state of decomposition of the body it would appear the victim had been dead for at least a week when discovered. As the forensic pathologist on the crime investigation team you need to present evidence for the cause of death of the deceased.
You have the following to use to construct your evidence for cause of death:
autopsy report
blood test report from the toxicology lab.
You will need to summarise the relevant findings and conclusions from your work at the crime investigation team meeting.
✂
Scene of Crime Officer (SOCO) briefing document
Your work is mainly conducted at the scene of the crime.
You will need to summarise the relevant findings and conclusions from your work at the crime investigation team meeting.
You are the SOCO for a murder at a house in Redhill, Surrey. The body was found outside, near the house on May 11, 2009. The immediate cause of death was thought to be a stab wound to the
abdomen. From the state of decomposition of the body it would appear the victim had been dead for at least a week when discovered. As the SOCO for this case you need to present a report at the crime investigation meeting detailing the evidence collected at the crime scene. You can give your report using the Microsoft PowerPoint presentation available.
The presentation contains notes that can be seen by viewing or printing as notes pages.
✂
Crime Investigation Officer briefing sheet
As the investigating officer you must chair the crime investigation meeting. You should ask for reports from the following forensic experts:
Scene of Crime Officer (SOCO)
Forensic pathologist
Entomologists
DNA profiling experts
Fingerprinting experts.
Ask the whole team to make notes on key points during the talks. You then lead a discussion about the possible course of events that led to the murder and make a decision as a team about the course of action to be taken.
Salters-Nuffield Advanced Biology Resources
Activity 6.5 Student SheetAutopsy report
Autopsy on: Mr Patrick Barrett-Hughes
Date of birth: 16/06/75
Address: Greyfriars, Whitepost Hill, Redhill, Surrey
Date of death: Unknown
Date autopsy: 13/05/09
PM done by: Dr U.C. D’Eath
Cause of death: Ruptured spleen and blood loss from abdominal stab wound.
HISTORY
Body was found on May 11, 2009 at home address, outside, near a garage block.
EXTERNAL EXAMINATION
Body bloated and discoloured green/black over most of the surface. It is estimated one week to 10 days must have elapsed since death. Abrasions and small cuts were present on face and knuckles of right hand. There was a stab wound to upper left abdomen, which had bled profusely internally and externally. Bleeding shows that death took place up to 30 minutes after the stabbing.
Weight: 98.3 kg
Height: 184 cm
INTERNAL EXAMINATION Central nervous system:
Scalp Normal Skull Normal Meninges Normal Middle ears Not examined
Dural sinuses Normal Vessels at base of brain Normal
Brain Normal Spinal cord Not examined
Respiratory system:
Hyoid bone and larynx Normal Trachea and main bronchi Normal Pleural cavities Normal
Lungs (left, right) Both lungs were normal. Diaphragm Normal
Cardiovascular system:
Pericardial sac Normal
Heart Normal
Salters-Nuffield Advanced Biology Resources
Activity 6.5 Student SheetGastrointestinal system:
Mouth, tongue, pharynx Normal
Oesophagus Normal
Stomach The stomach contained a small quantity of clear liquid. The mucosa was normal.
Duodenum Normal Small intestine Normal
Large intestine Normal
Appendix Normal Rectum Normal
Liver The liver tissue was normal. The capsule was intact. Gall bladder and biliary tree Normal
Pancreas Normal Peritoneal cavity Normal
Genito-urinary system:
Kidneys The renal capsules stripped easily to reveal normal renal surfaces. The renal cortex, medulla, calyces and pelvis of both kidneys were normal. Ureters Normal
Bladder Normal Prostate gland Normal
Testes Normal
Reticuloendothelial system:
Spleen Ruptured capsule, resulting in extensive internal bleeding Lymph nodes Normal
Thymus gland Normal fatty change
Endocrine system:
Thyroid gland Normal Parathyroid glands Normal Adrenal glands Normal
Pituitary Not examined
Musculoskeletal system:
The spinal column, ribs and pelvis were intact.
Specimens retained:
Blood sample.
Comment:
—
Salters-Nuffield Advanced Biology Resources
Activity 6.5 Student SheetBlood test report from the toxicology lab
Redhill and South Laboratory
Toxicology Report
76 Wanefield Park Road Redhill
Surrey
Enquiries concerning this report should be directed to: Ms I.M. Analyst
__________________________________________________________________________________
Case name: Mr Patrick Barrett-Hughes Report number: 2009/1762
Age: 34 Sex: M DoB: 16/06/75
SAMPLES RECEIVED TEST DONE ANALYTICAL FINDINGS
Lab No. Date Time Type Compound Concentration
14.5.09
Blood Cocaine Cocaine 0.6 mg l–1
Urine Drugs of abuse screen Cocaine Detected
Cocaine metabolite(s) Detected
Blood Cocaine metabolite Benzoylecgonine >1000 μg l–l
INTERPRETATION
Following a recreational dose, blood cocaine concentrations are usually below 0.3 mg l–l;
concentrations above 1 mg l–l are often associated with serious toxicity. Benzoylecgonine (cocaine
metabolite) was identified and quantified by immunoassay; concentrations above 500 μg l–l are
Salters-Nuffield Advanced Biology Resources
Activity 6.5 Student SheetDNA profiling briefing document
A body has been discovered at a house in Redhill, Surrey, on May 11, 2009. The body was found outside, near the house and the immediate cause of death was thought to be a stab wound to the abdomen. From the state of decomposition of the body it would appear the victim had been dead for at least a week when discovered.
As the expert on DNA profiling on the crime investigation team you need to present any evidence linking items from the crime scene with the people implicated in the crime.
You have been given four samples for DNA analysis:
1 Victim’s blood from body.
2 Blood from shirt found in outside rubbish bin at 35a Garlands Rd, on May 11 during police search.
3 Cheek cell sample from Mr A. Gifford, sole resident of 35a Garlands Rd.
4 Hair found in wrappings around cocaine in briefcase found at 35a Garlands Rd.
You have given the samples to your laboratory assistants to run genetic profiles. You instructed your assistants to amplify the DNA samples using PCR and suggested that they analyse three satellites, which in this case should be sufficient to match the forensic samples with the blood of either of the two individuals.
Go to the interactive tutorial (DNA analysis simulation) that accompanies this activity. This will allow you to find the results of the DNA analysis of the samples.
Prepare a report explaining your findings and your conclusions. There is a presentation template with some images that may help in your presentation. You need to remember that the detectives you are addressing will not necessarily be familiar with DNA profiles.
You will have to explain the following:
The peaks on the DNA profile represent the alleles of non-coding DNA called STRs selected for analysis.
The alleles for each STR have different numbers of repeat sequences, so are different lengths.
The DNA from a sample is prepared so that the alleles can be separated by electrophoresis. As any individual has two copies of each gene, there will be two peaks for each gene if the individual is heterozygous and one peak if the individual is homozygous.
The three STRs you have selected to analyse have 7, 10 and 13 possible alleles respectively (i.e. the number of different repeat sequences in the STRs), so there is only an extremely small chance of two individuals having exactly the same combination of alleles for all three genes.
Salters-Nuffield Advanced Biology Resources
Activity 6.5 Student SheetForensic entomologist briefing document
A body has been discovered at a house in Redhill, Surrey. The body was found on May 11, 2009 outside, near the house and the immediate cause of death was thought to be a stab wound to the abdomen. From the state of decomposition of the body it would appear the victim had been dead for at least a week when discovered.
As the forensic entomologist on the crime investigation team you need to present evidence for the estimated time of death of the deceased.
You have also been given some maggots from a blood-stained shirt that was found at a nearby address, but may be connected with the death. The shirt was found outside in the bin, wrapped in a plastic bag.
You have the following to use to construct your evidence for time of death:
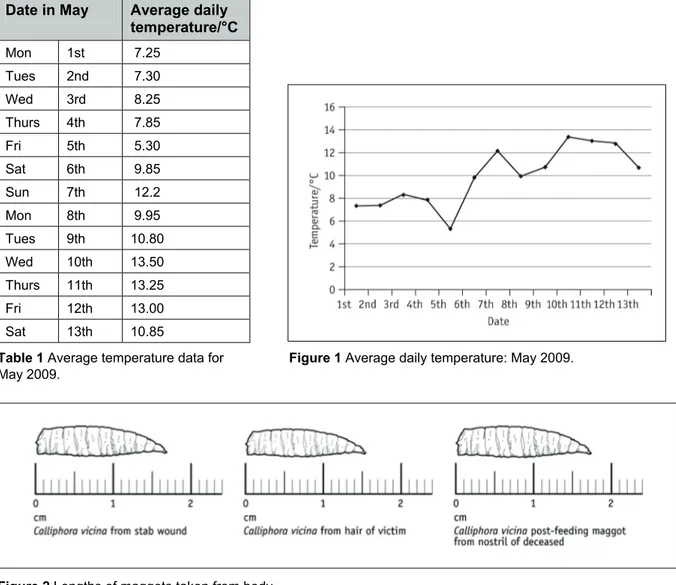
Average temperature data for May 2009. The average temperature for the week before the body was found can be calculated from this.
Diagrams of some preserved maggots taken from the body at the crime scene.
Diagram showing length of Calliphora vicina with age for different temperatures.
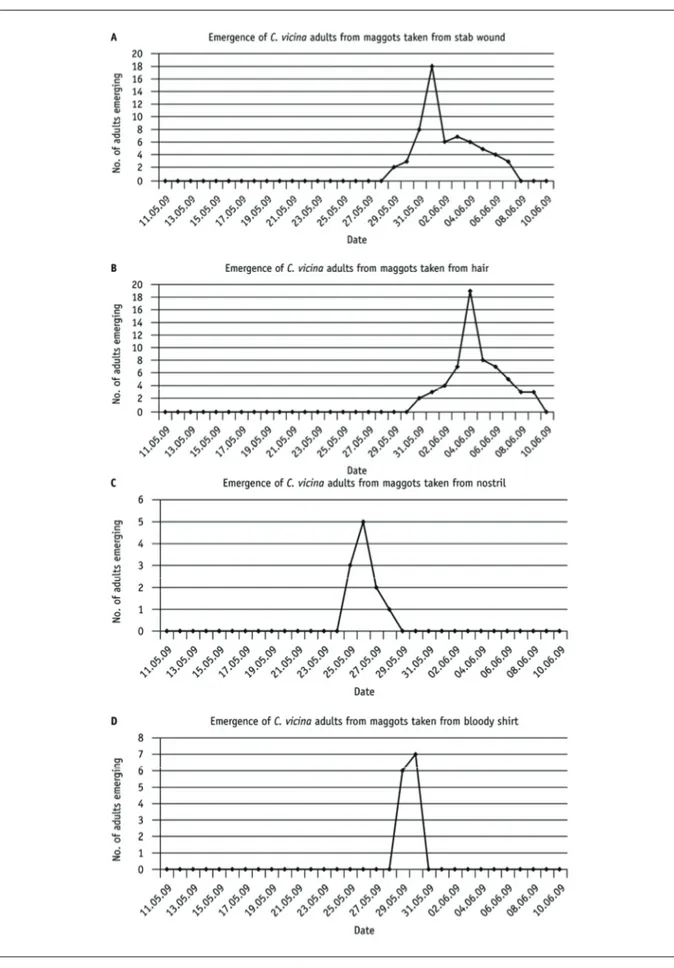
Graphs showing emergence of adults from maggots from the body and from a bloodstained shirt that may be linked with the murder. The maggots have been incubated on liver until emergence of adults, so as to simulate the conditions where the dead body was found. The maggots were incubated at the average temperature for the week before the body was found.
You need to do the following:
Calculate the average temperature for the period 4–10 May, i.e. the period of a week before the body was found. Round up to the nearest whole °C. This is the temperature used to incubate maggots found on the body and shirt.
Measure the length of the maggots and estimate the age of the maggots using the graph of length variations over time with temperature (Figure 6.20, page 82 of Student Book 2). Use the average temperature you have calculated above and the line for the measured maggot length to read the age of the maggot from the x-axis.
Calculate the estimated date of death, using the time for a complete life cycle, as discovered from incubation of the second generation, and the dates of emergence of the first adults from each sample. The time between eggs being laid on the dead body and discovery of the body can be calculated using the formula:
time of discovery of = (time taken for complete life cycle) –
body after egg laying (time taken for emergence of first adults from the maggots found on the body)
It is assumed that this gives an approximate estimate of time of discovery of the body after death. Does this agree with the estimated age of the maggots using their length?
You need to account for the difference in the apparent age of the maggots from the nostril and those from the body and shirt. Find out what factors can accelerate maggot development so that you can make suggestions. If you want to suggest any toxicology tests are performed on the body, ask the lab performing the autopsy to do this for you.
You need to present your findings to the next crime investigation team meeting. There is a presentation with some pictures and data that should help as a basis for your presentation.
C. vicina complete life cycle determination
Salters-Nuffield Advanced Biology Resources
Activity 6.5 Student SheetDate in May Average daily temperature/°C Mon 1st 7.25 Tues 2nd 7.30 Wed 3rd 8.25 Thurs 4th 7.85
Fri 5th 5.30
Sat 6th 9.85
Sun 7th 12.2
Mon 8th 9.95 Tues 9th 10.80 Wed 10th 13.50 Thurs 11th 13.25
Fri 12th 13.00
Sat 13th 10.85
Table 1 Average temperature data for Figure 1 Average daily temperature: May 2009. May 2009.
Salters-Nuffield Advanced Biology Resources
Activity 6.5 Student SheetSalters-Nuffield Advanced Biology Resources
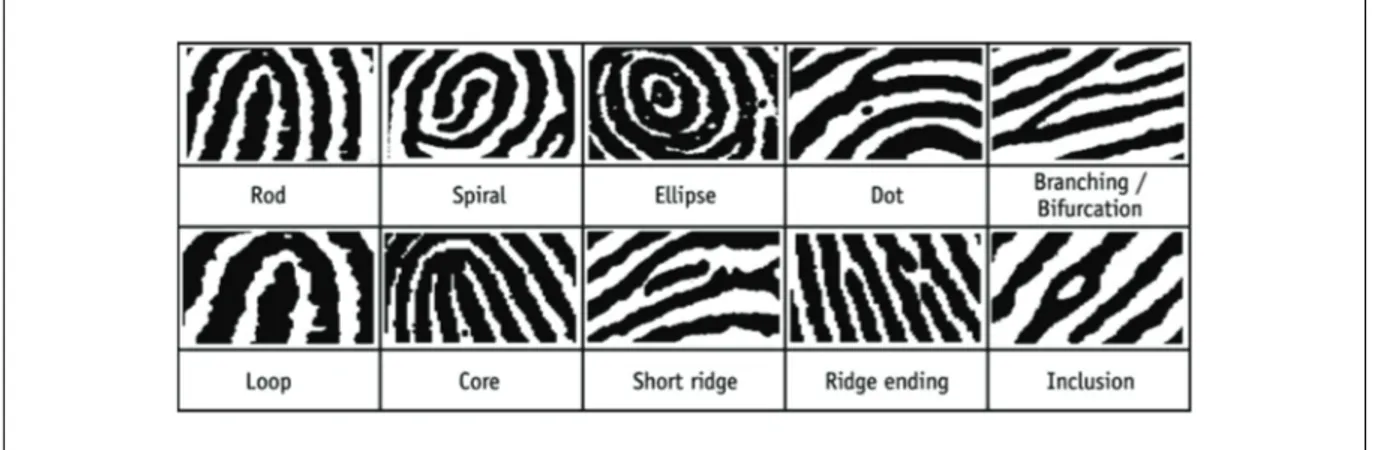
Activity 6.5 Student SheetFingerprint expert briefing document
A body has been discovered at a house in Redhill, Surrey, on May 11, 2009. The body was found outside, near the house and the immediate cause of death was thought to be a stab wound to the abdomen. From the state of decomposition of the body it would appear the victim had been dead for at least a week when discovered. As the fingerprint expert on the crime investigation team you need to present evidence matching either the deceased (Mr Patrick Barrett-Hughes) or the main suspect (Mr Anthony Gifford) to other items from the crime scene.
You have a selection of fingerprints from the crime scene, along with ‘ten print’ fingerprint cards taken from Mr Patrick Barrett-Hughes and the prints of Mr Anthony Gifford from the central records department.
Each member of your team can be given one crime scene fingerprint to match. Sixteen or more matching points should be found in order to confirm the match between a crime scene print and one of the people implicated in the crime, but fewer are accepted in court if the expert considers the features to provide sufficient evidence of a match. Use the weblinks that accompany Extension 6.1 to find any more information you need about fingerprint matching.
You need to make a short presentation to the crime investigation team, summarising any conclusions you come to about the fingerprints you have been given to match. The images of the fingerprints can be found in the mediabank on SNAB Online and can be downloaded for use in a Microsoft
PowerPoint presentation.
Matching fingerprints
The basic fingerprint patterns must first be matched and then the point details are identified.
Figure 1 Fingerprint patterns.
Salters-Nuffield Advanced Biology Resources
Activity 6.5 Student SheetLeft hand: Mr Anthony Gifford
Little finger Ring finger Middle finger Index finger Thumb
Right hand Mr Anthony Gifford
Thumb Index finger Middle finger Ring finger Little finger
Left hand: Mr Patrick Barrett-Hughes
Little finger Ring finger Middle finger Index finger Thumb
Right hand: Mr Patrick Barrett-Hughes
Thumb Index finger Middle finger Ring finger Little finger
Print from the briefcase containing cocaine found at 35a Garlands Road, Redhill.
Salters-Nuffield Advanced Biology Resources
Activity 6.5 Teacher SheetCRIME INVESTIGATION
Purpose
To explore the use of forensic techniques in an applied context.
Body discovered
In this role play students investigate a murder. A body has been discovered at a house in Redhill, Surrey, on May 11, 2009. The body was found outside, near the house and the immediate cause of death was thought to be a stab wound to the abdomen. From the state of decomposition of the body it would appear the victim had been dead for at least a week when discovered.
Experts investigate
The crime is investigated by various forensic ‘experts’. The students should be allocated places in expert groups and given the appropriate handouts. Fingerprinting is not mentioned in the specification so you may decide to give the results to the group or combine this role with the Scene of Crime Officer (SOCO) or Crime Investigation Officer. The students need to be given some preparation time (for example, a week’s homework allocation) before a simulated crime investigation team meeting. The Crime Investigation Officer chairs the investigation team meeting. He/she should introduce the crime investigation, then call upon the SOCO to speak, then call upon the various experts to speak. The Crime Investigation Officer should lead the discussion, as described in his/her brief.
Handouts for each group are provided and in some cases there is a presentation to facilitate the report, which can be accessed from the interactive tutorial that accompanies this activity.
The various jobs could be combined if there are only a few in the group. Conversely, if you have a large group there could be two separate investigation teams. They may not necessarily come to the same conclusion. Fingerprint analysis is relatively simple so can easily be combined with the forensic pathologist and/or the SOCO. The Crime Investigation Officer could be a person selected from any team or could be combined with the SOCO’s role as the Crime Investigation Officer does not have preparation to complete.
The outcome
The details of the final conclusion are not that important, but it should be possible to deduce that the victim, Patrick Barrett-Hughes, met with Gifford at Garlands Rd, where he could have handed over the briefcase and accepted money. The witness statements suggest a row broke out. If the stabbing took place at this point, it could have been possible for the victim to get home as it can be seen from the map that Whitepost Hill is only around the corner from Garlands Rd. A likely conclusion is that the case should be passed on for a murder trial. See the hints column on page 2 for detailed outcomes for each expert group.
Follow up
The Crime Investigation Officer will suggest that the team take notes during the expert reports, in order to discuss the outcome at the end.
In order to draw all the information together, the students could be asked to write an article for a popular scientific journal on ‘The biological basis for forensic analysis’. The DNA profiling animation and the PCR animation from Activity 6.1 may be useful to reinforce understanding of these two aspects. Students may need to be prompted to include information on genetic variation as the basis for some forensic analyses.
Salters-Nuffield Advanced Biology Resources
Activity 6.5 Teacher SheetExpert team Handouts Hints
Forensic pathologist (one student)
● Forensic scientist’s brief ● Autopsy report ● Toxicology report
The autopsy report suggests that, although fatally stabbed, the victim could have survived long enough to get home after the events that may have taken place in Garlands Rd. Cuts and bruises suggest the victim had been fighting recently. The toxicology report shows the victim had been using cocaine recently.
SOCO (one student) ● SOCO brief ● SOCO Microsoft
PowerPoint presentation for report
This role could be combined with that of the Crime Investigation Officer.
DNA analysis (small group)
● DNA profile brief ● DNA profiling animation ● DNA profiling Microsoft
PowerPoint presentation
The DNA analysis shows that the blood on the shirt found at Garlands Rd was stained with the blood of the victim and the suspect, Gifford. The hair in the cocaine packaging in the briefcase belonged to the victim, suggesting that the victim handled the cocaine at some point.
Entomologist (small group)
● Entomology briefing sheet Entomology Microsoft PowerPoint presentation
The average temperature for 4–10 May was 9.9 °C. Maggot measurements suggest those in the stab wound are about 11 days old; those from the hair are 9.5 days old; the post-feeding maggot is about 17 days old. The earliest emergence of adult flies from the stab wound area, along with measurement of the maggots, suggests that the body had been dead for 10 days. (The complete cycle of development takes 28 days, and the first emergence of adults was after 18 days.)
It would be expected that flies would oviposit first in a wound before the rest of the body, attracted by the blood. This accounts for the delayed development of the maggot from the hair of around one day. The accelerated development of the maggot from the nostril could either indicate that the body had been covered apart from the head at first, or that there is a substance, for example, a drug, present that has stimulated the accelerated development. To find out, the team can ask for a toxicology report from the pathology team. (This could actually be carried out on the maggot itself, but in this case the report is on blood from the body.)
Fingerprint analysis (small group)
● Fingerprint analysis briefing sheet
The briefcase containing cocaine has a print that can be matched with the right middle finger of the victim, Patrick Barrett-Hughes. The money package can be matched with the victim’s right index finger and with the thumb and index finger from Gifford’s left hand. This backs up the evidence from the DNA analysis that the victim may have been involved in a transaction related to the cocaine.
Salters-Nuffield Advanced Biology Resources
Activity 6.6 Student SheetBACTERIA AND VIRUSES
Purpose
To compare the structure of bacteria and viruses.
Viruses – neither eukaryotic nor prokaryotic
The interactive cell, accessed through Activity 3.1, concentrates on the structure and function of eukaryotic cells. Your task is to remind yourself about prokaryotic cells, and then produce either an animation brief for an additional section on bacteria and viruses that could be added to the website, or a presentation.
The animation brief/presentation should include details of the size and structure of bacteria and viruses. Some illustration of the size of prokaryotes relative to eukaryotic cells and possibly organelles, or other objects that can be easily visualised, would be useful. You could include a diagram so that when each component part is clicked, more detailed information, diagrams and/or a photograph appears. You should also include a section on viruses. You could incorporate animations to show division, viral infection of a cell or any other appropriate events.
Salters-Nuffield Advanced Biology Resources
Activity 6.6 Student SheetSalters-Nuffield Advanced Biology Resources
Activity 6.6 Teacher SheetBACTERIA AND VIRUSES
Purpose
To compare the structure of bacteria and viruses.
Viruses – neither eukaryotic nor prokaryotic
Students revisit Topic 3, which introduced prokaryotic cell structure, and extend their knowledge to include the structure of viruses. There is a section of Student Book 2 that includes this information. The Student Sheet asks students to design a section for the interactive cell or a presentation.
Alternatively, an extended piece of writing could be done that distinguishes between the structure of bacteria and viruses, and provides practice of extended writing.
The Microbiology Society has published Viruses: a practical resource for post-16 biology teachers, which details practicals using a safe, easy to handle bacteriophage. The publication is free to members or can be purchased via the Society’s website Microbiology online, which includes a wealth of resources and valuable information.
Key features of prokaryotes
No nucleus DNA in simple ring structure free in cytoplasm
Cell wall present, not made of cellulose, but peptidoglycans (polymer of sugars and amino acids)
Cell membrane controlling passage of substances into and out of the cell
Lack of membrane-bound organelles
Infolding of the cell membrane called mesosome is the site of respiration
Ribosomes (18 nm diameter) free in the cytoplasm are the sites of protein synthesis
Cell size is between 0.5 and 5 μm
Cells divide by binary fission with no spindle formation.
Key features of viruses
Have an outer protein coat (capsid) around a core containing a nucleic acid (can be RNA or DNA) and some virus enzymes
Lack internal membranes, cytoplasm and ribosomes
Some also have an outer envelope taken from the host cell’s surface membrane, so that the envelope contains lipids and proteins
Viral envelopes also have glycoproteins from the virus itself – these are antigens recognised by the host’s immune system. This envelope helps the virus attach to the cell and penetrate the surface membrane
Vary in size between 20 nm and 1 μm.
Salters-Nuffield Advanced Biology Resources
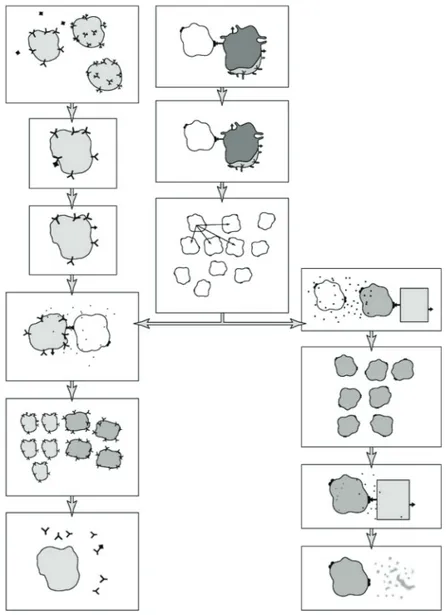
Activity 6.7 Student SheetPHAGOCYTOSIS
Purpose
To describe phagocytosis and the body’s non-specific response to infection.
A vital role in the immune response
Watch the interactive tutorial that accompanies this activity. It shows in detail what happens when a phagocytic cell, such as a macrophage, encounters a bacterium or any other foreign object. This process is not only important for the destruction of pathogens at the site of infection, but is important in the activation of the specific immune response.
Salters-Nuffield Advanced Biology Resources
Activity 6.7 Teacher SheetPHAGOCYTOSIS
Purpose
To describe phagocytosis and the body’s non-specific response to infection.
A vital role in the immune response
The interactive tutorial that accompanies this activity is referred to twice during the topic. Here, the aim is to help students understand the importance of phagocytosis for destruction of bacteria and presentation of antigens. The student makes notes on the sequence of events that occur in the
animation. Student Book 2 describes phagocytosis simply in the section on non-specific responses to infection and again within the specific immune response, including the detail of antigen presentation. The description may include the following points.
Membrane processes surround the bacterium.
The bacterium is engulfed by the macrophage.
The bacterium is inside the cell, contained within a vacuole.
The bacterium is digested within the vacuole.
A second vacuole containing some small fragments of the bacterium buds from the main vacuole.
The vacuoles fuse with the cell surface membrane.
The digested contents are released from the cell. The bacterial fragment (antigen) combines with the cell surface membrane.
The antigen is said to have been presented on the surface of the macrophage.
Salters-Nuffield Advanced Biology Resources
Activity 6.8 Student SheetTHE SPECIFIC IMMUNE RESPONSE
Purpose
To explain the body’s specific immune response.
The role of T and B cells
Q1 Use the interactive tutorial that accompanies this activity to label and annotate Figure 1, showing the specific immune response.
Figure 1 The specific immune response.
Q2 In patients suffering from Severe Combined Immuno-deficiency (SCID) the T helper cells do not produce cytokine. Use the diagram to explain why SCID patients are vulnerable to both viral and bacterial infections.
Q3 What is the role of macrophages in the specific immune response?
Salters-Nuffield Advanced Biology Resources
Activity 6.8 Teacher SheetTHE SPECIFIC IMMUNE RESPONSE
Purpose
To explain the body’s specific immune response.
The role of T and B cells
Students use the interactive tutorial that accompanies this activity and/or Student Book 2 to annotate the diagram on the Student Sheet, which summarises the specific immune response.
Student Book 2 and the interactive tutorial focus on the role of B and T cells in the immune response. The detail on antigen presentation is important in terms of specificity of the response.
The animation includes major histocompatibility complex proteins (MHCs) as purple bars presenting red triangular antigens. Detail of MHCs is not required and the term is not included in the Student Book and animation text. The blue bar-shaped protein still occurs in the animation diagrams. If students enquire about them, they could be referred to as special antigen presenting proteins. Complement proteins and details of immunoglobulins are not required.
The British Society of Immunology has a website called Bite-Sized Immunology which provides detailed accounts of all aspects of immunology. Take care if recommending to students as it is very detailed and some of the terminology used is different to that in SNAB, for example it refers to CD4+ and CD8+.
Answers
Q1 See labelled diagram on page 2.
Q2 The cytokines secreted by T helper cells stimulate cloning of T killer cells and B cells. The T killer cells destroy any body cells infected with a virus. Without cytokines, there would not be enough T killer cells produced to fight a viral infection. B cells produce antibodies that bring about destruction of viruses or bacteria in the blood and lymph. This process relies on B cell cloning, stimulated by cytokines. Without T cell production of cytokines, the specific immune response is not sufficient to respond to any type of infection successfully.
Q3 Macrophages engulf bacteria or viruses and become antigen-presenting cells. They then activate T helper cells with complementary receptors. The debris from cell lysis by T killer cells is cleared away by macrophages.
Antibodies mark antigens on bacteria or viruses in the blood and lymph, making them recognisable by macrophages for phagocytosis and destruction.
Salters-Nuffield Advanced Biology Resources
Activity 6.8 Teacher SheetSalters-Nuffield Advanced Biology Resources
Activity 6.9 Student SheetTUBERCULOSIS
Purpose
To consider the sequence of symptoms of tuberculosis (TB).
To apply knowledge of the specific immune system.
Abstract the relevant information on TB
Read the following passages about TB, then answer the questions that follow, using both the information in the passages and the Student Book.
Case study: TB
Tuberculosis (TB) is a bacterial disease caused by Mycobacterium tuberculosis.
Centuries ago, TB was known as ‘consumption’ and was thought to be incurable. Today, TB is treatable with antibiotics, but over a million people with the disease still die every year, mostly in poorer countries.
Not everyone who gets infected with M. tuberculosis develops symptoms, which include coughing and weight loss. Worldwide, as many as 1 in 3 people may carry the bacterium. The bacteria are
phagocytosed in the lungs by immune cells called macrophages. In most people, tuberculosis infection remains latent, kept in check by T cells. But in people with weakened immune systems, such as caused by HIV, it can reactivate to cause post-primary TB, usually affecting the lungs.
If the bacterium that causes TB gets into the brain through the bloodstream it can cause an infection called tuberculous meningitis. The live Bacille Calmette–Guérin (BCG) vaccine can prevent this in children, but it does not protect against lung disease in adolescents or adults. The BCG vaccination is no longer routinely given to teenagers in the UK because cases there are so rare, but it is used in the UK for at-risk babies.
Blood tests for TB measure the levels of an immune molecule called interferon-gamma, which is produced by an infected person’s white blood cells when they are mixed with antigens from M. tuberculosis. Treatment for TB involves taking a combination of different drugs for several months, but this regimen must be strictly adhered to or the bacterium can become drug-resistant and harder to treat. Drug-resistant forms of TB are a serious health concern.
Extract from ‘Big picture: Immune System’ published by the Wellcome Trust, in January 2015 on bigpictureeducation.com.
Tuberculosis
After tuberculosis bacteria have been inhaled by a person who has not previously been infected with, or vaccinated against, M. tuberculosis, they may start to multiply within the lung. They often infect the upper part of the lung, where the oxygen supply is greatest. This infection sets up an inflammatory response directed by the immune system. This area of inflammation is known as a granuloma; macrophages congregate here in an attempt to ingest the bacteria and contain the infection.
After around 3–8 weeks, the lymph nodes draining this infected area of lung enlarge in response to the infection. At this point the infection is either contained, or it spreads. Usually, in an otherwise healthy person, the immune system controls the infection and the area heals.
Salters-Nuffield Advanced Biology Resources
Activity 6.9 Student SheetIf the person’s immune system is not functioning properly, then the disease is more likely not to be contained. The bacteria spread throughout the infected lung causing a tuberculous pneumonia. The bacteria can then also spread in the bloodstream to affect other organs.
Extract from the article Gull, D. (2001) Tuberculosis. Biological Sciences Review, 13(1), 30–33.
XDR-TB: A disaster in the making?
In April 2014 the leading medical journal the ‘Lancet’ published a study of 107 patients who were diagnosed with and treated for ‘extensively drug-resistant TB’ (XDR-TB) in South Africa. XDR-TB is a form of TB that is resistant to both first- and second-line drugs, meaning there are very few
treatment options left for patients.
By comparison, multidrug-resistant (MDR) TB is resistant to first-line treatment – that given initially to patients. After two years nearly half of the patients in the study were dead and after five years almost three-quarters had died. Most samples of bacteria (though only available for around half of the patients) were resistant to at least eight different drugs. Some were resistant to ten.
The World Health Organization (WHO) has called for an end to the global TB epidemic and has developed an End TB Strategy, which provides a framework for tackling TB from 2016 onwards, with priority actions for stamping out MDR-TB (including XDR-TB). Currently around 4 per cent of new cases and 21 per cent of previously treated cases of TB are multidrug-resistant, and 9 per cent of patients with MDR-TB have what is classed as XDR-TB.
In South Africa, multidrug-resistant disease is on the rise. In 2011 there were 500 confirmed cases of XDR-TB and many more that were not confirmed. The editorial accompanying the recent study in the ‘Lancet’ described MDR-TB, including XDR-TB, as ‘an out of control problem with potentially vast and devastating repercussions for global public health’.
Extract from ‘Big Picture: Immune System’ published by the Wellcome Trust, in January 2015 on bigpictureeducation.com.
Q1 Explain how ‘tuberculosis infection remains latent, kept in check by T cells’.
Q2 Describe the role of interferon in the immune response.
Q3 Compare and contrast MDR-TB and XDR-TB.
Q4 Why is it advantageous to tuberculosis bacteria to ‘infect the upper part of the lung’?
Q5 What is meant by a granuloma? What are they like in a TB-infected lung?
Q6 Name the process by which macrophages ingest bacteria.
Q7 State two conditions that can reduce the proper functioning of the immune system.
Q8 Tuberculosis bacteria can invade and replicate inside the macrophages. Explain how this enables them ‘to evade the body’s defence mechanisms’.
Q9 Once in the bloodstream, what other parts of the body could be infected by TB bacteria?
Q10 For most cases the WHO recommends 20 months of drug treatment for MDR-TB. However, shorter treatment regimes have been introduced in Bangladesh and several African countries. Although cheaper and better tolerated by patients, concerns have been expressed about using shorter treatments. What might be the consequences for drug resistance of introducing shorter treatment periods?
Salters-Nuffield Advanced Biology Resources
Activity 6.9 Teacher SheetTUBERCULOSIS
Purpose
To consider the sequence of symptoms of tuberculosis (TB).
To apply knowledge of the specific immune system.
Abstract the relevant information on TB
This activity draws together ideas about TB and the development of drug resistance which will be studied in detail later in the topic. The activity provides students with practice in abstracting
information from a published article. Students will need to refer to the Student Book for some details that do not appear in the articles.
Answers
Q1 In latent TB, live TB bacteria remain within the granuloma/tubercules, but there are no signs or symptoms of disease. T killer cells destroy any infected macrophages, preventing spread beyond the tubercule.
Q2 Interferon is produced by cells infected by the microbe. It prevents the microbe multiplying by inhibiting protein synthesis.
Q3 Both are drug resistant forms of TB; XDR-TB is resistant to a wider range of available treatments than MDR-TB; MDR-TB is resistant to the first-line drug treatments, but not the second-line treatments, whereas XDR-TB is resistant to both first- and second-line treatments.
Q4 M. tuberculosis is an aerobic bacterium so needs oxygen. The upper part of the lung will have the highest oxygen content.
Q5 A granuloma is a growth of tissue produced around an area of infection. In TB they are anaerobic. They contain dead bacteria and macrophages. They are microscopic structures called tubercules.
Q6 Phagocytosis is the process by which macrophages ingest bacteria.
Q7 Malnutrition; infection with another disease; immune-suppressing drugs; general ill health (for example, resulting from excessive stress).
Q8 Macrophages are key cells in the immune system. By infecting the macrophages the bacteria prevent the macrophages from killing them. Inside the macrophages, other white blood cells cannot detect them; their antigens are hidden (or words to that effect).
Q9 Brain, bones, intestine, lymph nodes, kidneys (almost any part of the body is at risk of infection).
Salters-Nuffield Advanced Biology Resources
Activity 6.10 Student SheetGRAM STAINING BACTERIA
Purpose
To stain bacterial cells.
To distinguish between Gram-positive and Gram-negative bacteria.
To develop practical skills.
YOU NEED
● A mixed bacterial culture of a Gram-positive bacterium (e.g. Bacillus subtilis) and a Gram-negative bacterium (e.g. Escherichia coli) ● Crystal violet solution
● Gram’s iodine solution ● Ethanol
● Safranin solution (counterstain) ● Microscope slide
● Cotton wool bud ● Forceps ● 4 teat pipettes ● Wash bottle ● Blotting paper
● Stopclock
● Bunsen burner and matches
● Wax pencil or sticky label for labelling slide ● Microscope (×400 magnification) and
microscope lamp
● Immersion oil if using oil immersion lenses (ask your teacher/lecturer)
● Disinfectant and cloths to wipe down practical area
● Large beaker of disinfectant for dirty slides and used cotton wool buds
● Access to a sink and staining racks
SAFETY
Wear eye protection during the preparation and use of the stains and iodine solution. Ensure the room is well ventilated. Skin contact should be avoided at all times. Consult CLEAPSS Student Safety Sheet 70.
Solid crystal violet is toxic. Only use the solution provided. Gram’s iodine solution is harmful.
The bacterial cultures present a biohazard, and should be handled with care. Disinfect work
surfaces with Virkon™ solution (leave in place for 10 minutes). Any slides and used cotton wool
buds should be placed into the beakers of disinfectant provided.
Hands should be washed thoroughly before leaving the lab, and after clearing away at the end of the practical work.
Ethanol and safranin solution are highly flammable. Ensure that everyone has completed ‘heat-fixing’ slides and all Bunsen burners are turned off before either ethanol or safranin are used.
Gram-positive or Gram-negative?
One of the major differences between types of bacteria is the ability to stain their cell walls using a procedure called Gram staining, developed by C. Gram in 1884. Bacteria that have cell walls that take the stain are called Gram-positive; those that are not stained are called Gram-negative. The distinction matters because it is the first step in identifying bacteria, and because negative and Gram-positive bacteria respond differently to antibiotics.
This activity shows how stains can be used to distinguish between the two different types of bacteria. Gram-positive bacteria take up a dye called crystal violet and retain it even when they are rinsed with alcohol. Gram-negative bacteria lose the violet dye in alcohol, but stain with a dye called safranin.
Planning and experimental design
Salters-Nuffield Advanced Biology Resources
Activity 6.10 Student SheetProcedure
1 Dip a cotton wool bud into the bacterial culture, then smear a little onto a slide. Put the cotton wool bud into the beaker of disinfectant for disposal.
2 Air dry the slide.
3 Hold the slide with forceps and ‘heat-fix’ the bacterial smear by passing the slide quickly, smear side up, through the hottest part of a Bunsen flame (just above the pale blue cone). Only do this once. Do not overheat, as this will burn the bacteria and ruin your result. If the slide is too hot to handle after heating, it has been heated too much. If this is the case start again. Leave the slide to cool.
4 Switch off the Bunsen burner. The ethanol used in Step 8 is highly flammable. Do not use it if anyone still has a lit Bunsen.
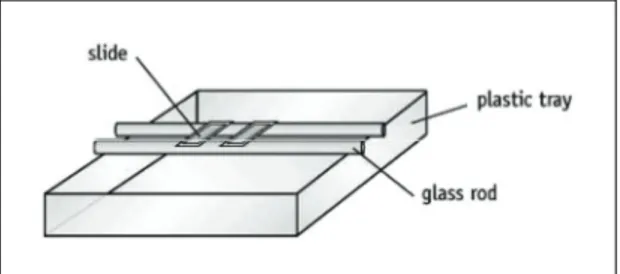
The remaining steps are best done with the microscope slide suspended on a pair of rods over a sink or tray (see Figure 1).
Figure 1 Microscope slide suspended on glass rods.
5 Using a teat pipette, flood the slide with crystal violet solution. Leave for exactly one minute.
6 Pour off any excess stain. Use forceps to handle the slide and gently wash the slide with running water.
7 Flood with Gram’s iodine solution to fix the stain. Leave for just over a minute.
8 Tilt the slide and add drops of ethanol until no more violet stain appears in the liquid that runs off.
9 Quickly wash with drops of water from a wash bottle.
10 Pour off the water and add safranin to counterstain. Leave for 15 seconds, then pour off any excess stain and wash with water from a wash bottle. Pour off the drops of water and allow the preparation to dry naturally.
11 Whilst the slide is drying, set up your microscope.
12 Place the slide on a microscope. Roughly focus the slide under low power and then switch to ×400 magnification to view the stained bacteria. Blue/purple = positive; red/pink = Gram-negative.
If you are using an oil immersion lens, place a drop of immersion oil directly onto the smear. Carefully lower the lens until it touches the oil. Now focus by moving the lens away from the specimen.
Describe what you see, using diagrams if helpful, and relate this to the technique used.